Abstract
The use of power ultrasound within the food industry is an innovative subject. Application of sound to monitor a process or product is common, e.g. in quality assurance. However, the use of ultrasound to directly improve processes and products is less popular in food manufacturing. In the present work, ultrasound-assisted immersion freezing was investigated on apple samples. Because the apple parenchyma is mechanically anisotropic, the influence of applying ultrasound on radial or tangential orientated samples was also examined. Apple cylinders were immersed in an ultrasonic bath system, which operates at 40 kHz frequency. Experiments were carried out at a power level of 131.3 W (0.23 W/cm2), and ultrasound was applied intermittently for different times from temperatures below and close to the initial freezing point. Results showed that ultrasound application at 0°C or −1°C for 120 s in total, with 30 s intervals, significantly improved the freezing rate represented by the characteristic freezing time up to 8% (P < 0.05), compared to immersion freezing without ultrasound. Results of the effect of ultrasound waves applied on radial or tangential cut samples sonicated for 120 s from −1°C and/or 0°C indicated that at the power level considered there were no significant differences among the ultrasonic radial or tangential irradiated samples of these treatments, though the freezing rates were enhanced and different (P < 0.05) from the control treatment. Some evidence of the influence of ultrasound to induce primary nucleation was also observed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freezing is a well-known preservation method widely used in the food industry. The freezing process combines the favourable effect of low temperatures with the conversion of water into ice. The water–ice transition has the advantage of fixing the tissue structure and separating the water fraction in the form of ice crystals in such a way that water is not available either as solvent or reactive component (Delgado and Sun 2001). However, the size and location of the ice crystals may damage cell membranes and break down the physical structure. Thus, the cause of the undesirable physico-chemical modifications during freezing is the crystallisation of water and sometimes solutes (Delgado and Sun 2001). It is well-known that the crystallisation of ice has two steps: the formation of nuclei and the later growth of the nuclei to a specific crystal size, the final crystal size being a function of the rates of nucleation and crystal growth, and also of the final temperature (Martino et al. 1998). Slow freezing generally leads to large ice crystals formed exclusively in extracellular areas that could damage cell structure and have an effect on the thaw behaviour as well as on the sensory properties and nutritional value of foodstuffs, while high freezing rates produce small crystals evenly distributed all over the tissue. Therefore, extended research has been carried out to control the crystal size.
Conventional cooling methods such as air blast, plate contact, circulating brine and liquid nitrogen (ordered in increasing values of the heat transfer coefficient) are the most common methods used for food freezing (Heldman 1992). Among these traditional methods, the immersion freezing process offers numerous advantages, e.g. high-heat transfer coefficients, individualised freezing, good product quality, energy savings, etc. Within the last few years, new methods are being developed and analysed, e.g. the high pressure assisted freezing, which, based on the lowering of water melting point with pressure, allows a nucleation of ice throughout the whole volume of the product (Sanz et al. 1999), or ultrasound-assisted technologies for product modification or process improvement, which has attracted considerable interest due to its promising effects in food processing and preservation (Knorr et al. 2004).
The sound ranges employed can be broadly divided into high frequency, low energy, diagnostic ultrasound in the MHz range, and low frequency, high energy, power ultrasound in the kHz range (Mason and Chemat 2003). Power ultrasound in particular, a kind of ultrasound wave with frequencies in the range from 20 to 100 kHz and high sound power or sound intensity (generally higher than 1 W/cm2), has proved to be extremely useful in crystallisation processes (Mason 1998). Acton and Morris (1992) determined that when subjecting a liquid to ultrasound during its solidification, it is possible to modify both the nucleation and the crystal growth stages of solidification. Compared to other nucleation methods (e.g. chemicals, aminoacids, ice nucleation bacteria, seed crystals, etc.) power ultrasound offers several advantages: the initial nucleation temperature of the liquid can be adjusted, the technique is chemically non-invasive and does not require direct contact with the product to be frozen, and furthermore, the use of power ultrasound does not present legislative difficulties (Acton and Morris 1992). Acton and Morris (1992) also presented a number of applications where the solidification process can be improved by the application of power ultrasound (e.g. chocolate tempering, freeze drying, freezing of oil-in-water emulsions, etc.). It is assumed that acoustic cavitation defined as the growth, oscillation and collapse of small micro-bubbles, and the behaviour of the bubble of cavitation upon the propagation of the acoustic waves constitute the essential events which induce the majority of the acoustic effects by means of several possible mechanisms still under discussion (Zeqiri et al. 2006; Leighton 1998; Knorr et al. 2004).
