Abstract
The effectiveness of a chitosan-coating treatment on the physical and chemical, nutraceutical, and sensorial traits of three sweet cherry cultivar (Prunus avium L., namely cvs. “Ferrovia,” “Lapins,” “Della Recca”) harvested at the commercial ripening stage was evaluated. Fruits were treated with a 0.5 % chitosan coating, stored at 2 °C for 14 days, and sampled weekly followed by storage at 24 °C for 3 days to assess the shelf life of the fruit. Physical-chemical (weight loss, soluble solid content, and titratable acidity), nutraceutical (total polyphenol, anthocyanin, flavonoid, ascorbic acid content, antioxidant capacity), and sensory (fruit juiciness, sweetness, sourness, texture, and taste) evaluations were performed. A chitosan coating significantly reduced the water loss and delayed the changes in color, titratable acidity, ascorbic acid content, and respiration rate in a cultivar-dependent manner. Additionally, changes in the total polyphenol, anthocyanin, and flavonoid content and in the antioxidant capacity of the chitosan-coated sweet cherry fruits were delayed. This treatment extended the postharvest life, improved the storability, and enhanced the nutraceutical value of sweet cherry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sweet cherry (Prunus avium L.) is an important crop from temperate areas that has been valued since ancient times (Vavilov & Starr Chester 1951). This fruit is very widespread and appreciated by the consumers and it is mainly consumed as not processed (Usenik et al. 2008).
Consumer fruit preferences and choices are influenced not only by different factors, such as convenience, price, and appearance, but also by the nutrient value and bioactive compound content. Health-promoting compounds are the most recently demanded in sweet cherry fruits. This fruit, as others, is an excellent source of minerals, vitamins, antioxidants, and bioactive elements, which are important nutritional components for a healthy diet. Recent epidemiological studies have demonstrated that sweet cherry consumption decreases the concentration of serum inflammatory biomarkers (Kelley et al. 2013). Changes in these identified biomarkers suggest that sweet cherry consumption may reduce the risk or modify the severity of inflammatory diseases, such as arthritis, diabetes, cardiovascular disease, high blood pressure, and cancer (Kelley et al. 2013). The health-protective effects of sweet cherry fruits have been attributed to phytochemical compounds, such as carotenoids, flavonoids, isoflavonoids, and phenolic acids, which are the non-nutrient plant compounds (Ferretti et al. 2010). Phytochemical composition varies greatly among the different cherry cultivars, with small changes due to pre- and postharvest factors (Serrano et al. 2005; Diaz-Mula et al. 2012).
Sweet cherries are a non-climacteric fruit with a high transpiration rate and a susceptibility to fungal rots and physiological disorders (Alique et al. 2005). Harvested sweet cherry fruits are highly perishable and often do not reach consumers at an optimal quality. Cold storage is the main postharvest treatment to reduce the rate of many metabolic processes in perishable fruits, to maintain quality, and to extend the storability of cherries considered to be non-chilling sensitive. To extend the shelf life of postharvest fruit, recently, several technologies, including modified atmosphere packaging, irradiation, and coating in combination with cold storage, have been applied (Xanthopoulos et al. 2012; Castagna et al. 2013). Edible coating with semipermeable films can provide an alternative to modified atmosphere storage by reducing quality changes and quantitative losses through the modification and control of the fruit internal atmosphere (Dhall 2013).
Chitosan, a deacetylated derivate of chitin, is a high-molecular-weight cationic linear polysaccharide composed of d-glucosamine and, to a lesser extent, N-acetyl-d-glucosamine with a β-1,4-linkage (Rinaudo 2006; Shahidi 2007; Baez-Sañudo et al. 2009). Chitosan is normally extracted from an abundant source of shellfish exoskeletons or the cell wall of some microorganisms and fungi (Hirano et al. 1976). Chitosan-based coatings are considered the best edible and biologically safe preservative coatings for different types of food, owing to their lack of toxicity (Jayakumar et al. 2005; Prabaharan & Mano 2006), biodegradability (Arvanitoyannis, IS 1999), film-forming properties (Arvanitoyannis et al. 1998), and antimicrobial action (Aider 2010; No et al. 2007; Yang et al. 2012). Chitosan-based coatings have been used to improve the storage and to extend the shelf life of several fruits, including the following: strawberry (El Ghaouth et al. 1991; Wang & Gao 2013), tomato (El Ghaouth et al. 1992), litchi (Zhang & Quantick 1997), kiwifruit (Du et al. 1997), pear (Du et al. 1997), apple (El Ghaouth et al. 2000), mango (Kittur et al. 2001), peach (Li & Yu 2001), longan (Jiang & Li 2001), papaya (Lin et al. 2011), table grape (Romanazzi et al. 2012), and guava (Hong et al. 2012). In sweet cherries, the effectiveness of some edible coatings based on chitosan (Romanazzi et al. 2003; 2013), chitosan acetate (Dang et al. 2010), alginate (Diaz-Mula et al. 2012), Aloe vera gel (Martinez-Romero et al. 2006), sucrose esters of fatty acid, sodium carboxymethyl cellulose, and mono-diglycerides of fatty acids (Semperfresh™) (Yaman & Bayindirh 2001, 2002) have been tested.
There are many studies reporting the effects of chitosan-edible composite coatings on the postharvest quality of cherry fruits. Previous reports suggested that chitosan-based coatings alone or combined with short hypobaric treatment decreased the microbial contamination level, thereby reducing the postharvest decay of sweet cherries (Romanazzi et al. 2003).
