Abstract
Ber fruit is highly nutritious and is rich in bioactive compounds that are known to have several health benefits. Maintaining the quality of fruits during long term storage with polyphenolic extract in combination with edible coatings could be a novel way. Therefore, the present study is investigated to evaluate the potential application of pectin (1%) coating impregnated with karonda polyphenolic extract (KPPE) at concentrations of 0.5%, 1% & 1.5% on bioactive compounds, antioxidant capacity and quality of ber fruits during cold storage for 28 days. The results revealed that coating of ber fruits with pectin enriched in karonda polyphenolic extract markedly reduced spoilage, and maintained the firmness during the storage. The coating has resulted in higher retention of ascorbic acid, flavonoids, phenolics and antioxidant activity during storage. The treated fruits showed delayed ripening by slowing down the synthesis of carotenoids as compared to the control fruit. Further, the coating resulted in the lower activities of cell wall degrading enzymes, pectin methyl estarase (PME), and cellulase in the stored ber fruits. Moreover, the concentrations of karonda polyphenolic extract have worked in a dose-dependent manner in the coating system as pectin with 1.5% KPPE was found to be more effective in maintaining the quality and nutritional potential of ber fruit during storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ber (Zizyphus mauritiana Lamk.) oftenly called as poor man’s fruit is indigenous to India and is an important minor fruit of arid as well as semi-arid regions of the country. It is enriched in natural antioxidants, ascorbic acid, anthocyanins and antimicrobials. It is also a good source of vitamin C and minerals like calcium, potassium and vitamin A and B complex (Jain et al. 2019). Among the commercially important varieties of ber in India, ‘Umran’, a high-yielding cultivar is commercially cultivated in Punjab across large regions. Ber fruits can not be stored for an extended amount of time at room temperature due to its perishable nature. Average Shelf life of ber fruit ranged from four to seven days at room temperature (Meena et al. 2009).
The ripening and senescence of fruits is triggered by ethylene, which results in its softening, browning and decay. All these factors reduce the shelf life of ber to 3–4 days at ambient temperature. Surplus fruit in the market during the peak harvesting period results in a drop in the market price, reducing monetary benefits to growers. Most available post-harvest treatments used for retaining the quality of fruits are either highly expensive or chemical based and create environmental concerns. ‘Edible coating technology’ is one of the most promising post harvest technology to extend the shelf life of fresh horticultural produce. Pectin, an amorphous, colloidal carbohydrate, is applied as an edible coating owing to its excellent properties of aroma maintenance, barrier to oxygen, carbon dioxide, mechanical and oil properties (Dhall 2013). Edible coatings can be used with a variety of plant extracts and oils to enhance their properties. Polyphenolic extracts are used for their anti-browning, high antioxidant and antimicrobial properties. Pomegranate peel extract and moringa leaf extract enriched chitosan effectively maintains fruit quality of avocado and guava as well as enhances the phytochemical status of the fruits (Nair et al. 2018). Similarly, olive leaf extract infused with alginate and chitosan coatings enhanced the shelf life of sweet cherry fruits (Zam 2019).
Karonda fruits (Carissa carandus), a rich source of polyphenols, anthocyanins, phenols and flavonoids relate positively with the antioxidant activity (Mahajan et al. 2022). However, the effect of karonda polyphenolic extract (KPPE) infused into edible coatings for shelf-life improvement of fruits remains unexplored. Therefore, the present work aims at studying the effect of karonda polyphenolic extract enriched pectin coatings on quality parameters of ‘Umran’ ber fruits under cold storage conditions.
Materials and Methods
Experimental Details
Healthy and uniform trees of ber fruit cv. ‘Umran’ were tagged at the Fruit Research Farm at RRS Bathinda. Mature and healthy fruits of uniform size were free of spots and scars were harvested from the tagged trees and collected in plastic crates. Fruits were sorted, graded and washed with chlorinated water (100 ppm) by dipping the fruits for 5 min and then air-dried in shade at room temperature.