Although the potential of power ultrasound to assist/accelerate food freezing was described, it has not yet been sufficiently exploited (Zheng and Sun 2006). Results from a previous research work (Li and Sun 2002; Sun and Li 2003) pointed to promising results when immersion freezing of potatoes with the aid of power ultrasound was studied. In the present work, ultrasound-assisted immersion freezing is investigated on apple samples focusing on the effect of ultrasound on the freezing rate and nucleation temperature. Because the apple parenchyma is mechanically anisotropic, the influence of applying ultrasound on radial or tangential orientated samples is also examined.
Materials and Methods
Sample Preparation
Fresh apples (Malus × domestica) of the Granny Smith variety (86.9% w/w moisture content, wet basis, soluble solids content ≅ 12 °Brix) were purchased in a local market and stored at 4 ± 1°C until use. Because of the uniformity of their tissues and their structural stability in storage, apples were chosen as the test material (Sterling 1968). Granny Smith variety is on the other hand available all over the year at a fairly constant quality. Not all apples could be selected at the same time, and since they might have not been necessarily in the same physiological state, some fruit-to-fruit variability was inevitable. Apple cylinders measuring 1.8 cm diameter and 2.5 cm high were cut using a cork borer in radial and tangential orientation from the middle parenchyma of the fruit (Fig. 1). Fruit size was chosen to allow for the cut of five to six cylinders from each apple. What remained of the fruit tissue were used for initial water content and soluble solids determination. Each sample was loosely wrapped in tissue paper saturated with water to prevent browning reactions, and kept in a refrigerator at a temperature of 4 ± 1°C to achieve uniform initial temperature until measurements were taken. Moisture lost or water uptake during this period was considered to have little effect.
Experimental Apparatus and Procedures
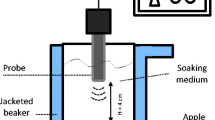
An ultrasonic bath system (Elma MC 300 LC, Elma GmbH & Co KG, Germany), which operates at 40 kHz frequency, and dissipated powers from 130.6 to 201.2 W was used. Experiments were carried out at a power level of 131.3 W or acoustic intensity of 0.23 W/cm2. The calorimetric method was used to determine the actual dissipated acoustic power, by measuring the rate of temperature increase due to the conversion of ultrasound energy into heat and calculating the dissipated power according to the following expression (Ratoarinoro et al. 1995):
where m is the mass of liquid (kg), c p is the heat capacity of the liquid (J/kg°C) and (dT/dt)0 is the initial slope of the curve of temperature versus time (°C/min). Water was used to calibrate the actual acoustic power of the ultrasonic bath system. Then acoustic intensity (W/cm2) was determined dividing the ultrasonic power by the cross-section area of the bottom of the vessel (Li and Sun 2002). The ultrasonic bath was well insulated in order to prevent heat losses to the surroundings.
Because the application of ultrasound produces heat, which is adverse to the freezing process, ultrasound was applied intermittently at 30 s intervals. The ultrasonic exposure time was referred to the sum of every treatment time (Li and Sun 2002). Ultrasound treatments were applied for different exposure times and from different initial temperatures (Table 1). At least four replications were carried out for each treatment.