Chitosan can be applied either during the preharvest or postharvest period and is one of the most effective means to delay the storage decay of sweet cherries. A recent study on antifungal activity of chitosan in vitro and in field trial applications demonstrated that its antimicrobial activity is comparable to synthetic fungicide (Romanazzi et al. 2013). Postharvest application of chitosan acetate on sweet cherry-delayed water loss maintained the quality attributes during storage and induced peroxidase and catalase activity in fruits (Dang et al. 2010).
Today, nutritional and functional fruit quality has gained great interest as a component of the overall quality that is highly valued by consumers. Therefore, postharvest technologies should maintain both functional and nutritional cherry quality until the cherries reach the consumer. The majority of literature studies provide information on the effects of edible coating on the physical-chemical and sensory quality, but few studies report the effects of chitosan-based coating on sweet cherry nutritional values.
The objectives of this study were to investigate the effectiveness of a chitosan-coating treatment on the physical-chemical and sensory traits of three sweet cherry cultivars and its influence on the fruit nutritional quality by monitoring the changes in the total phenol, anthocyanin, flavonoid, and ascorbic acid content and in the antioxidant capacity during cold storage.
Materials and Methods
Fruit Samples
Three sweet cherry cultivars were selected for this study: “Ferrovia,” “Lapins,” and “Della Recca.” The fruits were collected in 2013 from trees grown under standard commercial practices and in the same experimental orchard located in Pignataro Maggiore (Caserta, Southern Italy; 41° 09′ N, 14° 08′ E) owned by the CRA Research Unit on Fruit Trees. Fruits were randomly harvested at the commercial ripening stage and were immediately transported to the laboratory and screened for uniformity, appearance, and absence of physical defects or decay. Subsequently, the fruits were randomly distributed in two groups prior to treatment.
Chitosan with 90 % deacetylation and a molecular weight of 360 kDa was prepared at a concentration of 0.5 % (w/v) in an aqueous solution of acetic acid (0.5 % v/v). The solution was warmed to 45 °C and stirred on a magnetic stirrer for complete dissolution of chitosan, adjusting its pH to 5.2 with NaOH. After cooling at 20 °C, the sweet cherry fruits were dipped in the chitosan solution for 60 s to allow the chitosan to adhere to the whole fruit surface to create a uniform film. Samples were dried at 25 °C for 1 h, and 48 lots (≅500 g each) were prepared in 750-mL PET boxes. The same number of lots was prepared with the control fruits dipped in distilled water. The fruits were stored in a controlled chamber at 2 °C and 95 % relative humidity; they were removed after 7 and 14 days of cold storage and, subsequently, held at 24 °C for 3 days to evaluate the shelf life (SL). All analyses were conducted at harvest (day 0), after each period of cold storage, and after keeping fruit for 3 days at 24 °C.
Pomological, Physical, and Chemical Analyses
The pomological and qualitative traits were evaluated according to the International Union for the Protection of New Varieties of Plants (UPOV 2006) descriptors. All reagents and chemicals used in this study were purchased from the Sigma Aldrich (Milano, Italy) and were of analytical grade. All the solutions were prepared using distilled water.
For the physical and chemical determinations that took 20 whole fruits of each lot (i.e., 500 g), they were cut in small pieces, suspended in distilled water, homogenized in a grinder, centrifuged (15,000g, 4 °C, 15 min), and then the flesh juice was analyzed immediately. The physical and chemical harvest indices were estimated as follows: (i) The total soluble solid content (TSS, °Brix) was determined in the flesh juice using a digital refractometer (Sinergica Soluzioni, DBR35, Pescara, Italy) and (ii) the total acid content (TA) was determined by titrating 10 mL of flesh juice with 0.1 N NaOH (Wills et al. 1983). The results are expressed as gram of malic acid per liter of juice. All analyses were performed in triplicate.
Skin color was assessed using a Minolta colorimeter (CR5, Minolta Camera Co., Japan) to determine chromaticity values L* (Lightness), a* (green to red), and b* (blue to yellow) on the opposite sides of 30 fruits for treatment. The hue angle (h) was calculated from chromaticity values a* and b* as reported earlier by McGuire (1992).
The weight loss was expressed as the percentage loss compared with the initial weight.
Respiration Rate
Five hundred grams of fruits for each replicate was placed inside a 1-L air tight glass container equipped with a septum for sampling oxygen and carbon dioxide; analyses were performed in triplicate, following the trend of the same fruits throughout the test.
The rate of CO2 and O2 production was monitored by a gas chromatographic method using a GC Clarus 400 (ShinCarbon ST 80/100 column, TC detector, Perkin Elmer). The activity was expressed as milliliters of CO2 per kilogram of cherries in 1 h.
Total Phenol, Total Monomeric Anthocyanins, and Total Flavonoid Content
The total phenol content in sweet cherry was determined by the Folin-Ciocalteu method (Singleton & Rossi 1965). A phenolic extraction was performed as described by Tomás-Barberán & Espín (2001), and the total phenol content was expressed as milligrams of gallic acid equivalents (GAE) per 100 grams of fresh weight (FW).
The total monomeric anthocyanins were estimated by a pH-differential method (Giusti & Wrolstad 2001) and expressed as cyanidin-3-glucoside equivalent (CGE) per 100 grams of fresh weight (FW) (molar extinction coefficient of 26,900 L cm−1 mol−1 and molecular weight of 449.2 g mol−1). Absorbance measurements were conducted at 520 and 700 nm.
The total flavonoid content was determined by the aluminum chloride colorimetric method (Zhishen et al. 1999) using catechin as a standard. The total flavonoid content was expressed as milligrams of catechin equivalent (CE) per 100 grams of fresh weight (FW). All analyses were performed in triplicate.
