Abstract
The development of edible coatings has been lauded with respect to their safety and effectiveness. In this study, we researched the effects of edible coatings (2% CaCl2, 1% chitosan and 1% pullulan) on the nutrient content and antioxidant abilities of jujube (Zizyphus jujuba Miller cv. Dongzao). Using the new analysis technique for order of preference by similarity to ideal solution (TOPSIS), we evaluated the effects of these coatings. Compared with the control fruit group, test results showed that coating treatment significantly delayed fruit senescence. Specifically, CaCl2 treatment not only maintained fruit storage quality and antioxidant activity but also restrained the production and accumulation of malondialdehyde in jujube. Chitosan treatment delayed decreases in secondary metabolites and superoxide dismutase and catalase activity. Pullulan coatings performed better in terms of proanthocyanidin and cyclic adenosine monophosphate (cAMP). We also used TOPSIS to evaluate the preservation effect of different film coatings and found 2% CaCl2 to be the best treatment for jujube, followed by 1% chitosan and 1% pullulan. Based on the appropriate materials and concentration of the film coatings, edible coatings have the potential to retain the quality and antioxidant capacity of the Chinese jujube cv. Dongzao.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The jujube is an evergreen shrub and tree of the buckthorn family. The Zizyphus species (Rhamnaceae family) is indigenous to China where its history of cultivation goes back more than 4000 years. There are many species of Chinese jujube, including ‘Jinsixiaozao’, ‘Junzao’, ‘Dongzao’ and ‘Budaizao’, among others. Chinese jujubes are rich in phenolics, flavonoids, organic acids, sugar, cyclic adenosine monophosphate and ascorbic acid [1]. Not only do the edible tissue (peel and pulp) extracts of jujube have high antioxidant activity, but its seeds are a source of flavonoids and potent antioxidant activity [2]. Some researchers have reported higher immunological activity in the water soluble polysaccharides of ripe jujubes [3]. Chinese jujubes (Zizyphus jujuba Miller) are commonly used in folklore medicines in the treatment of various diseases for their anticancer, antiepileptic, anti-inflammatory and neuroprotective effects in cells, animals and humans [4]. Gao et al. [5] provided a reference for jujube’s other medicinal uses, its potential for drug applications, and food interactions.
Zizyphus jujuba Miller cv. Dongzao is very perishable and highly susceptible to postharvest colour fading, browning, decay and water loss [6]. As such, effective storage and preservation are essential to preserve its quality and nutrient values. Currently, synthetic chemical fungicides are the primary means for controlling postharvest diseases in jujube fruit in China [7], but there is growing public concern about the adverse impact of synthetic chemical fungicides on human health and the environment.
Carbohydrate-based edible coatings are being intensively investigated [8]. These coatings can control the internal gas atmosphere of fruit and minimize its respiration rate [9]. Semi-permeable chitosan coatings protect food from fungal decay and modify the atmosphere within the fruit [10]. Studies have reported that the pre- and post-harvest application of chitosan can effectively control the decay of table grapes [11] and extend the shelf lives of tomatoes [12] and blueberries [13]. Treatment with exogenous calcium increases the calcium levels in fruit and decreases the respiration rate, as well as the rates of ethylene production and pectin degradation [14]. Calcium dips have been used to reduce postharvest physiological disorders in mangoes [15]. Madani et al. [16] investigated the use of calcium on papaya fruit, which resulted in greater firmness, thus reducing the incidence and severity of anthracnose disease. Pullulan is synthesized from starch by the ubiquitous fungus Aureobasidium pullulans, which mainly consists of maltotriose units interlinked by 1–6 glycosidic bonds [17]. The pullulan film is colourless, transparent and has low oil and oxygen permeability [18, 19]. Xu et al. [20] found that edible pullulan films composed of optimum formula (Pul: SA: SPI = 0.08:0.165:0.755) could extend the shelf life of kiwifruit by a factor of three.