The Fruits Were Then Provided the Following Treatments
Coated fruits were then air dried in shade and stored in corrugated fibreboard boxes (5% perforation) with paper lining and kept in cold storage at 7.5 ± 1 °C and 90–95% relative humidity for 28 days. Treated fruits were analysed after an interval of 7, 14, 21 and 28 days of cold storage for physico-chemical characteristics. There was a total of four replications per treatment (Table 1).
Physical Characters
Physiological Loss in Weight (PLW)
The weight of ber fruits after each storage interval was recorded and percent physiological loss in weight (PLW) was calculated on the basis of final weight and initial weight of fruits. The given formula was used to calculate PLW:
Fruit Firmness
Fruit firmness was measured with the help of fruit tester penetrometer (Model 53203, capacity of 5 kg) having 8 mm diameter of stainless-steel probe. The pressure exhibited was expressed in kg/cm2.
Palatability Rating
The fruits were evaluated by a panel of four judges for the assessment of general taste, texture, flavour and appearance of the fruits with the help of 9‑point Hedonic scale. The scale rating was given as under: 1. Dislike extremely, 2. Dislike very much, 3. Dislike moderately, 4. Dislike slightly, 5. Neither like nor dislike, 6. Like slightly, 7. Like moderately, 8. Like very much, 9. Like extremely.
Spoilage
Per cent spoilt fruits were calculated by counting the number of spoilt fruits and the total number of fruits on each storage interval. Percent spoilt fruits were calculated as under:
Biochemical Analysis
Sample Preparation for Biochemical Analysis
Ber fruits were peeled and grated using a grater. The pulp was then strained using a muslin cloth to collect the juice. Peel was preserved for chlorophyll estimation.
Total Soluble Solids (TSS)
Total soluble solids (TSS) were determined from the fresh juice extracted, using a had refractometer. The observations were recorded as percentage. Temperature corrections were made using standard chart.
Titratable Acidity
Titratable acidity was determined by titration method. Titration of fresh juice of known quantity against 0.1 N NaOH with phenolphthalein (1%) as the indicator (AOAC 2005) was done. The end point is determined by appearance of slightly pink colour in the solution. Acidity was expressed as citric acid percentage using the given formula:
- N:
-
= Normality of standard NaOH used
Ascorbic Acid
Ascorbic acid of the fruits was estimated using the Indophenol method by Ranganna (1986). Ten grams of fruit pulp from fresh fruits was taken and finely crushed and mixed with 3% Meta phosphoric acid (HPO3) to make a volume of 100 ml with HPO3 solution. An aliquot of 10 ml was obtained and titrated against a standard dye solution (2, 6 dichlorophenol indophenol dye) till end point of pink colour was obtained which lasted for 15 s. For standardization of the dye, standard ascorbic acid was used which was diluted in 3% HPO3 solution. The ascorbic acid content in fruits was determined using the formula below and represented as mg ascorbic acid per 100 g of fresh fruit weight.
Total Sugars
Twenty-five milllitre of aliquot was used for determination of total sugars. Five ml of 60% HCl was added to the aliquot to hydrolyse the solution. This solution was kept undisturbed overnight at room temperature. The next day, the solution was placed in water bath operating at 68 °C for 10 min. the solution was then neutralised using 40% NaOH using 2–3 drops of phenolphthalein as indicator. This was followed by addition of 10% NaOH until yellow colour appears. Then the volume was raised to 100 ml using distilled water. This solution was titrated against boiling Fehling solution (5 ml Fehling A + 5 ml Fehling B) using methylene blue as the indicator. Appearance of brick red colour was marked as the end point of titration (AOAC 1980). Total sugars were calculated using the following formula:
Total Phenolics
Total phenolics content in the edible portion of fruit was determined using Folin-Ciocalteu reagent (Singleton et al. 1999). 100 μl of sample extract (in 80% ethanol) was taken out in a test tube. 2.9 ml of distilled water, 0.5 ml of Folin–Ciocalteu reagent and 2.0 ml of 20% Na2CO3 solution was then added to sample extract. Mixture was allowed to stand for 90 min and absorbance was recorded at 760 nm wavelength in a spectrophotometer against blank. The total phenolic content was expressed in microgram of gallic acid equivalent per gram of fresh weight (mg GAE/g Fresh weight).