Ascorbic Acid Content
The ascorbic acid content from sweet cherry fruit was determined following the method of Malik & Singh (2005), with some modifications. Sweet cherry fruits (5 g) was homogenised with 20 mL 16% (v/v) metaphosphoric acid solution containing 0.18 % (w/v) disodium ethylene diamine tetraacetic acid. The homogenate was centrifuged at 14,400g for 10 min. The assay mixture contained 400 μL of supernatant 0.3 % (v/v) metaphosphoric acid and (5:1, v/v) diluted Folin’s reagent, in a final volume of 2 mL. After 10 min, the absorbance of the sample was recorded at 760 nm with a UV–vis spectrophotometer (Model V-630, Jasco, Japan). The ascorbic acid concentration was calculated against a 100 % (w/v) ascorbic acid standard curve and was expressed as milligrams of ascorbic acid (AA) per 100 grams of fresh weight (FW).
Total Antioxidant Activity
The free radical scavenging activity of sweet cherry extract was measured by 1,1-diphenyl-2-picryl-hydrazil (DPPH) according to the method of Brand-Williams et al. (1995), with some modifications. Fruit extracts (75 μL) were allowed to react with 1425 μL of the DPPH solution, monitoring the decrease in absorbance for 15 min at 515 nm. The results were expressed in millimolar of Trolox equivalent (TE) per gram of fresh weight (FW).
Sensory Analysis
The fruits were subjected to sensory analysis by a panel of 12 trained assessors (tasters), who evaluated the fruit appearance, juiciness, sweetness, sourness, taste, and texture at harvest time, after cold storage, and after shelf life. Sweet cherry fruits were prepared immediately before being served and were placed into previously coded, labeled disposable plastic cups. The tasters were asked to taste three coded fruits (for each replicate) and, for each sample, to indicate the degree of like or dislike on an unstructured hedonic scale, expressing the attributes on a 10-cm line scale with the anchor points 0-dislike and 10-like, which was quantified by measuring the distance of the mark from the origin (Dever et al. 1996; Navarro da Silva et al. 2013). A score greater than 3, in the 50 % of analyzed parameters, was used as the threshold of sweet cherry fruits acceptability.
Statistical Analysis
All of the data are expressed as the means ± standard deviation (S.D.). To determine the difference between uncoated and chitosan-coated fruit in each cultivar, one-way ANOVA and the least significant difference (LSD) test for mean comparisons were used. Differences at P < 0.05 were considered significant and are indicated with different letters.
Correlations among the physical-chemical, qualitative, and sensory traits were analyzed using Pearson’s correlations (P < 0.05 and P < 0.01, respectively). A principal component analysis (PCA) was applied to describe the relationship between the physical-chemical traits and sensory attributes and to identify the principal components that accounted for the majority of the variation within the dataset. PCA is a widely used multivariate analytical statistical technique that can be applied to reduce a set of dependent variables to a smaller set of underlying variables, called factors, based on the patterns of correlation among the original variables (Chapman et al. 2001). The factors were orthogonally rotated following the “Varimax” method with Kaiser normalization, thus maximizing the sum of the variances of the squared loadings (Davis & Sampson 2002).
All analyses were performed using the SPSS software package, version 20.0 (SPSS Inc., Chicago, IL, USA).
Results and Discussion
Quality Attributes
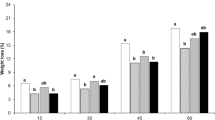
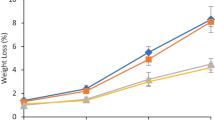
Chitosan coating reduced the changes of the most important quality parameters involved in the sweet cherry acceptability by consumers (Table 1). The weight loss increased significantly throughout cold storage and after the shelf life period. In sweet cherry cultivars, chitosan-coating treatment limited the fruit weight loss compared with uncoated fruits (Tables 1 and 2). The water loss may also vary significantly among coated and uncoated sweet cherry cultivars, resulting in significantly different weight loss values, even under similar storage conditions.
Weight loss in fruit and vegetables is mainly due to the water loss caused by transpiration and respiration processes (Zhu et al. 2008). Sweet cherry fruit is a highly perishable commodity with a low skin diffusion resistance (Serrano et al. 2005) and high surface/volume ratio (Conte et al. 2009; Wani et al. 2014), which promotes rapid water loss both from the fruit and stem (Romano et al. 2006).
Chitosan coatings form a semipermeable film that regulates gas exchange and reduces the transpiration rate, which is generally determined by the gradient of water vapor pressure between the fruit and the surrounding air (Bautista-Banos et al. 2006). This effect on sweet cherries is similar to that obtained with other edible coatings (Yaman & Bayindirli 2002; Martinez-Romero et al. 2006; Dang et al. 2010; Diaz-Mula et al. 2012). During cold storage, respiration rate was very low and no differences were observed among uncoated and coated fruit (Fig. 1). Respiration rate during the shelf life period was lower in chitosan-coated fruits than uncoated with values, after 14 days at 2 °C plus 3 days at 24 °C of 2.03 ± 0.04, 2.33 ± 0.06, and 1.65 ± 0.02 mL kg−1 h−1 in Ferrovia, Lapins, and Della Recca chitosan-coated fruits, respectively, and 1.88 ± 0.02, 1.96 ± 0.03, and 1.57 ± 0.05 mL kg−1 h−1 in Ferrovia, Lapins, and Della Recca uncoated fruits, respectively. Our results are in agreement with previous studies on alginate-coated sweet cherries (Diaz-Mula et al. 2012).