In this study, we investigated the effects of postharvest coating treatments with calcium chloride, chitosan and pullulan on the storage quality, bioactive compounds and antioxidant capacity of Chinese jujube cv. Dongzao harvested at white maturity to provide reference for the improved storage of Chinese jujube cv. Dongzao.
Materials and Methods
Fruits, Treatments and Storage
We performed experiments on the white-stage Chinese jujubes (Z. jujuba Mill. cv. Dongzao) grown in a commercial orchard in Cangzhou, Hebei Province, China. We selected fruits for their uniformity of size, ground colour and freedom from defects and mechanical damage. After harvesting, we immediately transported the fruits to Tianjin Agricultural University and then randomly distributed them into four groups of 400 fruits each. We assigned three groups to the three treatments and retained the fourth as a control. As in our previous studies [21, 22], we chose different concentrations of the three coating materials: (a) 2% calcium chloride, (b) 1% chitosan and (c) 1% pullulan. We also immersed the control samples in deionized H2O. The fruits were allowed to dry for 2 h at room temperature and were then sealed in polyethylene (PE) bags and put into cold storage (at 0 °C and 85–90% relative humidity). We tested 40 fruits every 15 days in each of the three groups.
Storage Quality
Fruit Firmness and Titratable Acid (TA)
We measured the firmness of fruit using a GY-I firmness tester with a 3.5-mm diameter head (Mudanjiang, China). We used the titration method to determine the presence of the titratable acid (TA), as anhydrous citric acid [10].
Weight Loss and Redness Index
To determine the weight loss associated with each treatment, we weighed eighty fruits using a digital scale [10]. All experiments were replicated in triplicate. We gave different scores based on the total red area of the fruit, where 0: 0%, 1: ≤1/4, 2: ≤1/2, 3: ≤3/4 and 4: >3/4 of the fruit was red. We developed redness index scoring as follows:
Freshness and Softening Rates
We measured the freshness and softening rates by counting the number of fresh fruits, as defined by having good flavour, colour, a glossy peel and no decay or browning.
Determination of Bioactivity
Measurement of TSS and Ascorbic Acid
We measured the total soluble solid (TSS) content using a handheld refractometer (Fisher Scientific, Ottawa, Canada). To measure ascorbic acid, we immediately homogenized tissue in 100 mL of 2% oxalic acid solution and then filtered it. The filtrate was then assayed by 2,6-dichlorophenolindophenol titration [17].
Measurement of cAMP and Total Polysaccharides
We performed adenosine 3′, 5′-cyclic monophosphate (cAMP) analysis using an Agilent 1260 infinity high-performance liquid chromatography (HPLC) system with a UV–Vis detector (Agilent Corp., Chicago, IL, USA) monitored at 254 nm. We ground 3 g of fresh tissue with 15 mL distilled water and boiled it for 30 min three times. We then centrifuged the extracts at 20,000g for 5 min and combined and standardised the filtrates to 50 mL for assay. We passed the extracts through a 0.45-µm polytetrafluoroethylene (PTFE) filter and used a Venusil MP C18 analytical column of 4.6 mm × 250 mm × 5 µm (Bonna-Agela Technologies Inc., Wilmington, DE, USA) at room temperature. The injection volume was 15 µL and the flow rate was 1.0 mL/min. The volume ratio of the mobile phase methanol to 20 mmol/L potassium dihydrogen phosphate was 20:80.
Next, we skimmed a fresh sample (15 g) with petroleum ether for 1 h, and then pretreated the residue twice with 80% ethanol, as well as ultrasound for 30 min to remove oligosaccharides and other coloured or small molecular substances. Then we ultrasonically extracted the pretreated samples for 1.5 h with distilled water (90 °C) twice. We then brought the supernatants to a final volume of 250 mL and stored them at −40 °C until use. We measured the total content using the phenol–sulfuric acid colorimetric method [23]. We calculated the polysaccharide content based on the equation for the standard curve.
Measurement of Secondary Metabolites
We ground 2 g of fresh tissue with 80% methanol and sonicated it for 30 min three times. We then pooled and evaporated the supernatants under vacuum at 45 °C and standardised the evaporated filtrate to a final volume of 25 mL with 80% methanol. We stored all extracts in the dark at −20 °C until use.