For the freezing process, the ultrasonic tank was filled with a mixture of ethylene glycol and water at the proportion of 50% to 50% in volume, which was cooled to the desirable temperature by circulating the fluid from a refrigerated circulator (Julabo FP50 model, Julabo Labortechnik GmbH, Germany). The circulator temperature was set up to reach an average solution temperature of −25°C for all the experimental runs.
Samples were removed from the refrigerator, and a T-type thermocouple was inserted into the centre before dipping the apple cylinder in the freezing solution. In order to prevent solution infiltration, the point of the thermocouple entry into the sample was sealed with enamel. Care was taken to keep the experimental set-up constant, as the velocities of the refrigerant medium and the intensity of ultrasound might vary with the position in the tank. Apple cylinders were first placed standing (either for the radial or tangential orientation) in a cage made by a thin wire so as to simulate individual freezing, and then they were laid on the bottom of the ultrasonic vessel, that is, there was no free space between the samples and the bottom of the ultrasonic unit, except for that of the wire of the cage, being the sample well below the air–liquid interface as well (Fig. 2).
Temperature readings were recorded at 2 s intervals with a data logger (Squirrel 1600, Grant Instruments, Cambridge, UK), which was operated with battery, due to some problems of ground loop connection experienced when recording temperatures with a data acquisition system connected to a computer. Thermocouple readings were not affected either by electromagnetic interference. The solution temperature was determined from the average readings of two thermocouples placed close to the sample. Thermocouples were calibrated at 0°C using a mixture of distilled water and crushed ice, and at 20°C, 10°C, −10°C, −20°C and −30°C by using the refrigerated circulator. A calibration curve was obtained and corrections were made over the temperatures recorded during each experimental run.
The freezing rate was represented by the characteristic freezing time (t cf) that is, the time for the centre to change the temperature from the initial freezing point to a temperature for which, for example, the 80% of the total water content is converted to ice (Zaritzky 2006). Although the initial freezing temperature experimentally determined was −1.9°C (±0.2), the temperature range considered for determining the characteristic freezing time was from −1°C to −7°C and then supercooling was also taken into account. Freezing was considered complete when the centre temperature reached −18°C.
Statistical Analysis
Significant differences in the treatments due to the application of ultrasound were assessed by one-way analysis of variance (ANOVA, 95% significance level) and a Tukey range test using the SPSS software package (SPSS, Chicago, IL, USA, Version 12.0.2, 2004). Means of the characteristic freezing times obtained from at least four replications were compared.
Results and Discussion
Influence of Ultrasound on the Freezing Rate and Nucleation Temperature
Minimising the time of the phase change period contributes to better maintain the product quality (Brennan et al. 1990). Li and Sun (2002) found that when ultrasound was applied to the phase change period during the immersion freezing process of potato samples, the freezing rate was increased significantly, while when power ultrasound was applied to the pre-cooling and tempering phases, the freezing rates were almost equal to those without ultrasound application. Table 2 shows the average characteristic times obtained when apple samples cut in tangential orientation were frozen without ultrasound (control) and when ultrasound was applied during the phase transition period at an average coolant temperature of −25.1°C (±0.2). The values in brackets are the standard deviations.
Analysis of the characteristic time showed that the freezing rates were improved (P < 0.05) only when treatments A and E were applied, that is when power ultrasound was applied for 120 s from −1°C and 0°C, respectively. Figure 3 shows typical freezing curves corresponding to treatments A and E with characteristic times of 266.4 and 270.6 s, respectively, which resulted in an increase in the freezing efficiency of about 10–11%. Also, an increase in the bath fluid temperature occurred during the application of ultrasound that raises in turn the temperature of the sample. It is important to point out here that because fruits and vegetables are non-homogeneous and highly attenuated materials, and furthermore, the tissue of some of them like apple consists of 20–25% air, and ultrasound is extremely sensitive to the presence of gas bubbles, it might be difficult to transmit ultrasound unless higher ultrasonic intensities and lower frequencies (<100 kHz) (Mizrach et al. 1994; McClements 1997; Khan and Vincent 1993), that is, appropriate and specific ultrasound variables, are used.