The TSS values of the coated and uncoated fruits increased over the 14 days of cold storage and the following shelf life period (Tables 1 and 2). This increase could be attributed to the breakdown of starch to sugar (Arthey & Philip 2005), to the decrease in respiration rate and conversion of sugars in CO2 and H2O (Ghasemnezhad et al. 2011), to the hydrolysis of cell wall polysaccharides (Comabella & Lara 2013), and to the increase of dry matter due to water loss (Dris & Niskanen 1999). The increase of TSS values on chitosan-coated fruits, of the three sweet cherry cultivars, was likely due to changes in the internal atmosphere of the fruit, with a reduction in the O2 level and/or an increase in the CO2 level and a suppression of ethylene production (Dong et al. 2004). These changes reduce respiration and metabolic activity in the chitosan-coated fruits, resulting in a lower TSS value due to the reduced hydrolysis of starch to sugars as a source of energy (Rohani et al. 1997).
Our results are consistent with other studies concerning coated treatment effects on different commodities, such as mango, banana, papaya, and guava (Kittur et al. 2001; Ali et al. 2011; Hong et al. 2012).
TA estimates the organic acid content of fleshy fruits. In uncoated fruits, TA decreased significantly with increasing storage time, but this decrease in TA values was not observed in chitosan-coated fruits (Table 1). The TA values decreased significantly with increasing shelf life period in uncoated and coated fruit, although these TA changes were less noticeable in coated fruits (Table 2). The higher acidity loss in uncoated fruits could be due to the use of organic acids as substrates for the respiratory metabolism (Díaz-Mula et al. 2009; Diaz-Mula et al. 2012).
The lower acidity loss in chitosan-coated fruits during storage was also reported by other studies on strawberry, peach, tomato, guava, and litchi (Hernandez-Munoz et al. 2008; Li & Yu 2001; El Ghaouth 1992; Hong et al. 2012; Dong et al. 2004), suggesting that chitosan treatment may play a role in delaying fruit ripening.
TSS and TA are important components of fruit organoleptic quality, and the ratio between them is one of the most important parameters determining sweet cherry acceptability by consumers (Crisosto et al. 2003). Coated fruits exhibited a smaller increase in the TSS/TA ratio during cold storage and the shelf life period due to slower ripening than that in uncoated sweet cherries (Tables 1 and 2).
At harvest, the three sweet cherry cultivars exhibited significant differences in fruit color (L* and hue angle) (Table 1). During cold storage, the cherry color tended to darken with a reduction in hue angle values, although these color changes were less noticeable in coated fruits; in coated fruits, the final hue angle value was significantly higher than that in uncoated fruit for every cherry cultivar analyzed.
The decrease in the hue angle was more pronounced during the shelf life period because these color changes are due to over-ripening and senescence processes in fruit postharvest life.
Nutraceutical Compounds and Antioxidant Activity
Genetic, preharvest, and postharvest factors can affect the nutraceutical compound content in sweet cherries. These compounds possess many biological activities, including antioxidant, anticancer, and anti-inflammatory properties (Ferretti et al. 2010). Phenolic compounds are concentrated in the skin and contribute to the sensory and organoleptic qualities of sweet cherries, such as taste and astringency (Ferretti et al. 2010).
Our study reveals that at harvest, the total phenolics content differed significantly among the analyzed sweet cherry cultivars with low values in Della Recca (54.8 ± 3.2) than in Ferrovia and Lapins (101.7 ± 7.5 and 99.6 ± 3.9 mg GAE/100 g FW, respectively). During cold storage and the subsequent shelf life period, we observed a decrease in total phenolics content values influenced both by cultivar and chitosan treatment. During cold storage, coated fruits exhibited a lower decrease in total phenolics content values than did uncoated fruits in the Ferrovia and Della Recca samples, whereas in the Lapins samples, the total phenolics content values did not exhibit changes in coated fruits (Fig. 2a). Changes in total phenolics content exhibited the same trend during shelf life period with higher total phenolics content values in coated fruit (Fig. 2b). Chitosan treatment influences the total phenolics content during postharvest life, improving the nutraceutical properties of sweet cherries. Our study demonstrated that postharvest polyphenol changes are dependent on the cultivar analyzed, as suggested by Goncalves et al. (2004), and on the treatment used (Diaz-Mula et al. 2012).
At harvest, the total anthocyanins content was lower in the bicolor sweet cherry Della Recca (3.5 ± 0.2 mg CGE/100 g FW) and higher in the red sweet cherry Ferrovia and Lapins (64.6 ± 2.1 and 45.1 ± 2.7 mg CGE/100 g FW, respectively). No significant changes in the total anthocyanins content were observed in coated fruit (Ferrovia and Lapins) over the 14 days of cold storage compared with uncoated fruit. Della Recca exhibited a decrease in the total anthocyanin content, but chitosan treatment delayed this decrease (Fig. 3a). During the shelf life period, the chitosan coating improved the total anthocyanin content (Fig. 3b). Among the analyzed cultivars, different types of anthocyanins have been identified; the most highly represented included cyanidin 3-rutinoside, followed by cyanidin-3-glucoside, pelargonidin 3-rutinoside, and peonidin 3-rutinoside (Usenik et al. 2008). The concentration and distribution of different anthocyanins in the skin influences the color of sweet cherry fruit (Esti et al. 2002; Goncalves et al. 2007).
The genotype influences the total flavonoids content in the sweet cherry fruits. At harvest, Ferrovia, Lapins, and Della Recca exhibited values of 3.6 ± 0.2, 3.4 ± 0.3, and 1.4 ± 0.1 mg CE/100 g FW, respectively. The total flavonoid content decreases during cold storage (Fig. 4a) and the shelf life period (Fig. 4b), with a higher flavonoid content in coated compared with uncoated fruits. Flavonoids have an important role in the human diet (Cooper-Driver 2001), reducing oxidative stress in biological systems due to their antioxidant capacities (Kim et al. 2005). In biological systems, most flavonoids are detected as O- or C-glycosides because the glycosylation reduces the reactivity and increases the water solubility of flavonoids, which in turn prevents their cytoplasmic damage and guarantees their storage in the cell vacuole (Cuyckens & Claeys 2005). The presence of glycosides attached to flavonoid aglycons, such as flavonol or anthocyanidin, decreases the antioxidant activity of flavonoid (Prvulović et al. 2011).
