Abstract
The use of Oscillating Magnetic Fields (OMF) during freezing has been advocated by some for preserving the intrinsic ‘fresh’ qualities of the subsequently thawed food. This paper describes studies to investigate the effects of a range of different OMF freezing settings using an ABI Cells Alive System (CAS) freezer on characteristics of the freezing process and food-quality parameters. The effects of short-term frozen storage on sample properties were also evaluated. Apple and potato samples were frozen at − 30 or − 45 °C with a range of OMF settings whilst measuring sample temperatures. Samples were then either immediately thawed or stored frozen for 1 month. Effects of OMF on freezing temperature curve texture parameters, sample dimensions, sample weights and sample texture parameters of thawed samples were determined. Overall, results showed no statistically significant continuum of effect in these trials that prevails across all CAS settings and/or temperatures for apple and potato for any parameter measured. However, significant effects were seen at some settings for some parameters for each product, and this may suggest that OMF may possibly be tuneable for specific situations. Where significant changes did occur, these only appeared at isolated settings, temperatures or storage periods. This suggests that OMF may not affect all foods in an equal manner and that any effect depends on an interrelationship of food type, freezing rate, magnetic field frequency and storage conditions. We conclude that there is no major effect of OMF on freezing parameters and there is no overall pattern of OMF on freezing processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freezing is one of the most popular and widely used methods for the preservation of foods. One limitation of freezing as a method of food preservation is that changes occur during freezing and thawing that render the quality of frozen food different from that of the fresh unfrozen product. There is growing interest in finding freezing methods that will give a long shelf life but preserve the intrinsic ‘fresh’ qualities of the food. One method advocated for this is the use of Oscillating Magnetic Fields (OMF) during the freezing process (Otero et al. 2016). A commercial system utilising OMF is the Cells Alive System (CAS) patented and marketed by the ABI Corporation Ltd. (Chiba, Japan), which claims to provide a process where a product can be frozen in such a manner as to “approximate the quality of a fresh product” once thawed (Owada and Kurita 2001; ABI 2007; Owada 2007; Kelly 2008). The ABI CAS uses permanent magnets and induction coils to produce a weak oscillating magnetic field within the freezing chamber. CAS is not a refrigeration process itself but is claimed to assist in improving existing freezing and frozen storage processes (both in terms of process speeds and product quality). The CAS patent literature claims that the oscillating magnetic fields act on polarised water molecules to delay formation of ice crystals (Owada and Kurita 2001; Owada 2007). Whether this is due to inducing super-cooling, or movement of the molecules during the ice formation process, is unclear as conflicting explanations are given in the ABI literature, patents and in discussions with the manufacturers. However, the claimed consequence is that because of the delay in ice formation, most of the ice crystals will form at the same time thus restricting their size. It is assumed that smaller ice crystals will result in less cellular damage to the food during freezing and frozen storage, and therefore causing less structural damage to the product after thawing.
Water is the key component in food freezing and there is evidence that water, being a diamagnetic material, can be magnetised in a magnetic field. Woo and Mujumdar (2010) cite a number of independent studies that have demonstrated that freezing water under the influence of a magnetic field can change its freezing characteristics. For example, Zhou et al. (2012) looked at the super-cooling and crystallisation of tap water under DC magnetic fields. They found that increasing the magnetic field intensity increased the degree of super-cooling.
Despite the claims for CAS (Kelly 2008), there are few peer-reviewed studies on the freezing, storage and thawing of food under magnetic fields. Otero et al. (2016) recently reviewed what has been published. Whilst some studies have reported advantages of OMF-assisted freezing of foods (Yamamoto et al. 2005; Kim et al. 2013a, b; Ku et al. 2014; Choi et al. 2015), other studies (Suzuki et al. 2009; Watanabe et al. 2011; James et al. 2015a) have reported OMF to have no effect on freezing characteristics or frozen food quality.
The findings of Suzuki et al. (2009) and Watanabe et al. (2011) were also negative. Using a ‘copy’ of the ABI CAS system, they found that oscillating weak magnetic fields had no effect on the freezing curves of sweet potato, spinach, fish, agar gel or water and had no visible effect on cellular microstructures of the tissues they examined. James et al. (2015b) describe a short study carried out to investigate the effect of freezing under OMF conditions on the degree of super-cooling and characteristics of the freezing curve of individual cloves within whole garlic bulbs when compared with freezing under the same conditions without OMF. Overall, these results clearly indicate that the OMF had little effect on the freezing characteristics, or degree of super-cooling, of garlic bulbs, in comparison with freezing under the same conditions without OMF.