We measured the proanthocyanidins content using the vanillin assay method [24] and assayed the total triterpene content according to the procedure reported in Ni et al. [25]. We prepared crude extracts of total polyphenol and total flavonoids as proanthocyanidin, which we analysed using a modified Folin–Ciocalteu colorimetric method [26]. We determined the total flavonoids using the colorimetric assay method [27]. We express the proanthocyanidin contents as catechin equivalents in mg/100 g FW, the total triterpene contents as ursolic acid equivalents in mg/g FW, the total polyphenols contents as gallic acid equivalents in mg/g FW and the total flavonoid contents as rutin equivalents in mg/g FW.
Antioxidant Enzyme Activity
We performed enzyme extraction in an extracted buffer containing 50 mmol/L of sodium phosphate buffer (pH 7.8), 3 mmol/L EDTA and 0.5% insoluble polyvinylpolypyrrolidone (PVPP), along with some modification of the method, as described in Kochhar et al. [28]. For the SOD, CAT and peroxidase (POD) enzymes, we homogenized 2 g of frozen ground tissue using a precooled mortar and pestle in 8 mL of the extracted buffer described above. For the ascorbate peroxidase (APXs) [29], we ground 2 g of flesh tissue with the extracted buffer to which 0.6 mmol/L of ascorbic acid had been added. After homogenization, we centrifuged the extract at 12,000g at 4 °C for 30 min and collected the supernatant for assay. We assayed the enzyme according to the method described in Yan et al. [30] and determined the presence of SOD, CAT and POD according to the procedure described in Lee et al. [31].
Antioxidant Capacity Assays
DPPH Scavenging Activity
We evaluated the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging capacity of the jujube extracts according to a previously reported protocol [32]. Next, we blended 0.1 mL of the jujube extracts with 3 mL of the prepared DPPH· reagent solution, left them to react in the dark for 30 min, and detected them at 517 nm against a methanol blank. We express the DPPH scavenging activity as millimoles of Trolox equivalent per 100 g of fresh weight (mmol Trolox eq./100 g FW).
ABTS Scavenging Activity
We assayed the 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) scavenging ability according to a method described in Ref. [32]. We reacted 7 mmol/L of ABTS and 20 mmol/L of sodium acetate buffer (pH 4.5) (V:V = 1:1) to create a stable radical solution after 12 h of incubation in the dark. We then diluted the test solution to an absorbance of 0.7 ± 0.01 at 734 nm. The reaction mixtures contained 50 μL of extract and 3 mL of test solution. We assessed the absorbance of the mixture after 1 min of reaction time.
FRAP Determination
We performed the ferric reducing ability of plasma (FRAP) assay according to the method reported in Ref. [5]. We mixed the samples (1 mL) with 2 mL of 0.2 mol/L phosphate buffer (pH 6.6) and 2 mL of 1% potassium ferricyanide. After incubation in a 50 °C water bath for 20 min, we added 2 mL of 10% trichloroacetic acid. Then, we moved 1 mL of the above mixture to a test tube and added and thoroughly incorporated 0.2 mL of 0.1% ferric chloride and 1 mL of distilled water. After incubation in the dark for 30 min, we measured the absorbance at 700 nm. We express the results as milligrams of vitamin C equivalent antioxidant capacity per 100 g fresh weight (mg vitamin C eq./100 g FW).
Measurement of MDA
We assayed the MDA content according to the method reported in Ref. [33] with some modification. We determined the absorbance at 532 nm and corrected for unspecified turbidity by subtracting the absorbance of the same at 600 nm.
Statistical Analysis
We replicated the coating experiments three times under each experimental condition, and then examined the data obtained from our analysis of variance (ANOVA) and TOPSIS analyses. We used the statistical software program SPSS 19.0 for Windows (Version 14) to determine the parameters, and we expressed values as means ± standard deviations. We compared significant differences using Duncan’s new multiple range tests and determined the level of statistical significance to be 95%.