The ascorbic acid content decreased in uncoated fruit during the cold storage and shelf life period, while the chitosan coating delayed the decrease in ascorbic acid content (Fig. 5a, b). This trend was observed in a study by Dang et al. (2010), who hypothesized that the reduction of ascorbic acid loss in coated sweet cherries could be due to the low oxygen permeability of the chitosan coating, which lowered the activity of the enzymes and prevented the oxidation of ascorbic acid (Dang et al. 2010).
The antioxidant activities of sweet cherry fruits, measured by DPPH assay, are presented in Fig. 6. During cold storage, we observed a decrease in the antioxidant activity in sweet cherry cultivars, with higher values in coated compared with uncoated fruits (Fig. 6a). The antioxidant activity decreased throughout the shelf life period, with higher values in coated compared with uncoated fruits (Fig. 6b).
Antioxidative capacity can be attributed to synergistic and additive effects between different phytochemicals (Liu 2003; Kim et al. 2005; Vangdal & Slimestad 2006).
Sensory Analysis
A sensory analysis is relevant for fruit consumption. The sensory evaluation approach was used to evaluate the effect of chitosan coating on the three sweet cherry cultivars during cold storage and the following shelf life period. A sensory test was conducted to characterize the properties of sweet cherry fruits using a quantitative descriptive analysis. The results of the sensory tests are presented in Tables 3 and 4 as a function of the cold storage period and the shelf life in coated and uncoated fruits. Cold storage of uncoated fruits slightly affected the sweetness perception, with a slight increase in its values. The sourness perception exhibited the same trend, with a slight decrease with chilling that was most likely due to a decrease in the acid content. Sweetness and sourness are due to the soluble sugar and organic acid composition and to the volatile substances that are very important for fruit quality because they convey the typical character to the flavor (Eccher-Zerbini 2002). Aroma is one of the most important sensory attributes of fruit (Zhang et al. 2008) and results from the combination of sugars, acids, and aromatic materials within the fruit (Kingston 1991). In this study, the aroma values decreased as a function of the different cold storage and shelf life periods and exhibited statistically significant differences among coated and uncoated fruits. The low and decreasing aroma perception may be caused by the unfavorable ratio between the total soluble solid content and titratable acidity and by the small amount of aromatic compounds due principally to genetic characteristics. Texture was the most important quality factor, decreasing significantly with the progress of time both during cold storage and shelf life, with higher values in coated compared with uncoated fruits.
A chitosan coating and low-temperature storage reduced the darkening process and adverse changes in sweet cherry fruit quality, improving the sensorial traits.
PCA and Pearson Correlation After Cold Storage and Shelf Life Period
To assess the efficacy of chitosan coating on the three sweet cherry cultivars analyzed, a principal component analysis (PCA) with physical and chemical and sensorial parameters and the nutraceutical compounds was performed during cold storage and the following shelf life period. The cross-validation technique revealed that three principal components are necessary to explain the total variability of the analyzed traits. The eigenvalues of the covariance matrix revealed that the set of three principal components (PCs) accounted for 89.5 % of the total variance in the dataset with respect to cold storage. PC1 explained 33.5 % of the variance in the dataset, whereas PC2 and PC3 explained an additional 31.0 and 25.0 % of the variance, respectively. The lightness, hue angle, and texture were highly positively correlated with PC1, whereas the polyphenol, anthocyanin, and flavonoid content were negatively correlated. In contrast, the second axis was driven by TSS, titratable acidity, TSS/TA ratio, sweetness, and sourness. The third axis was driven by weight loss, ascorbic acid content, antioxidant activity, appearance, juiciness, and taste. A plot illustrating the overall trends of the quality of the three chitosan-coated and uncoated sweet cherry cultivars is presented in Fig. 7a.
3D principal component analysis plot of the pomological, nutraceutical, and sensory attributes in the three sweet cherry cultivars Ferrovia (F), Lapins (L), and Della Recca (DR) at harvest (C0) and 7 (C7 uncoated fruit, T7 chitosan-coated fruit), and 14 days (C14 uncoated fruit, T14 chitosan-coated fruit) of cold storage (a) or after a 3-day period of shelf life (b) (WL weight loss, TSS total soluble solid content, TA total titratable acidity, TSS/TA: TSS/TA ratio, L L* value, h hue angle, POL polyphenol content, FLAV flavonoid content, AA acid ascorbic content, DPPH radical scavenging activity)
PCA performed on the dataset concerning the shelf life period revealed three PCs. The PC1, PC2, and PC3 principal components accounted for 88.1 % of the total variance (32.1, 31.8, and 24.2 %, respectively). The variables highly correlated with PC1 accounted for the lightness, hue angle, and polyphenol, anthocyanin, and flavonoid content, whereas the variables correlated with PC2 and PC3, particularly PC2, included weight loss, TSS, TA, TSS/TA ratio, sweetness, juiciness and sourness of fruit, and PC3 ascorbic acid content, antioxidant activity, texture, taste, and appearance (Fig. 7b).
The 3D-PCA plots of the physiochemical, sensory, and nutraceutical traits during cold storage and shelf life identified a wide variation in these traits among the three coated and uncoated sweet cherry cultivars. With increasing time in cold storage and of the shelf life period, the uncoated fruits exhibited a lower storability than chitosan-coated fruits, as identified by the average PC scores, with a shift in the different PCs. The results of PCA suggest that the chitosan treatment delays the decay in fruit quality during the 14 days of cold storage in a cultivar-dependent manner.