Yamamoto et al. (2005) observed longer freezing plateaus when freezing chicken breasts in an ABI freezer (1.5 to 2 mT at 20, 30 and 40 Hz) compared with no OMF application. Yamamoto et al. (2005) attributed these longer freezing times to additional heat load from the OMF generators inside the freezer. After 1 week of storage at − 30 °C, no differences between the different freezing methods were detected in drip and cooking losses and fracture properties (rupture stress and strain) of the chicken breasts. After 6 months of storage, OMF frozen samples were significantly softer than those conventionally frozen, and when examined microscopically, showed less damage than conventionally frozen samples. However, there was little difference in drip and cooking losses between OMF frozen and conventionally frozen samples.
Kim et al. (2013a, b), Ku et al. (2014) and Choi et al. (2015) reported reduced total freezing times, lower drip losses and improved quality attributes in pork, chicken and beef samples frozen in an ABI OMF-assisted freezer at − 55 °C compared with samples conventionally frozen in an air blast freezer at − 45 °C. However, as freezer temperatures were not the same, it is not possible to deduce whether the improvements detected were produced by the OMF or by the lower-temperature freezing process.
This paper describes studies to investigate the effects of a range of different OMF freezing settings using an ABI CAS freezer on characteristics of the freezing process and food quality parameters. The effects of short-term frozen storage on sample properties were also evaluated. Apple and potato samples were frozen at a range of OMF settings whilst measuring core temperatures. Samples were then either immediately thawed (4 ± 1 °C) or stored frozen (− 19 ± 1 °C) for 1 month. Sample dimensions and weights were measured before and after freezing, and after thawing, and drip loss calculated. Texture parameters were determined for samples before freezing and after thawing. Apple and potato were chosen as the test materials because of the uniformity of their tissues and their structural stability in storage and because these test materials have been used in other studies that assessed the effect of freezing (e.g., Tregunno and Goff 1996; Delgado et al. 2009; Comandini et al. 2013).
Materials and Methods
OMF-Assisted Blast Freezer
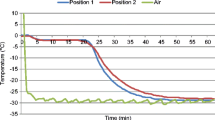
These trials used the same OMF-assisted blast freezer (ABI Corporation Ltd., Chiba, Japan) as used by James et al. (2015b). The performance of the OMF system was checked by an ABI engineer before starting the trials and certified as being within the manufacturer’s specified operating parameters. The minimum temperature demand setting was − 45 °C. Air velocities in the working space were turbulent and variable (between 0.5 and 5 ms−1). To provide more reproducible conditions, a secondary ‘inner working section’ was constructed to permit control of air velocity over the samples (Fig. 1). In the CAS-assisted-blast freezer used, the CAS can be set between 0 and 100% where the settings produce conditions as shown in Fig. 2. Trials were carried out at demand air temperatures of − 30 and − 45 °C, a mean air velocity of 0.9 ms−1 (SD = 0.08) and CAS settings of 0, 25, 50, 75 and 100%. The control conditions (‘NoCAS’) were carried out in the same ABI CAS system but with the OMF system switched off. It is important to note that the 0% CAS setting is not the same as a ‘NoCAS’ setting (Fig. 2). In all cases, freezing was continued until core sample temperatures were below − 25 °C.
Sample Preparation
‘Granny Smith’ cultivar apples and ‘White’ potatoes (precise cultivar was unknown as it was a generic product) were sourced from the same local supermarket for all trials. Practically, this gave the best uniformity of raw material source and temperature history as the same retailer supply chain was used for all source products, but precise details were not available. Products with longest stated shelf lives were selected in each case. Samples preparation was based on the method used by Delgado et al. (2009). A no. 12 cork borer was used to cut cylindrical samples (nominal diameter 17 mm) from the apples and potatoes. The cylinders were then cut normal to the main axis into 20 mm long cylinders. Samples were cut to contain no skin, core or pips. Following the practice of Delgado et al. (2009), during preparation, each sample was loosely wrapped in tissue paper saturated with water to prevent browning reactions whilst awaiting freezing. Moisture lost or water uptake during this period was considered to have little effect.
Experimental Protocol
For each particular experiment, 13 apple and 13 potato samples were cut.
A T-type wire thermocouple was inserted into three samples from each apple and potato group at the approximate geometric centre to measure the core temperatures (±0.5 °C) during the freeze-thaw process. Sample core and process air temperatures were recorded at 15-s intervals using a Comark Diligence EV N2014 logger (Comark Instruments Ltd., Hitchin, UK) at the locations indicated in Fig. 1.