Results
Storage Quality
Firmness and TA
Reduction in firmness was significantly inhibited by the fruit coating treatments (Fig. 1a). Firmness in the control fruit began declining and occurred significantly faster than in the coating-treated fruit after 30 days (p < 0.01), but we found no significant difference between calcium-treated and chitosan-treated fruit stored for 75 days. The firmness of the calcium-treated fruit (7.45 × 105 Pa) was significantly higher than that of the control fruit (5.04 × 105 Pa) and the chitosan-treated fruit (6.02 × 105 Pa) at the end of the storage period.
During cold storage, we observed a slow increase of TA in the jujube (Fig. 1b). TA in the control fruit increased up to 45 days and then declined rapidly. However, TA of the coating-treated fruit increased for 75 days and there was a significant (p < 0.05) difference in TA between the control and coating-treated fruits. TA in the control sample decreased to 0.09% at the end of the storage period.
Weight Loss, Redness Index, Freshness-Keeping Rate and Softening
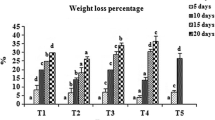
As shown in Fig. 1c, the control fruit lost significantly more weight than the coating-treated fruit (p < 0.01). When the weight loss rate reached 0.85% on day 60, the fruit began to wilt. After day 60, the weight loss rate in the control fruit significantly sped up, which coincided with the softening of the fruit. The weight loss rate reached 3.2% in the control group, which was about 2.6 times higher than that of the coating-treated fruit at the end of the storage period, for which fruits treated with calcium chloride, chitosan and pullulan were just 1.06, 1.17 and 1.28%, respectively. Consequently the best freshness-keeping effect was realized by calcium chloride, followed by chitosan and pullulan.
During storage, appearance of the jujubes underwent a series of changes (Fig. 2). After 60 days, the freshness of the fruit in the control group began to decrease rapidly, whereupon the softening and redness index rapidly increased (Fig. 1d–f). The amount of inedible fruit totalled more than 50%. In contrast, the coating-treated fruit maintained its freshness and quality, except for those treated with pullulan. At day 105, there was 26.25% fresh fruit in the control group, but about 68% in the calcium- and chitosan-treated fruits. We infer that the pullulan concentration was too high for Chinese jujube cv. Dongzao. On the other hand, as a polysaccharide, pullulan may promote fruit reddening. There was a significant difference between the control and coating-treated groups (p < 0.01). These results indicate that edible coatings have a significant effect on the storage quality of the jujube.
Bioactive Compounds
Total Soluble Solids and Ascorbic Acid
As shown in Fig. 3a, the TSS increased as the fruit ripened. Up to 60 days, the TSS was significantly (p < 0.05) higher in the control fruit than in the coating-treated fruit. A sharp TSS decline occurred in the control sample after storage day 60 and the TSS content then decreased to 7.64%. However, the decline of TSS was delayed by about two weeks in the coating-treated fruit and its content was above 8.5%.
The edible coatings demonstrated the best effect on the maintenance of the ascorbic acid content (Fig. 3b). We observed no significant difference between the control and coating-treated fruit before 30 days (p > 0.05). After that the vitamin C (Vc) content in the treated samples was significantly higher than in the control group (p < 0.05), especially in the calcium-treated fruit (p < 0.01). The Vc content in the control fruit decreased rapidly due to the faster rate of senescence, whereas the Vc content of coating-treated fruit was 1.45 times higher.
cAMP and Polysaccharide Contents
In this study, in the initial storage stage, the total polysaccharide content increased and reached a peak in the control and calcium-treated fruit (2.18, 1.89 mg/g) (Fig. 3c). As the fruit matured, the polysaccharide content continuously increased and that of the control fruit was significantly higher than in the calcium- and chitosan-treated fruit until day 90 (p < 0.05). We found no significant difference between the three coating treatments (p > 0.05). At the end of the storage period, the coating-treated fruit showed a slight decline, but the content was higher than in the control fruit. The cAMP content increased during cold storage (Fig. 3d). In the initial stage of storage, the cAMP content slightly increased in all the samples. As the fruit ripened, there was a higher cAMP content in the control fruit. After 60 days, the cAMP content of the control fruit accelerated and became significantly higher than that in the cotaing-treated samples (p < 0.05).