Conclusions
Chitosan coatings exhibit film-forming properties on fruit surfaces and can be used as protective barriers to reduce respiration and transpiration rates and to retard color changes, thereby improving fruit quality.
This study revealed the positive influence of a chitosan-coating treatment on the physical-chemical and sensory traits of the three sweet cherry cultivars analyzed. Chitosan-coated fruit can be stored for a maximum time of 14 days at 2 °C plus 3 days at 24 °C with changes in the bioactive compound content and antioxidant capacity in a cultivar-dependent manner.
References
Aider, M. (2010). Chitosan application for active bio-based films production and potential in the food industry: review. LWT--Food Science and Technology, 43, 837–842.
Ali, A., Muhammad, M. T. M., Sijam, K., & Siddiqui, Y. (2011). Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chemistry, 124, 620–626.
Alique, R., Zamorano, J. P., Martínez, M. A., & Alfonso, J. (2005). Effect of heat and cold treatments on respiratory metabolism and shelf-life of sweet cherry, type picota cv. ‘Ambrunés’. Postharvest Biology and Technology, 35, 153–165.
Arthey, D., & Philip, R. A. (2005). Fruit processing nutrition, product, and quality management (2nd ed.). Noida: Brijbasi Art Press Ltd.
Arvanitoyannis, IS. (1999). Totally and partially biodegradable polymer blends based on natural and synthetic macromolecules: preparation, physical properties, and potential as food packing materials. Journal of Macromolecular Science, Part C—Reviews in Macromolecular Chemistry and Physics, 39, 205–271.
Arvanitoyannis, IS, Nakayama, A., & Aiba, S. (1998). Chitosan and gelatin based edible films: state diagrams, mechanical and permeation properties. Carbohydrate Polymers, 37(4), 371–382.
Baez-Sañudo, M., Siller-Cepeda, J., Muy-Rangel, D., & Basilio Heredia, J. (2009). Extending the shelf-life of bananas with 1-methylcyclopropene and a chitosan-based edible coating. Journal of the Science of Food and Agriculture, 89, 2343–2349.
Bautista-Banos, S., Hernández-Lauzardo, A. N., Velázquez-del Valle, M. G., Hernández López, M., Ait Barka, E., Bosquez-Molina, E., & Wilson, C. L. (2006). Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Protection, 25, 108–118.
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of free radical method to evaluate antioxidant activity. LWT - Food Science and Technology, 28, 25–30.
Castagna, A., Chiavaro, E., Dall’Asta, C., Rinaldi, M., Galaverna, G., & Ranieri, A. (2013). Effect of postharvest UV-B irradiation on nutraceutical quality and physical properties of tomato fruits. Food Chemistry, 137, 151–158.
Chapman, C. L., Bourke, P. D., & Wright, J. J. (2001). Spatial eigenmodes and synchronous oscillation: coincidence detection in simulated cerebral cortex. Journal of Mathematical Biology, 45, 57–78.
Comabella, E., & Lara, I. (2013). Cell wall disassembly and post-harvest deterioration of ‘Sweetheart’ sweet cherry fruit: involvement of enzymic and non-enzymic factors. Pure and Applied Chemical Sciences, 1, 1–18.
Conte, A., Scrocco, C., Lecce, L., Mastromatteo, M., & Del Nobile, M. A. (2009). Ready-to-eat cherries: study on different packaging systems. Innovative Food Science & Emerging Technologies, 10, 564–571.
Cooper-Driver, G. A. (2001). Contributions of Jeffrey Harborne and co-workers to the study of anthocyanins. Phytochemistry, 56, 229–236.
Crisosto, C. H., Crisosto, G. M., & Metheney, P. (2003). Consumer acceptance of ‘Brooks’ and ‘Bing’ cherries is mainly dependent on fruit SSC and visual skin colour. Postharvest Biology and Technology, 28, 159–167.
Cuyckens, F., & Claeys, M. (2005). Determination of the glycosylation site in flavonoid mono-O-glycosides by collision-induced dissociation of electrospray-generated deprotonated and sodiated molecules. Journal of Mass Spectrometry, 40, 364–372.
Dang, Q. F., Yan, J. Q., Li, Y., Cheng, X. J., Liu, C. S., & Chen, X. G. (2010). Chitosan acetate as an active coating material and its effects on the storing of Prunus avium L. Journal of Food Science, 75, 125–131.
Davis, J. C., & Sampson, R. J. (2002). Statistics and data analysis in geology. New York: Wiley.
Dever, M. C., MacDonald, R. A., Cliff, M. A., & Lane, W. D. (1996). Sensory evaluation of sweet cherry. HortScience, 31, 150–153.
Dhall, R. W. (2013). Advances in edible coatings for fresh fruits and vegetables: a review. Critical Reviews in Food Science and Nutrition, 53, 435–450.
Díaz-Mula, H. M., Zapata, P. J., Guillén, F., Martínez-Romero, D., Castillo, S., Serrano, M., & Valero, D. (2009). Changes in hydrophilic and lipophilic antioxidant activity and related bioactive compounds during postharvest storage of yellow and purple plum cultivars. Postharvest Biology Technology, 51, 354–363.
Diaz-Mula, H. M., Serrano, M., & Valero, D. (2012). Alginate coatings preserve fruit quality and bioactive compounds during storage sweet cherry fruit. Food Bioprocess Technology, 5, 2990–2997.