The temperatures of ultrasound application were chosen to be close to the initial freezing point. As it is well-known, the nucleation of water to form ice in foods can occur within a temperature range called the supercooling range, and depends on the physico-chemical properties of the food. The initial freezing temperature of apple experimentally determined from the time–temperature curves was −1.9°C (±0.2), which is in the range of the experimental value of −2.4°C reported by Lucas et al. (1998) for apple of the Granny Smith variety as well. Various reports over the last 70 years or so have indicated that the nucleation of solids from liquids is influenced by the presence of an ultrasonic wave (Chow et al. 2003). Studies carried out on cavitation-induced nucleation suggested that the collapse of a cavitation bubble increases the equilibrium freezing temperature of water and then triggers the nucleation of ice (Zhang et al. 2003). In general, crystallisation in the presence of an ultrasonic wave or sonocrystallisation exhibits a number of features specific to the ultrasonic wave, which, for most materials, include: (1) faster primary nucleation (formation of a nucleus in a previously crystal-free solution), (2) the initiation of secondary nucleation (nucleation induced by the presence of pre-existing crystals), and (3) the production of smaller crystals with greater size uniformity (Chow et al. 2005).
Figure 4 shows the point at which the centre temperature of the sample rapidly increased caused by the latent heat of crystallisation, which was recorded as the nucleation temperature or initiation of (primary) nucleation of ice (Chow et al. 2003). The characteristic freezing times for the control and ultrasound treated samples shown in Fig. 4 were 294 and 282 s, with nucleation temperatures of −3.1°C and −2.4°C, respectively. Here, the initiation of nucleation occurred at a higher temperature for the ultrasound treated sample as was expected from earlier studies (Chow et al. 2003). Evidence of the ultrasonic-induced nucleation of ice under fixed ultrasonic conditions was reported by Chow et al. (2003) for sucrose solutions in the concentration range of 0–45% sucrose. However, no definitive conclusions could have been drawn about the significance of any differences between both ultrasonically irradiated and control samples. Nucleation temperatures were determined for all the experimental runs of this work and the results are shown in Fig. 5, where the supercooling, i.e. the number of degrees below 0°C, is plotted for the different treatments. Although there were no significant differences among the nucleation temperatures of the different treatments, it could be seen that the samples of treatments B, D and F, which were sonicated for lower time periods (80–90 s), exhibited a slightly increase in the nucleation temperature (lower supercooling). This effect was not observed in treatments A, C and E probably due to the heating effects caused by the ultrasound application for 120 s, which caused a raise in the temperature of the refrigerant medium (Fig. 3), and then sufficient supercooling for ice nucleation was not achievable. However, the cooling capabilities of the refrigerated circulator allowed to overcome the temperature raise soon after and the overall effect of applying ultrasound for a longer time resulted in a significant improvement of the freezing efficiency (P < 0.05) for treatments A and E. Many of the attempts to relate ultrasound with the increase in the primary nucleation of ice were carried out in pure water systems, or in sucrose solutions, while the results obtained in this work, using a more complex system as it is a fruit, suggest also some evidence of the stimulation of primary nucleation.
Ultrasound and Apple Tissue Orientation
The characteristic shape of apples is the result of lateral growth mainly in the plane at right angles to the core so that the fruit grows outwards from the centre (Khan and Vincent 1993). The cells size increases towards the centre, with cell sizes up to 200–300 μm in diameter and small cells of 50 μm beneath the surface (Khan and Vincent 1993). The inner cells are highly radially elongated and organised into radial columns, with numerous radially elongated spaces between these cell columns (apple flesh consists of 20–25% air) (Khan and Vincent 1990). The dashed lines in Fig. 1 represent the direction of orientation of cell columns and intercellular spaces. Like the cell columns, the intercellular spaces and the vascular strands have a diverging pattern resulting in a highly anisotropic tissue (Khan and Vincent 1993). Therefore, the influence of applying ultrasound on radial and tangential orientated samples was examined. Treatments A and E were analysed since the freezing rates corresponding to these treatments were significantly enhanced (P < 0.05) under sonication. For the control treatment, radial and tangential cut samples were also used, although there was no significant difference between them. Table 3 shows the average characteristic times, the nucleation temperatures and the standard deviations obtained at an average coolant temperature of −25.2°C. The large standard deviations in the nucleation temperature of 0.63 and 0.82 for treatments A-tangential and E-radial were due to some high supercooling values (up to −4.6°C), which were also included when averaging the data.