Plant Secondary Metabolites Content
We next investigated the plant secondary metabolite contents, including phenolics, total flavonoid, proanthocyanidin and triterpenoid, as presented in Fig. 4. We observed similar trends in the secondary metabolites, except for proanthocyanidin, in the control and coating-treated fruit. Specifically, the phenolics, total flavonoid and triterpenoid contents continuously decreased with ripening, but they sharply declined in the control fruit and were lower than in the coating-treated fruit during storage. At day 75, the phenolics content in the control fruit decreased to 5.59 mg GAE eq./g, whereas the content in the chitosan-treated fruit was 6.466 mg GAE eq./g. After 75 days of storage the flavonoid content tended to stabilise (about 25.54 mg RE eq./g). The content slightly increased in the calcium-treated fruit (41.47 mg RE eq./g). The secondary metabolite contents in the coating-treated fruit were significantly higher than in the control fruit (p < 0.05).
Figure 4d shows the observed proanthocyanidin content. In the initial storage stage, the proanthocyanidin content slightly increased in all the samples and there was no significant difference between the control and coating-treated fruit (p > 0.05). The rate of increase accelerated after 30 days, however, the content in the control fruit was higher than that in the coating-treated samples (p < 0.05).
Activity of Related Enzymes
On the whole, SOD activity increased during initial storage but declined at the end of the storage period (Fig. 5a). There was a rapid change in SOD activity in the control fruit, but it remained stable in the coating-treated fruit. Ultimately, the SOD activity of the coating-treated fruit was significantly higher than that of the control fruit (p < 0.05), which indicates that edible coating treatments may promote SOD activity and maintain high enzyme levels.
The CAT activity in all fruit groups decreased (Fig. 5b). During storage, CAT activity declined continuously and that in the control fruit was significantly lower than in the coating-treated fruit. The CAT activity decreased from 0.19 to 0.06 U/(min g) and the rate of decline slowed after 30 days. In addition, the CAT activity in the pullulan-treated fruit was significantly higher than in the control fruit (p < 0.01). There was no significant difference between the calcium- and chitosan-treated fruits (p > 0.05).
POD activity in the jujube increased up to storage day 30 and then decreased (Fig. 5c). The POD activities of the control and chitosan-treated fruits were significantly higher than in the other two coating-treated fruits (p < 0.05). During storage, the activity in the coating-treated fruit was lower than in the control fruit.
The APX activity in the jujube increased sharply during the initial 60 days of storage and then declined until the end of the storage period (Fig. 5d). We observed no significant difference between the control and coating-treated fruits (p > 0.05).
Antioxidant Capacity
Next, we observed antioxidant activity (DPPH, ABTS, FRAP and MDA) during storage and found different patterns of activity changes for different antioxidants. The DPPH of the control fruit was lower than in the coating-treated fruits throughout the storage period, as shown in Fig. 6a. Jujube stored at 0 °C showed an increasing trend of DPPH activity up to 45 days and then declined in the control fruit. We observed no significant difference (p > 0.05) between the control and coating-treated fruits. The DPPH activity in the coating-treated fruit began to decline at day 60, about two weeks later than in the control fruit. The jujube treated with pullulan exhibited the highest DPPH activity at day 60.
Generally, we observed similar change patterns in the control and calcium-treated fruits (Fig. 6b). We observed a drop in ABTS activity on day 15, but the activity of the control fruit was significantly higher than in the treated fruit (p < 0.05). This activity declined again and was 65.089 mmol/L Trolox eq./100 g on day 105. In contrast to the control fruit, on day 90 we observed an increase in the chitosan- and pullulan-treated fruits. Thus, we concluded that film coatings had no significant effect on ABTS activity for jujube stored at 0 °C.