The weight (±0.1 g) and dimensions (±0.1 mm) of all other samples was then measured and recorded individually using a digital balance (Sauter RC2022, Sauter KmbH, Albstadt, Germany) and digital callipers (Draper Tools, UK), respectively.
The blast freezer was set to the required CAS setting and temperature and the inner working section fan turned on. After the equipment had equalised to the required operating conditions, all samples were transferred to the air duct of the inner working section. After the core temperatures of all instrumented samples had fallen below − 25 °C, all the samples were removed. The frozen non-instrumented samples were re-weighed, dimensional data re-measured and separated into two groups of five samples for each produce type. All frozen samples were wrapped in absorbent paper, and one group of each produce type were placed in a refrigerator set at 4 ± 1 °C to thaw, whilst the other group were placed in a domestic chest freezer operating at − 19 ± 1 °C.
Samples in the refrigerator were left to thaw overnight (>14 h), and the thawed samples were re-weighed and re-measured.
After one calendar month, the frozen stored samples were transferred to the refrigerator set at 4 °C and left to thaw overnight (>14 h). The thawed samples were re-weighed, dimensions re-measured and texture analysis performed as with the immediately thawed samples.
Three complete replicate experiments were performed, each with CAS settings of 0, 25, 50, 75 and 100% and ‘NoCAS’ with 13 samples each per produce type/freezing temperature/frozen period at each trial. This produced 15 (3 × 5) replicates for weight, dimensional and texture data and 9 (3 × 3) temperature replicates at each condition.
Measurements and Data Analysis
Weight and Dimensional Changes
Individual sample weights and dimensions were recorded in each treated group before freezing, after freezing and after thawing. Proportional changes were assessed by the change in process in relation to the initial value.
Change (%) was calculated as
Texture Measurement
Texture measurements were made on samples using a TVT-150 texture analyser (TexVol Instruments, Viken, Sweden). Using a 4-mm diameter penetration head, each sample was subjected to a compression of 40%, a 30-s hold duration and release cycle with a head speed of 1 mm/s. The peak force and residual force (force at the end of the 30 s hold) were recorded for each sample tested.
The peak force is a measure relating to the overall firmness of the sample.
The ratio of peak to residual forces is calculated by dividing the peak force by the residual force. This is a measure of the elasticity with a higher ratio indicating a more resilient elastic texture and a lower ratio indicating a softer more plastic texture.
Temperature Curve Data Extraction
Core temperatures were recorded for three apple and three potato samples for each trial run.
The temperature data was processed in MS Excel to determine:
-
a.
Freezing time—the elapsed time for samples to cool from + 10 to − 18 °C. Sample starting temperatures were in the range 15–22 °C due to inherent variations in room temperature and the placement of the temperature sensor. Freezing time was taken to be from 10 °C to reduce the impact of these variations.
-
b.
Freezing plateau time—the elapsed time for the samples to pass from 0 to − 7 °C. This characterises the length of the freezing plateau where latent heat is extracted. All water in a product does not convert to ice instantly at the initial freezing point, as the temperature drops further a greater proportion of the water content becomes ice. The range 0 to − 7 °C is taken to allow for the majority of the water to become ice and the product to be predominately frozen.
-
c.
Initial freezing point—the transition point from the initial cooling to the freezing plateau. This is the temperature at which the linear portion of initial cooling merges into the predominantly horizontal freezing plateau of latent heat extraction.
Data Analysis
Statistical analyses were carried out using Excel (Microsoft Corporation, Microsoft Excel for Mac 2011, version 14.4.3). Two-tailed student t tests were performed to establish differences between any of the CAS settings and data from the NoCAS control freezing. The significance threshold was set at 0.05 for all tests.
Results and Discussion
Environmental air temperatures were recorded for all experimental runs. For the − 30 °C demand trials, the air temperatures typically cycled in the range − 32 to − 26 °C, and for the − 45 °C trials, temperatures typically cycled in the range − 47 to − 42 °C.
OMF Effects During Freezing
A data summary of OMF effects on temperature curves is shown in Table 1.
OMF-Assisted Freezing Effects on Freezing Time
At − 30 °C, none of the apple or potato freezing times were significantly different from the NoCAS control times. When freezing apple samples at − 45 °C the 0% CAS samples showed a significant increase in freezing time in comparison with the control (and all other CAS settings) conditions. For potato samples at − 45 °C, however, this relationship was reversed with the 0% CAS showing no significant difference to the control setting, and all other CAS settings being significantly different to the control conditions.