Analysis of the characteristic time indicated that there were no significant differences (P > 0.05) between samples cut in radial or tangential orientation under treatments A and E, but they were different from the control tangential orientated sample (t cf = 291 s), or from the control sample when both orientations, radial and tangential, were considered in one group (t cf = 288.6 s). Slightly lower characteristic time was determined when ultrasound was applied for 120 s from −1°C to samples cut in radial orientation.
The primary nucleation temperature of ice for the radial samples under treatment E differed (P < 0.05) from treatment A-radial and from both control samples. Nucleation occurred at a higher temperature only for the radial orientated samples of treatment A, while treatment E-radial showed higher supercooling (Table 3). Although no significant differences were found between radial and tangential treatments A and E from the freezing rate efficiency point of view, the influence of ultrasound on the nucleation temperature was noticeable on the radial orientated samples. Such finding may point to a relation between the direction of orientation of cell columns and intercellular spaces (Fig. 1) and ultrasound transmission. Dražeta et al. (2004) found, when studying the radial distribution of air in apples of the Braeburn variety, that there is a gradient of declining air content from just beneath the skin to the centre of the fruit. It seems possible that the Granny Smith variety might exhibit a similar behaviour, and since ultrasound is sensitive to the presence of intercellular air spaces, radial samples could have been more affected by ultrasound than tangential ones.
The ultrasonic induction of crystal nucleation allows a comparison with seeding, a common practice in industrial crystallisation processes (Ruecroft et al. 2005), which can help to explain the results with respect to the low nucleation temperature of treatment E-radial (−3.63°C). In a batch process, seeds have to be added at the correct time, that is, during the development of the supersaturation profile. Addition too soon to a solution that is undersaturated will result in the seeds dissolving, while too late will also be ineffective because the material may already have crystallised (Ruecroft et al. 2005). In treatment E ultrasound was applied from 0°C, which might indicate that sonication was applied earlier and before the development of a supersaturation profile than when sonication was applied from −1°C. On the other hand, it is known that higher supercooling (lower nucleation temperature) causes the formation of a large number of nuclei, while a lower degree of supercooling favours crystal growth (Zheng and Sun 2006). The above concepts are useful for selecting an ultrasound treatment in relation to a particular food process.
It has been demonstrated that ultrasound can improve some transport operations (Floros and Liang 1994; Haydock and Yeomans 2003; Mulet et al. 2003; Cárcel et al. 2007). Acoustic streaming has been cited by many authors as a mechanism for enhancing mass transfer processes in membranes, porous materials and foods once an ultrasound intensity threshold is achieved (Floros and Liang 1994; Haydock and Yeomans 2003; Mulet et al. 2003; García-Pérez et al. 2006; Cárcel et al. 2007). Since immersion freezing is a simultaneous heat-and-mass transfer process where cross-transfer of water and solute takes place, and mass transfer is much slower at lower temperatures, it is expected that this process can be improved by the effects generated from the application of power ultrasound. However, the solute gain by the food product can have a positive or negative effect and will depend on the particular process. For example, during the immersion freezing of fruits and vegetables in brine or sugar solutions, control of the solute uptake is very important. Studies of the immersion chilling and freezing process (ICF) on apple cylinders dipped into a sodium chloride solution showed that as long as the product remains unfrozen, for example in freezing conditions when supercooling persists, mass transfer is relatively high (Lucas et al. 1998), so the levels of salt impregnation can be inconveniently high, and then treatment A may provide more adequate conditions for this situation. Treatment E will probably be a better choice if, for example, a higher sugar gain is desired. Analysis on the relation size/distribution of ice crystals to the microstructure and freezing conditions should also be taken into account.