FRAP activity decreased during storage and there was no significant difference between the control and coating-treated fruits (p > 0.05) in the first 15 days of storage (Fig. 6c). The FRAP activity ranged from 305.42 to 141.75 mg Vc eq./100 g in the control fruit. At the end of the storage period, the FRAP activity in the coating-treated fruit was higher than in the control fruit (p < 0.05).
We observed a continuous increase in the MDA content in both the control and coating-treated fruits (Fig. 6d). However, the coating-treated fruit significantly inhibited the increase in MDA (p < 0.05). On the 45th day of storage, the MDA content of the control fruit was 5.245 µmol/g FW, which was significantly higher than that of the coating-treated fruit (p < 0.05). Coating treatments inhibited MDA accumulation, especially calcium chloride treatment. The MDA contents of the calcium-treated and chitosan-treated fruits were about 24 and 13% lower, respectively, than the control fruit at the end of the storage period.
Comprehensive Evaluation of Different Coatings
Based on our TOPSIS analysis results, we determined the following composite score order for the different jujube treatments (Fig. 7): 2% calcium chloride > 1% chitosan > 1% pullulan > control. These results indicate that the use of film coatings is an efficient method for maintaining the storage quality of postharvest jujube and that TOPSIS analysis is an efficient ranking method for use as a data-based foundation for manufacturing companies to make optimal coating selections.
Discussion
Edible coatings can form a transparent film that inhibits water loss, limits respiration, and defends against fungal disease. Chitosan has two main characteristics—film-forming and bacteriostasis—which cause it to stimulate beneficial enzymes and inhibit harmful compounds in fruit. Therefore, it has a better fruit preservation effect in terms of enzymes. The application of chitosan-coating treatment to Indian jujube fruit has shown to have beneficial effects on the fruit’s firmness, rate of weight loss, TSS, ascorbic acid and TA [10]. This may be due to the lowered respiration rate and its inhibiting effect on spoilage organisms. Chitosan coatings have also exhibited good effect on the evolution of the colour characteristics and parameters of fresh-cut mushrooms [34].
Calcium acts as an inter-molecular binding agent that can stabilise pectineprotein complexes of the middle lamella [35]. Exogenous calcium chloride treatments improve fruit storage quality and protect some antioxidants in fruit and vegetables by increasing their Ca2+ content. Madani et al. [16] observed the higher firmness effect of calcium in papaya fruit. In our previous study [22], we found 2%-calcium chloride and 1%-pullulan treatments to significantly decrease (p < 0.01) the production of MDA in pears compared to control groups. In this research, coating-treated jujube exhibited higher SOD and CAT activities than the control fruit and the MDA content declined significantly. This result agrees with that of Kou et al. [22]. As such, we can conclude that fruit treated with calcium chloride maintains its firmness and good sensory qualities.
We also investigated plant secondary metabolites in this study. The contents of phenolics, total flavonoids and proanthocyanidins have been reported to decrease with the fruit ripening process [36]. These change trends are consistent with those reported for ‘Fuji’ apples [37] and the total flavonoid contents in our experiment were higher than those reported in Zhang et al. [2]. A comparison with ten other kinds of jujube fruits [1] shows that the phenolic content is highest in Dongzao jujube, but proanthocyanidin and total flavonoid contents are lower. The DPPH and ABTS scavenging activities were ten times higher in our study than those of the other ten jujube fruits, while their reduction levels were lower than those of unripe apples [37]. Our experimental results confirm that film coatings can protect secondary metabolites and have a good preservation effect on the Dongzao jujube. As reported by Choi et al. [4], during ripening, fruit antioxidant capacity declines. Despite this, the DPPH scavenging activities we observed in the coating-treated fruits in our study were significantly higher than those in the control fruit. In summary, film coatings can enhance antioxidant activity in jujube during cold storage.
In our previous study, we found 1%-pullulan treatment to inhibit brown spot activity and prolong the shelf life of ‘Huang guan’ pears [21]. However, a different phenomenon occurred in jujube fruit treated with pullulan which had a high redness index and softening effect. We infer that the peel of Dongzao jujube is thinner compared to that of pears, so the pullulan concentration was relatively high such that the carbon dioxide and ethylene could not be released. In future studies, the effects of different concentrations of pullulan on fruit preservation will be important issues to address.