Total Antioxidant Activity
The antioxidant activity of the ber fruit extract was determined using the method proposed by Brand-Williams et al. (1995) using the method of inhibition of the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) and measuring the obtained absorbance at 515 nm. Three millilitre aliquot of a 0.0635 mM of DPPH solution was prepared in methanol. It was added to 0.1 mL of each extract and shaken vigorously. The absorbance of the sample extract was monitored at 515 nm for 30 min until it achieved a steady state. The antioxidant activity was calculated using the following formula:
Total Carotenoids
Total carotenoids were determined from the fruit peel using the method suggested by Moran and Porath (1980). 1 g of fruit peel was chopped and ground using 10 ml of 80% acetone. The mixture was then centrifuged. The supernatant was collected and the colour developed was measured using a spectrophotometer at 663 nm and 645 nm.
For carotenoid estimation, the colour development was also measured at 470 nm.
The following formula was used to calculate total chlorophyll, chlorophyll a and chlorophyll b:
Where,
- A663:
-
is absorbance at 663 nm
- A645:
-
is absorbance at 645 nm
- A470:
-
is absorbance ta 470 nm.
Pectin Methylesterase Enzyme Activity
The method given by (Mahadevan and Sridhar 1982) was used with some modification to assess the activity of PME. Twenty grams of fruit pulp was taken and macerated in 60–100 ml of NaCl solution. The solution was then filtered using double layered muslin cloth. The filtrate was transferred to conical tubes and centrifuged at 4 °C at 2000 rpm for 30 min. the supernatant obtained was used for enzyme assay. For enzyme assay about twenty ml of 1% pectin solution was taken in 50 ml beaker. 10 ml of enzyme solution was taken and 1 N NaOH was used to adjust the pH. The samples were then put in water bath at 30 °C for 15 min. This was taken as zero time for reading. the pH of water bath was checked and adjusted at 7.0 using 0.02 N NaOH. The volume of NaOH used was noted.
Cellulase Activity
Twenty grams of fruit pulp was taken and macerated in 60–100 ml of NaCl solution. The solution was then filtered using double layered muslin cloth. The filtrate was transferred to conical tubes and centrifuged at 4 °C at 2000 rpm for 30 min. the supernatant obtained was used for enzyme assay. For estimation of cellulase enzyme activity of 2 ml of the extract, 4 ml of carboxy methyl cellulose solution and 1 ml of sodium acetate acetic acid buffer of 5.2 pH was used by injecting into a viscometer by syringe. Air was drawn in gently to mix the solution through large arm of viscometer by suction and subsequently the smaller arm. The efflux time was recorded as the zero time. The cellulase enzyme activity was expressed as per cent reduction in viscosity of the mixture (Mahadevan and Sridhar 1982).
Results and Discussion
The effect of edible coating of pectin enriched with karonda polyphenols on ber during storage was studied, and the results are discussed as follows. The visual effect of different treatments on ber fruit as been depicted through a pictograph which is shown in Fig. 1.