Conclusions
The use of ultrasound to assist the immersion freezing process was investigated on apple samples. Results showed that when ultrasound was applied from 0°C or –1°C for 120 s in total, with 30 s intervals, the average freezing rate represented by the characteristic freezing time was significantly improved up to 8% (P < 0.05). No significant differences were found between radial or tangential cut samples sonicated at –1°C and/or 0°C for 120 s, though the freezing rates were enhanced (P < 0.05) compared to the control treatment. When ultrasound was applied for 80–90 s either from −1°C or −2°C, or when sonication was applied from −1°C for 120 s for the radial orientated samples, some evidence of the influence of ultrasound to induce primary nucleation was observed.
References
Acton, E. & Morris, G. J. (1992). Method and apparatus for the control of solidification in liquids. WO/1992/020420, USA Patent Application.
Brennan, J. G., Butters, J. R., Cowell, N. D., & Lilly, A. E. V. (1990). Food engineering operations (3rd ed.). Amsterdam, The Netherlands: Elsevier.
Cárcel, J. A., Benedito, J., Rosselló, C., & Mullet, A. (2007). Influence of ultrasound intensity on mass transfer in apple immersed in a sucrose solution. Journal of Food Engineering, 78(2), 472–479.
Chow, R., Blindt, R., Chivers, R., & Povey, M. (2003). The sonocrystallisation of ice in sucrose solutions: primary and secondary nucleation. Ultrasonics, 41(8), 595–604.
Chow, R., Blindt, R., Chivers, R., & Povey, M. (2005). A study on the primary and secondary nucleation of ice by power ultrasound. Ultrasonics, 43(4), 227–230.
Delgado, A. E., & Sun, D.-W. (2001). Heat and mass transfer models for predicting freezing process—a review. Journal of Food Engineering, 47(3), 157–174.
Dražeta, L., Lang, A., Hall, A. J., Volz, R. K., & Jameson, P. E. (2004). Air volume measurement of “Braeburn” apple fruit. Journal of Experimental Botany, 55(399), 1061–1069.
Floros, J. D., & Liang, H. (1994). Acoustically assisted diffusion through membranes and biomaterials. Food Technology, December, pp. 79–84.
García-Pérez, J. V., Cárcel, J. A., de la Fuente-Blanco, S., & Riera-Franco de Sarabia, E (2006). Ultrasonic drying of foodstuff in a fluidized bed: parametric study. Ultrasonics, 44(Supplement 1), e539–e543.
Haydock, D., & Yeomans, J. M. (2003). Acoustic enhancement of diffusion in a porous material. Ultrasonics, 41(7), 531–538.
Heldman, D. R. (1992). Food freezing. In D. R. Heldman, & D.B. Lund (Eds.), Handbook of food engineering (pp. 277–315). New York, USA: Marcel Dekker..
Khan, A. A., & Vincent, J. F. V. (1990). Anisotropy of apple parenchyma. Journal of the Science of Food and Agriculture, 52(4), 455–466.
Khan, A. A., & Vincent, J. F. V. (1993). Compressive stiffness and fracture properties of apple and potato parenchyma. Journal of Texture Studies, 24(4), 423–435.
Knorr, D., Zenker, M., Heinz, V., & Lee, D.-U. (2004). Applications and potential of ultrasonics in food processing. Trends in Food Science and Technology, 15(5), 261–266.
Leighton, T. G. (1998). The principles of cavitation. In M. J. W. Povey, & T. J. Mason (Eds.), Ultrasound in food processing (pp. 151–182). London, UK: Blackie Academic & Professional.