Conclusions
Results from this study indicate that postharvest film coatings (calcium chloride, chitosan and pullulan) during cold storage can effectively inhibit increases in the numbers of soft fruits, redness index scores, weight loss rates and MDA, and reduce the loss of ascorbic acid and freshness. The storage life was prolonged by enhancing the number of defensive enzymes such as SOD and CAT and increasing antioxidant activity in coating-treated fruit. Furthermore, secondary metabolites (phenolics, flavonoids, triterpenoids and proanthocyanidin), cAMP and polysaccharide were maintained at high levels in the coating-treated fruit. In the long run, edible film coatings may represent a safe method for preserving fruit and vegetables. In the meantime, further study is required to determine suitable concentrations and film materials for different fruits and vegetables.
References
Gao QH, Wu CS, Yu JG et al (2012) Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill.) selections. J Food Sci 77:1219–1225
Zhang H, Jiang L, Ye S et al (2010) Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem Toxicol 48:1461–1465
Zhao ZH, Liu MJ, Tu PF (2008) Characterization of water soluble polysaccharides from organs of Chinese Jujube (Ziziphus jujuba Mill. cv. Dongzao). Eur Food Res Technol 226:985–989
Choi SH, Ahn JB, Kim HJ et al (2012) Changes in free amino acid, protein, and flavonoid content in jujube (Ziziphus jujube) fruit during eight stages of growth and antioxidative and cancer cell inhibitory effects by extracts. J Agric Food Chem 60:10245–10255
Gao QH, Wu CS, Wang M (2013) The jujube (Ziziphus Jujuba Mill.) fruit: a review of current knowledge of fruit composition and health benefits. J Agric Food Chem 61(14):3351–3363
Lin L, Tian SP, Wan YK et al (2004) Effects of temperature and atmosphere component on quality of stored jujube fruit. Acta Bot Sin 46:928–934
Wang LT, Wu H, Qin GZ et al (2014) Chitosan disrupts Penicillium expansum and controls postharvest blue mold of jujube fruit. Food Control 41:56–62
Zhang YC, Rempel C, Mclaren D (2014) Chapter 12-edible coating and film materials: carbohydrates. Innovations in food packaging, 2nd edn. Academic Press, United States
Park HJ (1999) Development of advanced edible coatings for fruits. Trends Food Sci Technol 10:254–260
Zhong QP, Xia WS (2007) Effect of 1-methylcyclopropene and/or chitosan coating treatments on storage life and quality maintenance of Indian jujube fruit. LWT 40:404–411
Romanazzi G, Nigro F, Ippolito A et al (2002) Effects of pre- and postharvest chitosan treatments to control storage grey mold of table grapes. J Food Sci 67:1862–1867
EI Ghaouth A, Arul J, Asselin A et al (1992) Antifungal activity of chitosan on post-harvest pathogens: induction of morphological and cytological alterations in Rhizopus stolonifer. Mycol Res 96:769–777
Duan JY, Wu RY, Strik Bernadine C et al (2011) Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol Technol 59:71–79
Singh BR, Kulshreshtha SB, Kapoor KN (1996) An orange juice outbreak due to enterotoxigenic Escherichia coli. J Food Sci Technol 32:504–506
Sheng JP, Shen L (2011) 13-Chinese jujube (Ziziphus jujuba Mill.) and Indian jujube (Ziziphus mauritiana Lam.). Postharvest biology and technology of tropical and subtropical fruits: cocoa to mango. Woodhead Publishing Ltd, England, pp 299–325
Madani B, Mirshekari A, Yahia E (2016) Effect of calcium chloride treatments on calcium content, anthracnose severity and antioxidant activity in papaya fruit during ambient storage. J Sci Agric 96(9):2963–2968
Madani B, Mohamed MTM, Biggs AR et al (2014) Effect of pre-harvest calcium chloride applications on fruit calcium level and post-harvest anthracnose disease of papaya. Crop Prot 55:55–60