OMF-Assisted Freezing Effects on Freezing Plateau Time
In all but one instance, there were no significant effects of CAS setting on freezing plateau times for either apple or potato at either − 30 or − 45 °C. Only potato frozen at − 30 °C with 0% CAS (highest magnetic field strength) showed a significant difference compared with the NoCAS control freezing conditions. At 0% CAS, the freezing plateau time was 18.9 min, 3.2 min (14%) faster than the NoCAS control.
OMF-Assisted Freezing Effects on Initial Freezing Point
At − 30 °C, whilst there were no statistically different results compared with the control setting, there were noticeable depressions of the mean initial freezing point at the 75% CAS setting for apple and the 50% CAS setting for potato. At − 45 °C, there were significantly lower initial freezing points than for the control seen for apple at 25% CAS and for potato at 25% CAS and 100% CAS settings.
OMF-Assisted Freezing Effects on Sample Weight Loss After Freezing
Apple and potato samples behave similarly at each temperature (Table 2), and in all but one instance, there were no significant effects of CAS setting on sample weight changes during freezing for either apple or potato frozen at either − 30 or − 45 °C. Only potato frozen at − 45 °C with 75% CAS setting showed a significant difference compared with the control freezing conditions, the weight loss being 0.4% greater than that in the control. An increase in weight loss is generally not a desirable trait for the commercial food sector.
OMF-Assisted Freezing Effects on Sample Length During Freezing
It can be seen that at all conditions, potato expands more than apple during freezing (Table 3). In all but two instances, there were no significant effects of CAS setting on sample length changes during freezing for either apple or potato samples frozen at either − 30 or − 45 °C. Only apple frozen at − 30 °C at a 25% CAS or 100% CAS setting showed a significant difference compared with the control freezing conditions.
OMF-Assisted Freezing Effects on Sample Diameter Change During Freezing
At each freezing temperature, both apple and potato samples show similar patterns of changes in diameter in relation to the CAS setting, but these patterns are different for each temperature (Table 4). For apple freezing at − 30 °C, three of the five CAS settings give a significant decrease of sample diameter in comparison with the control setting. However, for potato freezing at − 30 °C, three of the five CAS settings give a significant increase in sample diameter. Only the highest magnetic intensity setting (0% CAS) significantly affected the diameter of both the apple and potato sample types; however, this effect was for significant decreases and increases in diameter respectively.
At − 45 °C, freezing with CAS energised appears to give similar results for all CAS settings. Both sample types show an increase in diameter in comparison with the control freezing. This increase is significant for potato but not significant for apple.
Effects on Thawed Products After OMF-Assisted Blast Freezing
OMF-Assisted Freezing Effects on Sample Weight After Thawing
The majority of conditions are not significantly different to the control samples (Table 5). Only one condition (potato frozen at − 45 °C and stored for 1 month before thawing) was significantly different to the control results.
OMF-Assisted Freezing Effects on Sample Length After Thawing
The majority of settings do not produce results significantly different from the control NoCAS settings (Table 6). The exceptions are for 100% CAS apple and 0% CAS potato samples frozen at − 30 °C and immediately thawed where mean length changes are significantly greater than the NoCAS controls, and for 100% CAS apple samples frozen at − 45 °C and stored before thawing where mean length changes less than for the controls.
OMF-Assisted Freezing Effects on Sample Diameter After Thawing
Both sample types show mostly similar trends in relation to CAS setting (Table 7) apart from the higher magnetic intensities for potato frozen at − 30 °C and thawed immediately. OMF frozen potatoes samples frozen at − 30 °C and stored for 1 month before thawing all showed a significant difference to the control NoCAS setting, but there were no substantial differences due to CAS setting.
OMF-Assisted Freezing Effects on Peak Force After Thawing
In all cases, thawed potato samples were firmer (higher peak force) than apple. Raw unfrozen apple samples had a mean firmness of 1177 g (SD = 448), and raw unfrozen potato samples had a mean firmness of 2766 g (SD = 222). All thawed samples exhibited significantly less firmness than these unfrozen control values irrespective of the frozen storage period (Table 8). All apple samples frozen at − 45 °C showed a significant increase in firmness over the NoCAS control samples.