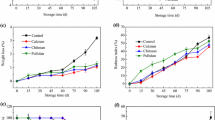
Variation in total phenols (a), total antioxidant activity (b), carotenoids (c), in ber fruits under cold storage conditions in relation to different edible coating treatments. Values are represented as mean ± S.E of 4 replicates. Mean values followed by similar superscript within a column are not significantly different at *p ≤ 0.05
Physiochemical Parameters
Physiological Loss of Weight (PLW)
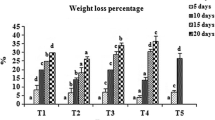
Physiological loss in weight (PLW) increased progressively with the increase in storage duration (Table 2). However, weight loss of treated fruits was significantly (p ≤ 0.05) less compared to untreated fruits during storage. The minimum average PLW of 3.77% was recorded in fruits treated with pectin (1%) + K-PPE (1.5%) and the maximum average PLW (5.06%) was recorded in untreated fruits. It was observed that K‑PPE (1.5%) infused edible coating of pectin significantly reduced the PLW over storage period. PLW of fruits over storage could be the result of loss of moisture from the fruits due to various physical and biochemical processes such as respiration, transpiration and oxidation. The use of edible coating enriched in polyphenolic extract preserves the antioxidant activity and reduce the respiration and transpiration, thereby reducing the loss of water from the fruits. Lower physiological loss in weight were also reported in apricot fruits treated with pomegranate peel polyphenolic extract incorporated in chitosan edible coatings (Gull et al. 2021).
Firmness
There was continuous decrease in fruit firmness as the storage period advanced. Coating of ber fruit with pectin showed a positive effect on retention of fruit firmness. Pectin (1%) + K-PPE (1.5%) treatment retained the maximum mean firmness throughout the storage period at 7.13 kg cm−2, which was significantly (p ≤ 0.05) higher than other treatments. Control fruits showed maximum loss in firmness and recorded the least firmness of 4.24 kg cm−2 on 28th day of storage. Retention of higher firmness in coated fruits might be due to decrease in transpiration and respiration rate and declined ethylene production, resulting in extra turgidity of fruit cell. Fruits treated with pectin (0.05% TCIN- and 0.1% TCIN-) retained significantly higher firmness as compared to untreated fruits (Sun et al. 2023).
Spoilage and Palatable Rating
An increase in the spoilage percentage of ber fruits was observed with the advancement of storage interval. No incidence of spoilage was noticed till day 14 in all the fruits (Table 2). By day 28, untreated fruits acquired the maximum spoilage percentage of 8.80%. However, the minimum spoilage of 2.32% on day 28 was observed in pectin (1%) + K-PPE (1.5%) treated fruits followed by 2.56% in pectin (1%) + K-PPE (1.0%) treated fruits. It could be because polyphenols form complexes with polysaccharides and proteins, thus, disabling the enzymes crucial for fungal growth. Similar observations were also given by Sinha et al. (2022) where edible coatings of salicyclic acid enriched beeswax exhibited antifungal, antimicrobial and antibacterial properties and induce a strong defence system. The significantly higher (p ≤ 0.05) sensory quality of 7.09 was retained in pectin (1%) + K-PPE (1.5%) treated fruits followed by pectin (1%) + K-PPE (1.0%) treated fruits till the end of storage period (Table 2). Untreated fruits showed the maximum decline by 28th day of storage. The sensory score was found to be 5.16 till tthat period. Due to biochemical changes occurring in the fruits over time, there was an increase in sensory quality rating with storage time which gradually declined once the peak was obtained. The late ripening in treated ber fruits could be due to decreased level of internal oxygen and delay in ethylene production (Gol et al. 2013).
Total Soluble Solids (TSS) and Titratable Acidity (TA)
By the 28th day, significantly higher TSS content of 12.90 was retained by pectin (1%) + K-PPE (1.5%) followed by pectin (1%) + K-PPE (1.0%) (Table 2). Minimum TSS content was noticed (11.89) under untreated fruits. The results are in concurrence with Meena et al. (2009) who also worked on coatings and observed high TSS in ber fruits under cold stoarge. A linear decline in titratable acidity percentage (TA) was noticed in all the fruits with the advancement in storage period. Maximum average TA of 0.138 was retained by the treatment pectin (1%) + K-PPE (1.5%) followed by 0.134 with pectin (1%) + K-PPE (1.0%) and the minimum average TA was observed in untreated fruits (0.120). The decrease in titratable acidity during storage is a result of utilization of organic acids in physiological activities taking place in the fruit. The rate of change in TA content of the fruits coated with beeswax and chitosan slowed down over due course of storage as compared to untreated fruits (Eshetu et al. 2019).