Dong, H., Cheng, L., Tan, J., Zheng, K., & Jiang, Y. (2004). Effects of chitosan coating on quality and shelf life of peeled litchi fruit. Journal of Food Engineering, 64, 355–358.
Dris, R., & Niskanen, R. (1999). Quality changes of ‘Lobo’ apples during cold storage. Acta Horticulturae, 485, 125–133.
Du, J., Gemma, H., & Iwahori, S. (1997). Effects of chitosan coating on the storage of peach, Japanese pear and kiwi fruit. Journal of the Japanese Society for Horticultural Science, 66, 15–22.
Eccher-Zerbini, P. (2002). The quality of pear fruit. Acta Horticolturae, 596, 805–810.
El Ghaouth, A. (1992). Chitosan coating to extend the storage life of tomatoes. HortScience, 27, 1016–1018.
El Ghaouth, A., Arul, J., Ponnampalam, R., & Boulet, M. (1991). Chitosan coating effect on storability and quality of fresh strawberries. Journal of Food Science, 56, 1618–1620.
El Ghaouth, A., Ponnampalam, R., Castaigne, F., & Arul, J. (1992). Chitosan coating to extend the storage life of tomatoes. HortScience, 27, 1016–1018.
El Ghaouth, A., Smilanick, J. L., & Wilson, C. L. (2000). Enhancement of the performance of Candida saitoana by the addition of glycolchitosan for the control of postharvest decay of apple and citrus fruit. Postharvest Biology and Technology, 19, 103–110.
Esti, M., Cinquanta, L., Sinesio, F., Moneta, E., & Di Matteo, M. (2002). Physicochemical and sensory fruit characteristics of two sweet cherry cultivars after cool storage. Food Chemisty, 76, 399–405.
Ferretti, G., Bacchetti, T., Belleggia, A., & Neri, D. (2010). Cherry antioxidants: from farm to table. Molecules, 15, 6993–7005.
Ghasemnezhad, M., Nezhad, M. A., & Gerailoo, S. (2011). Changes in postharvest quality of loquat (Eriobotrya japonica) fruits influenced by chitosan. Horticultural Environmental Biotechnology, 52, 40–45.
Giusti, M. M., & Wrolstad, R. E. (2001). Unit F1.2.1-13. Anthocyanins. Characterization and measurement with UV-visible spectroscopy. In R. E. Wrolstad (Ed.), Current protocols in food analytical chemistry. NewYork: Wiley.
Goncalves, B., Landbo, A. K., Knudsen, D., Silva, A. P., Moutinho-Pereira, J., Rosa, E., et al. (2004). Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). Journal of Agricultural and Food Chemistry, 52, 523–530.
Goncalves, B., Landbo, A. K., Knudsen, D., Silva, A. P., Moutinho-Pereira, J., & Rosa, E. (2007). Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chemisty, 103, 976–984.
Hernandez-Munoz, P., Almenar, E., Del Valle, V., & Gavara, R. (2008). Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria × ananassa) quality during refrigerated storage. Food Chemistry, 110, 428–435.
Hirano, S., Ohe, Y., & Ono, H. (1976). Selective N-acylation of chitosan. Carbohydrate Research, 47, 315–320.
Hong, K., Xie, J., Zhang, L., Sun, D., & Gong, D. (2012). Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Scientia Horticulturae, 144, 172–178.
Jayakumar, R., Prabaharan, M., Reis, R. L., & Mano, J. F. (2005). Graft copolymerized chitosan-present status and applications. Carbohydrate Polymers, 62, 142–158.
Jiang, Y., & Li, Y. (2001). Effect of chitosan coating on postharvest life and quality of longan fruit. Food Chemistry, 73, 139–143.
Kelley, K., Cosman, A., Belgrader, P., Chapman, P., & Sullivan, D. C. (2013). Detection of methicillin-resistant Staphylococcus aureus by a duplex droplet digital PCR assay. Journal of Clinical Microbiology, 51, 2033–2039.
Kim, S. Y., Lim, J. H., Park, M. R., Kim, Y. J., Park, T., Won Seo, Y., et al. (2005). Enhanced antioxidant enzymes are associated with reduced hydrogen peroxide in barley roots under saline stress. Journal of Biochemistry and Molecular Biology, 38, 218–224.
Kingston, C. (1991). Maturity indices for apple and pear. Horticultural Reviews, 13, 407–432.
Kittur, F. S., Saroja, N., & Tharanathan, H. R. N. (2001). Polysaccharide-based composite coating formulations for shelf-life extension of fresh banana and mango. European Food Research and Technology, 213, 306–311.
Li, H., & Yu, T. (2001). Effect of chitosan on incidence of brown rot, quality and physiological attributes of postharvest peach fruit. Journal of the Science of Food and Agriculture, 81, 269–274.
Lin, B., Du, Y., Liang, X., Wang, X., & Yang, J. (2011). Effect of chitosan coating on respiratory behavior and quality of stored litchi under ambient temperature. Journal of Food Engineering, 102, 94–99.
Liu, R. H. (2003). Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. American Journal of Clinical Nutrition, 78, 517–520.
Malik, A. U., & Singh, Z. (2005). Pre-storage application of polyamines improves shelf-life and fruit quality in mango. The Journal of Horticultural Science and Biotechnology, 80, 363–369.
Martinez-Romero, D., Alburquerque, N., Valverde, J. M., Guillén, F., Castillo, S., Valero, D., & Serrano, M. (2006). Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: a new edible coating. Postharvest Biology and Technology, 39, 93–100.
McGuire, R. G. (1992). Reporting of objective colour measurements. HortScience, 27, 1254–1255.
Navarro da Silva, A., Navarro da Silva, R., Ferreira, M. A. M., Rodrigues Minim, V. P., Teixeira da Costa, T., & Perez, R. (2013). Performance of hedonic scales in sensory acceptability of strawberry yogurt. Food Quality and Preference, 30, 9–21.