OMF-Assisted Freezing Effects on Peak Force to Residual Force Ratios (Elasticity)
There were no substantial effects of OMF seen in the elasticity data (Table 9) with similar values being seen at all settings and conditions. Potato samples were seen to be consistently more elastic than apple samples. Raw unfrozen apple samples had a mean elasticity of 2.4 (SD = 0.4), and raw unfrozen potato samples had a mean elasticity of 2.0 (SD = 0.1). All thawed samples were significantly more elastic than these unfrozen control values irrespective of the frozen storage period (Table 9).
Discussion
Throughout the dimensional data, there is a high level of variance. This is attributed to a combination of the small 20-mm length of the sample (where even a small absolute error in measurement has a large proportional effect) and inherent errors in cutting the sample ends truly square, misalignment of the callipers to the axis of the previous measurements and compression of thawed samples by the callipers during measurement.
Overall, results showed no statistically significant effects of CAS setting (magnetic intensity) on any measured parameter. Where significant changes were seen, these only appeared at one temperature or storage period. We conclude there is no major effect of OMF on freezing parameters and there is no overall pattern of effect on freezing processes. There is no continuum of increasing (or decreasing) effect of OMF with changes in CAS setting. However, there are some small pockets of significant effect at specific settings on specific parameters for a certain product and this may suggest that OMF may possibly be tuneable for specific situations. Future work focussed on reducing variations in samples and experimental conditions and preparation would be required to assess this possibility.
Zhou et al. (2012) reported a 40% increase in crystallisation growth time using OMF. Similar large decreases in freezing time (+ 10 to − 18 °C) are seen at the lower CAS settings for apple at − 30 °C and for both products at − 45 °C. However, the CAS setting does not appear to affect the length of the freezing plateau, suggesting that there is no change to the ice formation process as suggested in the ABI CAS marketing literature.
The initial freezing point data (Table 1) show individual CAS settings having a significant effect. This may suggest a possible ‘tuning’ effect where a specific CAS setting affects a specific property of a specific product in a specific manner; however, more detailed work is required.
The weight changes between thawed and initial weights may indicate differences in ice formation engendered by OMF, but also reflect any impacts of OMF on commercially important weight loss values. Only apple samples frozen at − 30 °C and a setting of 0% CAS showed any significant difference from the controls (Table 5), and this was to increase the weight loss, which is not a desirable trait.
ABI claim in their marketing literature (ABI 2007) that CAS freezing can inhibit enzymic tissue browning associated with ‘normal’ freezing. Although not instrumentally measured in these trials, no obvious evidence was seen of differences in enzymic browning at any CAS setting for either apple or potato when compared with the NoCAS control samples.
Overall, the results of this study are similar to studies by Yamamoto et al. (2005), Suzuki et al. (2009), Watanabe et al. (2011) and James et al. (2015b) in that OMF was not found to have any significant effect on the freezing characteristics of food samples. No significant differences were observed in the temperature-time history freezing curves of apple and potato samples frozen with or without OMF. There was no evidence of OMF enhancing super-cooling during freezing, as claimed in the patent literature (Owada and Kurita 2001; Owada 2007).
Conclusions
The aim of this work was to investigate any OMF-induced effects on the freezing process and/or quality parameters relating to frozen apple and potato samples. Overall, results showed no statistically significant effects of CAS setting (OMF magnetic intensity) on any measured parameter that appeared for all air temperatures. This data shows no strong continuum of effect that prevails across all CAS settings and/or temperatures for any of these products. Some results for specific products have suggested there are possibilities of ‘tuned’ temperature and CAS settings that do produce an effect but these results were not always repeatable and may simply be due to inherent variations in experimental samples and conditions.
The results of this study do not support the patent and marketing material claims that OMF-assisted freezing substantially improves the freezing process for foodstuffs. This does not necessarily invalidate OMF-assisted freezing as there does appear to be some evidence of OMF having some effect on the freezing characteristics of the foods examined at some of the settings used. This suggests that weak OMF may not affect all foods in an equal manner and that any effect will depend on a complex combination of food, freezing rate, magnetic field frequency and storage conditions.
References
ABI Co., Ltd. (2007). CAS: cells alive system. The CAS energy function has an international patent. Available in four parts at: http://www.rayswebstudio.co.uk/Lynton/download-pdf/English%20Brochure_01.pdf and ... _02.pdf, ... _03.pdf, ... _04.pdf. Accessed Mar 2017.
Choi, Y. S., Ku, S. K., Jeong, J. Y., Jeon, K. H., & Kim, Y. B. (2015). Changes in ultrastructure and sensory characteristics on electro-magnetic and air blast freezing of beef during frozen storage. Korean Journal of Food Science of Animal Resources, 35, 27–34.