Ascorbic Acid
The ascorbic acid content of fruits decreased significantly irrespective of the treatments given. Coatings had a significant (p ≤ 0.05) impact on the ascorbic acid content of fruits. The maximum average ascorbic acid content of 58.72 mg/100g was retained by pectin (1%) + K-PPE (1.5%) which was at par with the ascorbic acid content of 57.36 mg/100g with pectin (1%) + K-PPE (1.0%) treated fruits (Table 2). The maximum ascorbic acid was lost by the untreated fruits throughout the storage period. As polyphenols have good antioxidant activity, therefore coatings enriched in polyphenols slow down the oxidation process, and maintain the higher level of AA during storage as earlier observed in pear and papaya fruits (Prasad et al. 2022).
Total Phenolics
The total phenolic content (TPC) of fruits decreased significantly (p ≤ 0.05) throughout the storage interval irrespective of the treatment given (Fig. 2a). The lowest TPC of untreated fruits was recorded to be 0.55% on day 28. Coatings had a significant (p ≤ 0.05) effect on the TPC of the fruits. The maximum phenolic content of 0.75% was retained by pectin (1%) + K-PPE (1.5%) followed by pectin (1%) + K-PPE (1.0%) at 0.72%. The decline in TPC with the advancement in storage could be attributed to the breakdown of cell structure as senescence occurs (Ghasemnezhad et al. 2010). The high polyphenol content in coated fruits could be due to the decreased respiration in coated fruits that led to slow down the activity of polyphenol oxidase enzyme responsible for the degeradation of polyphenols.
Antioxidant Activity
There was a decline in antioxidant activity with advancement in storage period. Maximum antioxidant activity was retained by pectin (1%) + K-PPE (1.5%) at 54.33% throughout the storage period, followed by pectin (1%) + K-PPE (1.0%) at 51.61% (Fig. 2b). The minimum average antioxidant activity was observed in untreated fruits (35.42%), followed by pectin (1%) treated fruits at 40.12%. Studies have shown that edible coating modify the internal atmosphere of fruits, slows down the metabolism and increase the synthesis of phenolics that may be responsible for retention of higher antioxidant activity during the storage (Kaur et al. 2022).
Carotenoids
The carotenoid content of fruits increased as storage days progressed (Fig. 2c). Significantly (p ≤ 0.05) lesser carotenoid content of 2.50 µg/100g was recorded in pectin (1%) + K-PPE (1.5%) by the end of storage, untreated fruits however, retained the maximum carotenoid content of 4.18 µg/100g. The coating material act as oxygen restrictor and slow down the respiration rate and metabolic processes, thereby delay ripening process. Similarl results were obtained by Carillo-Lopez et al. (2000) that coatings of Aloe vera gel over the fruit surface delay the carotenoids formation.
Cell Wall Degrading Enzymes
Pectin methyl esteraseand cellulase are the enzymes responsible for fruit ripening and result into fruit softening. The data pertaining to pectin methyl esterase enzyme (PME) activity of fruits with respect to storage days is represented in Fig. 3a. PME enzyme activity increased till day 14 followed by a decline. Edible coating infused with karonda polyphenolic extract had a significant influence on the activity of PME during the storage of fruit. The minimum PME activity was observed on day 28 (1.30) under untreated fruits. Among the treatments, pectin (1%) + K-PPE (1.5%) showed the minimum average PME enzyme activity of 1.67, followed by pectin (1%) + K-PPE (1.5%). Rao et al. (2016) showed that sodium alginate enriched with olive oil slowed PME activity as a result of reduced gaseous exchange by the fruit surface. Cellulase enzyme plays a significant role in ripening of fruits by carrying out degradation of the cellulose matrix which leads to softening of the fruit (Li et al. 2010). An increase in cellulase activity was observed as the storage progressed upto 14th day of storage after which a decline was observed (Fig. 3b). Significantly higher (1.48) cellulase activity was recorded in fruits treated with Pectin (1%) + K-PPE (1.5%) which was at par with fruits treated with Pectin (1%) + K-PPE (1.0%) at the end of storage. Maximum mean cellulase activity of 1.92% was exhibited by control followed by pectin (1%). Sinha et al. (2022) have reported the low cellulase activity in pear fruits coated with salicyclic acid enriched beeswax during storage.