Li, B., & Sun, D.-W. (2002). Effect of power ultrasound on freezing rate during immersion freezing of potatoes. Journal of Food Engineering, 55(3), 277–282.
Lucas, T., François, J., & Raoult-Wack, A.-L. (1998). Transport phenomena in immersion-cooled apples. International Journal of Food Science and Technology, 33(5), 489–499.
Martino, M. N., Otero, L., Sanz, P. D., & Zaritzky, N. E. (1998). Size and location of ice crystals in pork frozen by high-pressure-assisted freezing as compared to classical methods. Meat Science, 50(3), 303–313.
Mason, T. J. (1998). Power ultrasound in food processing—the way forward. In M. J. W. Povey, & T. J. Mason (Eds.), Ultrasound in food processing (pp. 105–126). London, UK: Blackie Academic & Professional.
Mason, T. J., & Chemat, F. (2003). Ultrasound as a preservation technology. In P. Zeuthen, & L. Bøgh-Sørensen (Eds.), Food preservation techniques (pp. 303–337). Cambridge, England: CRC.
McClements, D. J. (1997). Ultrasonic characterization of foods and drinks: principles, methods, and applications. Critical Reviews in Food Science and Nutrition, 37(1), 1–46.
Mizrach, A., Galili, N., & Rosenhouse, G. (1994). Determining quality of fresh products by ultrasonic excitation. Food Technology, 48(12), 68–71.
Mulet, A., Cárcel, J., Benedito, C., Rosselló, C., & Simal, S. (2003). Ultrasonic mass transfer enhancement in food processing. In J. Welti-Chanes, F. Vélez-Ruiz, & G. V. Barbosa-Cánovas (Eds.), Transport phenomena of food processing (Chapter 18). Boca Raton, FL, USA: CRC.
Ratoarinoro Contamine, F., Wilhelm, A. M., Berlan, J., & Delmas, H. (1995). Power measurement in sonochemistry. Ultrasonics Sonochemistry, 2(1), S43–S47.
Ruecroft, G., Hipkiss, D., Ly, T., Maxted, N., & Cains, P. W. (2005). Sonocrystallization: the use of ultrasound for improved industrial crystallization. Organic Process Research and Development, 9(6), 923–932.
Sanz, P. D., de Elvira, C., Martino, M., Zaritzky, N., Otero, L., & Carrasco, J. A. (1999). Freezing rate simulation as an aid to reducing crystallization damage in foods. Meat Science, 52(3), 275–278.
SPSS (2004). Version 12.0.2. Chicago, IL, USA: SPSS.
Sterling, C. (1968). Effect of low temperature on structure and firmness of apple tissue. Journal of Food Science, 33, 577–580.
Sun, D.-W., & Li, B. (2003). Microstructural change of potato tissues frozen by ultrasound-assisted immersion freezing. Journal of Food Engineering, 57(4), 337–345.
Zaritzky, N. (2006). Physical-chemical principles in freezing. In D-W. Sun (Eds.), Handbook of frozen food processing and packaging (pp. 3–31). Boca Raton, FL, USA: Taylor & Francis.
Zeqiri, B., Hodnett, M., & Carroll, A. J. (2006). Studies of a novel sensor for assessing the spatial distribution of cavitation activity within ultrasonic cleaning vessels. Ultrasonics, 44(1), 73–82.
Zhang, X., Inada, T., & Tezuka, A. (2003). Ultrasound-induced nucleation of ice in water containing air bubles. Ultrasonics Sonochemistry, 10(2), 71–76.
Zheng, L., & Sun, D.-W. (2006). Innovative applications of power ultrasound during food freezing process—a review. Trends in Food Science and Technology, 17(1), 16–23.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delgado, A.E., Zheng, L. & Sun, DW. Influence of Ultrasound on Freezing Rate of Immersion-frozen Apples. Food Bioprocess Technol 2, 263–270 (2009). https://doi.org/10.1007/s11947-008-0111-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0111-9