Comandini, P., Blanda, G., Soto-Caballero, M. C., Sala, V., Tylewicz, U., Mujica-Paz, H., Valdez Fragoso, A., & Gallina Toschi, T. (2013). Effects of power ultrasound on immersion freezing parameters of potatoes. Innovative Food Science & Emerging Technologies, 18, 120–125.
Delgado, A. E., Zheng, L., & Sun, D.-W. (2009). Influence of ultrasound on freezing rate of immersion-frozen apples. Food and Bioprocess Technology, 2, 263–270.
James, C., Purnell, G., & James, S. J. (2015a). A review of novel and innovative freezing technologies. Food and Bioprocess Technology, 8, 1616–1634.
James, C., Reitz, B. G., & James, S. J. (2015b). The freezing characteristics of garlic bulbs (Allium sativum L.) frozen conventionally or with the assistance of an oscillating weak magnetic field. Food and Bioprocess Technology, 8, 702–708.
Kelly, T. (2008). Mr. Freeze. Forbes, http://www.forbes.com/forbes/2008/0602/076.html. Accessed Mar 2017.
Kim, Y. B., Jeong, J. Y., Ku, S. K., Kim, E. M., Park, K. J., & Jang, A. (2013a). Effects of various thawing methods on the quality characteristics of frozen beef. Korean Journal of Food Science of Animal Resources, 33, 723–729.
Kim, Y. B., Woo, S. M., Jeong, J. Y., Ku, S. K., Jeong, J. W., Kum, J. S., & Kim, E. M. (2013b). Temperature changes during freezing and effect of physicochemical properties after thawing on meat by air blast and magnetic resonance quick freezing. Korean Journal of Food Science of Animal Resources, 33, 763–771.
Ku, S. K., Jeong, J. Y., Park, J. D., Jeon, K. H., Kim, E. M., & Kim, Y. B. (2014). Quality evaluation of pork with various freezing and thawing methods. Korean Journal of Food Science of Animal Resources, 34, 597–603.
Otero, L., Rodríguez, A. C., Pérez-Mateos, M., & Sanz, P. D. (2016). Effects of magnetic fields on freezing: Application to biological products. Comprehensive Reviews in Food Science and Food Safety, 15, 646–667.
Owada, N. (2007). Highly-efficient freezing apparatus and highly-efficient freezing method. US Patent 7237400 B2.
Owada, N., & Kurita, S. (2001). Super-quick freezing method and apparatus therefore. US Patent US 6250087 B1.
Suzuki, T., Takeuchi, Y., Masuda, K., Watanabe, M., Shirakashi, R., Fukuda, Y., Tsuruta, T., Yamamoto, K., Koga, N., Hiruma, N., Ichioka, J., & Takai, K. (2009). Experimental investigation of effectiveness of magnetic field on food freezing process. Transactions of the Japan Society of Refrigerating and Air Conditioning Engineers, 26(4), 371–386.
Tregunno, N. B., & Goff, H. D. (1996). Osmodehydrofreezing of apples: Structural and textural effects. Food Research International, 29, 471–479.
Watanabe, M., Kanesaka, N., Masuda, K., & Suzuki, T. (2011). Effect of oscillating magnetic field on supercooling in food freezing. Proceedings of the 23rd IIR International Congress of Refrigeration; refrigeration for sustainable development, August 21–26, Prague, Czech Republic. 1, 2892–2899.
Woo, M. W., & Mujumdar, A. S. (2010). Effects of electric and magnetic field on freezing and possible relevance in freeze drying. Drying Technology, 28, 433–443.
Yamamoto, N., Tamura, S., Matsushita, J., & Ishimura, K. (2005). Fracture properties and microstructure of chicken breasts frozen by electromagnetic freezing. Journal of Home Economics Japan, 56, 141–151.
Zhou, Z., Zhao, H., & Han, J. (2012). Supercooling and crystallization of water under DC magnetic fields. CIESC Journal, 63, 1404–1408.
Acknowledgements
The authors gratefully acknowledge the funding support of Air Products in this study and the placement students Nabil Benarib and Hugo Mazet who collected much of the experimental data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Purnell, G., James, C. & James, S.J. The Effects of Applying Oscillating Magnetic Fields During the Freezing of Apple and Potato. Food Bioprocess Technol 10, 2113–2122 (2017). https://doi.org/10.1007/s11947-017-1983-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-017-1983-3