Variation in pectin methyl esterase activity (PME) (a) and cellulase activity (b) in ber fruits under cold storage conditions in relation to different edible coating treatments. Values are represented as mean ± S.E of 4 replicates. Mean values followed by similar superscript within a column are not significantly different at *p ≤ 0.05
Conclusions
The present study has concluded that pectin 1% with 1.5% karonda polyphenol extract is very effective in mainating the quality of ber fruits in terms of weight, phenolics, ascorbic acid, antioxidant activity through out the storage at low temperature. In addition, this composite coating has slow down the activity of cell wall degrading enzymes and maintain the sensory acceptability of ber fruit till 28 day sof cold storage. Therefore, it is recommended the use of karonda polyphenolic extract as a novel ingredient in pectin coating for maintaining the nutritional quality of ber fruits during low temperature storage of 28 days.
References
AOAC (1980) Official methods of analysis of analytical chemists. Association of the Official Analytical Chemists, Washington, DC
AOAC (2005) Official and Tentative Methods of Analysis, 18th edn. Association of Official Agric Chemists, Washington, DC
Carrillo-Lopez A, Ramirez-Bustamante F, Valdez-Torres JB, Rojas-Villegas R, Yahia EM (2000) Ripening and quality changes in mango fruit as affected by coating with an edible film. J Food Qual 23:479–486
Dhall RK (2013) Advances in edible coatings for fresh fruits and vegetables: a review. Critical Rev Food Sci Nutr 53:435–450
Eshetu A, Ibrahim AM, Forsido SF, Kuyu CG (2019) Effect of beeswax and chitosan treatment on quality and shelf life of selected mango (Mangifera indica L.) cultivars. Heliyon 9:11–16
Ghasemnezhad M, Shiri MA, Sanavi M (2010) Effect of chitosan coatings on some quality indices of apricot (Prunus armeniaca L.) during cold storage. Cas J Environ Sci 8:25–33
Gol NB, Patel PR, Rao TR (2013) Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol Technol 85:185–195
Gull A, Bhat N, Wani MS, Masoodi FA, Amin T, Ganai SA (2021) Shelf-life extension of apricot fruit by application of nanochitosan emulsion coatings containing pomegranate peel extract. Food Chem 349:129–149
Jain V, Chawla S, Choudhary P, Jain S (2019) Post-harvest calcium chloride treatments influence fruit firmness, cell wall components and cell wall hydrolyzing enzymes of Ber (Ziziphus mauritiana Lamk.) fruits during storage. J Food Sci Technol 56:4535–4542
Kaur J, Jawandha SK, Gill PS, Grewal SK, Singh H (2022) Effect of beeswax enriched with sodium nitroprusside coating on antioxidant properties and quality of lemon cv. PAU Baramasi Lemon-1 fruits during low temperature storage. J Food Pro Preser 46:e16319
Li X, Xu C, Korban SS, Chen K (2010) Regulatory mechanisms of textural changes in ripening fruits. Cri Rev Plant Sci 29:222–243
Mahadevan A, Sridhar R (1982) Methods in physiological plant pathology. Sivagami Publ, Madras, pp 25–63
Mahajan M, Bons HK, Dhillon GK, Aggarwal P (2022) Unlocking the impact of drying methods on quality attributes of an unexploited fruit, karonda (Carissa carandas L.): A step towards food and nutritional security. South Afr J Bot 145:473–480
Meena HR, Kingsly ARP, Jain RK (2009) Effect of post-harvest treatments on shelf life of ber fruits. Indian J Horticul 66(1):58–61