Shih FF, Daigle KW, Champagne ET (2011) Effect of rice wax on water vapour permeability and sorption properties of edible pullulan films. Food Chem 127:118–121
Singh RS, Saini GK, Kennedy JF (2008) Pullulan: microbial sources, production and applications. Carbohydr Polym 73:515–531
Xu SY, Chen XF, Sun DW (2001) Preservation of kiwifruit coated with an edible film at ambient temperature. J Food Eng 50:211–216
Kou XH, Guo WL, Guo RZ et al (2014) Effects of chitosan, calcium chloride, and pullulan coating treatments on antioxidant activity in pear cv. “Huang guan” during storage. Food Bioprocess Technol 7:671–681
Kou XH, Wu MS, Li L et al (2015) Effects of CaCl2 dipping and pullulan coating on the development of brown spot on “Huangguan” pears during cold storage. Postharvest Biol Technol 99:63–72
Xi X, Wei X, Wang Y et al (2010) Determination of tea polysaccharides in Camellia sinensis by a modified phenol–sulfuric acid method. Arch Biol Sci 62:669–676
Gunaratne A, Wu K, Li D et al (2013) Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem 138:1153–1161
Ni QX, Xu GZ, Wang ZQ et al (2012) Seasonal variations of the antioxidant composition in ground bamboo Sasa argenteastriatus leaves. Int J Mol Sci 13:2249–2262
Dewanto V, Wu X, Adom KK et al (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50:3010–3014
Yu YW, Zhang SY, Ren YZ et al (2012) Jujube preservation using chitosan film with nano-silicon dioxide. J Food Eng 113:408–414
Kochhar S, Watkins CB, Conklin PL et al (2003) A quantitative and qualitative analysis of antioxidant enzymes in relation to susceptibility of apples to superficial scald. J Am Soc Sci Hortic 128:910–916
Cao SF, Yang ZF, Cai YT et al (2014) Antioxidant enzymes and fatty acid composition as related to disease resistance in postharvest loquat fruit. Food Chem 163:92–96
Yan JK, Cao JK, Jiang WB et al (2012) Effects of preharvest oligochitosan sprays on postharvest fungal diseases, storage quality, and defense responses in jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit. J Sci Hortic 142:196–204
Lee J, Cheng LL, Rudell DR et al (2012) Antioxidant metabolism of 1-methylcyclopropene (1-MCP) treated ‘Empire’ apples during controlled atmosphere storage. Postharvest Biol Technol 65:79–91
Gao QH, Wu PT, Liu JR et al (2011) Physico-chemical properties and antioxidant capacity of different jujube (Ziziphus jujuba Mill.) cultivars grown in loess plateau of China. Sci Hortic 130:67–72
Moo-Huchin VM, Estrada-Mota I, Estrada-León R et al (2014) Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from Yucatan, Mexico. J Food Chem 152:508–515
Eissa HAA (2008) Effect of chitosan coating on shelf-life and quality of fresh-cut mushroom. J Food Nutr Sci 58:95–105
Mao L, Pang HQ, Wang GZ et al (2007) Phospholipase D and lipooxygenase activity of cucumber fruit in response to chilling stress. Postharvest Biol Technol 44:42–47
Dey PM, Brinson K (1984) Plant cell walls. Adv Carbohydr Chem Biochem 42:265–382
Zheng HZ, Kim YI, Chung SK (2012) A profile of physicochemical and antioxidant changes during fruit growth for the utilisation of unripe apples. Food Chem 131:106–110
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 3117 1769). We also gratefully acknowledge the cold storage support of Dr Yan Shijie of Tianjin Agricultural University and Zhu Zhiqiang of the National Engineering Technology Research Centre for the Preservation of Agricultural Products.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kou, X., Li, Y., Wu, J. et al. Effects of Edible Coatings on Quality and Antioxidant Activity of Zizyphus Jujuba Miller cv. Dongzao During Storage. Trans. Tianjin Univ. 23, 51–61 (2017). https://doi.org/10.1007/s12209-016-0021-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-016-0021-2