No et al. (2007). Applications of chitosan for improvement of quality and shelf life of foods: a review. Journal of Food Science, 72(5), R87–R100.
Prabaharan, M., & Mano, J. F. (2006). Chitosan derivatives bearing cyclodextrin cavities as novel adsorbent matrices. Carbohydrate Polymers, 63, 153–166.
Prvulović, D., Malenčić, D., Popović, M., Ljubojević, M., & Ognjanov, V. (2011). Antioxidant properties of sweet cherries (Prunus avium L.) role of phenolic compounds. World Academy of Science, Engineering and Technology, 59, 11–25.
Rinaudo, M. (2006). Chitin and chitosan: properties and applications. Progress in Polymer Science, 31, 603–632.
Rohani, M. Y., Zaipun, M. Z., & Norhayati, M. (1997). Effect of modified atmosphere on the storage life and quality of Eksotika papaya. Journal of Tropical Agriculture and Food Science, 25, 103–113.
Romanazzi, G., Nigro, F., & Ippolito, A. (2003). Short hypobaric treatments potentiate the effect of chitosan in reducing storage decay of sweet cherries. Postharvest Biology and Technology, 29, 73–80.
Romanazzi, G., Lichter, A., Gabler, F. M., & Smilanick, J. L. (2012). Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biology and Technology, 63, 141–147.
Romanazzi, G., Feliziani, E., Santini, M., & Landi, L. (2013). Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biology and Technology, 75, 24–27.
Romano, G. S., Cittadini, E. D., Pugh, B., & Rob, S. (2006). Sweet cherry quality in the horticultural production chain. Stewart Postharvest Review, 6, 1–9.
Serrano, M., Guillén, F., Martínez-Romero, D., Castillo, S., & Valero, D. (2005). Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. Journal of Agricultural and Food Chemistry, 53, 2741–2745.
Shahidi, F. (2007). Chitin and chitosan from marine by-products. In F. Shahidi (Ed.), Maximizing the value of marine by-products (pp. 340–373). Abington: Wood head Publishing limited.
Singleton, V. L., & Rossi, J. A. (1965). Colourimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158.
Tomás-Barberán, F. A., & Espín, J. C. (2001). Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. Journal of Agricultural and Food Chemistry, 49, 4748–4760.
UPOV, 2006: Guidelines for the conduct of tests for distinctness, homogeneity and stability. Sweet cherry (Prunus avium L.). TG/35/7. 31 pp.
Usenik, V., Fabcic, J., & Stampar, F. (2008). Sugars, organics acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chemistry, 107, 185–192.
Vangdal, E., & Slimestad, R. (2006). Methods to determine antioxidative capacity in fruit. Journal of Fruit and Ornamental Plant Research, 14, 123–132.
Vavilov, N. I., & Starr Chester, K. (1951). The origin, variation, immunity and breeding of cultivated plants. NewYork: Ronald Press.
Wang, S. Y., & Gao, H. (2013). Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system and postharvest fruit quality of strawberries (Fragaria × aranassa Duch). Food Science and Technology, 52, 71–79.
Wani, A. A., Singh, P., Gul, K., Wani, M. H., & Langowski, H. C. (2014). Sweet cherry (Prunus avium): critical factors affecting the composition and shelf life. Food Packaging and Shelf life, 1, 86–99.
Wills, R. B. H., Scriven, F. M., & Greenfield, H. (1983). Nutrient compositions of stone fruit (Prunus spp.) cultivars: apricot, cherry, nectarine, peach and plum. Journal of the Science of Food and Agriculture, 34, 1383–1389.
Xanthopoulos, G., Koronaki, E. D., & Boudouvis, A. G. (2012). Mass transport analysis in perforation-mediated modified atmosphere packaging of strawberries. Journal of Food Engineering, 111, 326–335.
Yaman, O., & Bayindirh, L. (2001). Effects of an edible coating, fungicide and cold storage on microbial spoilage of cherries. European Food Research and Technology, 213, 53–55.
Yaman, O., & Bayindirli, L. (2002). Effects of an edible coating and cold storage on shelf-life and quality of cherries. LWT- Food Science and Technology, 35, 146–150.
Yang, Y., Cui, J., Zheng, M., Hu, C., Tan, S., Xiao, Y., et al. (2012). One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chemical Communications, 48, 380–382.
Zhang, D., & Quantick, P. C. (1997). Effects of chitosan coating on enzymatic browning and decay during postharvest storage of litchi (Litchi chinensis Sonn.) fruit. Postharvest Biology and Technology, 12, 195–202.
Zhang, W. S., Li, X., Zheng, J. T., Wang, G. Y., Sun, C. D., Ferguson, I. B., et al. (2008). Bioactive components and antioxidant capacity of Chinese bayberry (Myrica rubra Sieb. and Zucc.) fruit in relation to fruit maturity and postharvest storage. European Food Research and Technology, 227, 1091–1097.
Zhishen, J., Mengcheng, T., & Jianming, W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, 64, 555–559.
Zhu, X., Wang, Q., Cao, J., & Jiang, W. (2008). Effects of chitosan coating on postharvest quality of mango (Mangifera indica L. cv. Tainong) fruits. Journal of Food Processing and Preservation, 32, 770–784.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petriccione, M., De Sanctis, F., Pasquariello, M.S. et al. The Effect of Chitosan Coating on the Quality and Nutraceutical Traits of Sweet Cherry During Postharvest Life. Food Bioprocess Technol 8, 394–408 (2015). https://doi.org/10.1007/s11947-014-1411-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-014-1411-x