Moran R, Porath D (1980) Chlorophyll determination in intact tissues using N, N dimethylformamide. Plant Physiol 65:478–479
Nair MS, Saxena A, Kaur C (2018) Effect of chitosan and alginate-based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem 240:245–252
Prasad K, Singh G, Singh SK, Pradhan I, Kumar U, Singh H (2022) Plant extract and essential oil coating prolongs shelf life and maintains keeping quality of papaya fruit during storage. J Food Proc Preser. https://doi.org/10.1111/jfpp.17015
Ranganna S (1986) Handbook of analysis and quality control for fruit and vegetable products. Tata McGraw-Hill Education
Rao RTV, Baraiya NS, Vyas PB, Patel DM (2016) Composite coating of alginate-olive oil enriched with antioxidants enhances postharvest quality and shelf life of Ber fruit (Ziziphus mauritiana Lamk. Var. Gola). J Food Sci Technol 53:748–756
Singleton VL, Orthofer R, Lamuela-Ranventos RM (1999) Analysis of total phenols other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth Enzymol 299:152–178
Sinha A, Gill PPS, Jawandha SK, Grewal SK (2022) Composite coating of chitosan with salicylic acid retards pear fruit softening under cold and supermarket storage. Food Res Int 160:1–16
Sun X, Wall M, Follett P, Liang P, Xu S, Zhong T (2023) Effect of pectin coatings containing Trans-cinnamaldehyde on the postharvest quality of Rambutan. Hortic Sci 58(1):11–15
Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30
Zam W (2019) Effect of Alginate and Chitosan Edible Coating Enriched with Olive Leaves Extract on the Shelf Life of Sweet Cherries (Prunus avium L.). J Food Qual 20:1–7
Weiterführende Literatur
Azevedo IG, Oliveira JG, Silva MG, Pereira T, Corrêa SF, Vargas H, Facanha AR (2008) P‑type H+-ATPases activity, membrane integrity and apoplastic pH during papaya fruit ripening. Postharvest Biol Technol 48:242–247
Jawandha SK, Singh H, Arora A, Singh J (2014) Effect of modified atmosphere packaging on storage of Baramasi lemon (Citrus limon L. Burm). Inter J Agric Environ Biotechnol 7(3):635–638
Kaur B, Jawandha SK, Singh H, Thakur A (2013) Effect of putrescine and calcium on colour changes of stored peach fruits. Int J Agric Environ Biotechnol 6:301–304
Acknowledgements
The authors would like to thank Department of fruit Science, Punjab Agricultural University and Regional Research Station (PAU), Bathinda for all the facilities provided to do the research work.
Author information
Authors and Affiliations
Contributions
Karandeep Kaur: Performed the experiments; Navjot Gupta: Conceived, designed and wrote the paper; Monika Mahajan: Analyzed and interpreted the data and wrote the paper; Sukhjit Kaur Jawandha: Conceived and designed the experiments; contributed reagents and materials experiments; Nirmaljit Kaur: Done the editing of the paper and gave valuable suggestions
Corresponding author
Ethics declarations
Conflict of interest
K. Kaur, N. Gupta, M. Mahajan, S. Kaur Jawandha and N. Kaur declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, K., Gupta, N., Mahajan, M. et al. Synergeistic Effect of Pectin and Karonda Polyphenols Composite Edible Coating On Post-Harvest Life of Ber (Zizyphus mauritiana Lamk.) Fruit. Applied Fruit Science 66, 1409–1416 (2024). https://doi.org/10.1007/s10341-024-01114-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-024-01114-8