Abstract
Emulsion-based edible films made of soy protein isolate (SPI), virgin coconut oil (VCO), and soy lecithin (SL) and plasticized with glycerol were prepared using the casting method. The effect of VCO and SL concentrations in SPI films and their in-between interaction were studied through the evaluation of physical (moisture and opacity), mechanical (elongation and tensile strength), water vapor permeability, and thermal properties. The response surface methodology was used to identify the most significant factors in the properties studied. The applicability of SPI emulsion-based films was evaluated as a package for olive oil to be used in small portions. The oxidative stability of the packaged olive oil was monitored by peroxide analyses during 28 days. The incorporation of VCO and SL decreased the moisture content and increased the elongation of the SPI emulsion-based films when compared to the SPI film without these components (control). The opacity of the films increased with the addition of VCO into the protein-based films, but not with the addition of SL or a combination of both constituents. By the other hand, the water vapor permeability was not improved by the incorporation of VCO, SL, or a combination of both. The peroxide value of the olive oil stored in SPI emulsion-based film sachets increased rapidly during the seven first days of storage. After this period, the peroxides increased relatively slow up to 28 days of storage. The peroxide values of the packaged olive oil did not reach the maximum limit recommended by the Codex Alimentarius. Based on these results, this work may be useful for the technological enhancement of emulsion-based films or for food packaging applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past few years, there was an increased interest in developing environmentally friendly packaging, as those produced from renewable resources, especially in the field of bioplastics. Bioplastics or edible films are commonly produced using proteins (Blanco-Pascual et al. 2014; Chang and Nickerson 2014; Hammann and Schmid 2014; Gofferje et al. 2014), lipids (Chiumarelli and Hubinger 2014), polysaccharides (Antoniou et al. 2014; Jost et al. 2014; Pan et al. 2014a), or mixed composite blends (Kowalczyk and Baraniak 2014; Marquez et al. 2014; Pan et al. 2014b; Winkler et al. 2015). In general, polysaccharides and proteins provide mechanical stability and good gas barrier properties, while fats are used to minimize water transmission (Pavlath and Orts 2009).
Proteins have several functional properties for film formation due their structure and intrinsic properties. They present multiple sites for chemical interactions due to the high diversity of amino acid functional groups and charged areas along the protein chains, which, in turn, result in interactive forces capable of improving the stability of films (Hammann and Schmid 2014; Dangaran et al. 2009). Recent reports suggested the use of soy protein isolate (SPI) as a matrix of edible films (Ou et al. 2005; Cho et al. 2007; Denavi et al. 2009; Atarés et al. 2010; Kokoszka et al. 2010; González et al. 2011; Friesen et al. 2015; Hopkins et al. 2015) due to the significant production of soy protein in the world and its recognized ability of forming edible films. The SPI comprises a mixture of proteins, of which approximately 90 % are globulins: 7S (β-conglycinin) and 11S (glycinin) are the main fractions of globulins, corresponding to approximately 37 and 31 % of the total extractable protein (Cho and Rhee 2004). The SPI is known for its good gelling capacity and emulsifying characteristics (Nishinari et al. 2014), which means it can improve the incorporation of hydrophobic components into solutions where it is contained, as in the case of solutions for making composite or blend films. Thus, the structural and functional properties of the SPI make it a potential element for the development of edible films.
The SPI edible films are transparent and flexible, exhibit good oxygen barrier (Krochta and Miller 1997; Cho et al. 2007), have low cost, and are of biodegradable origin and available worldwide. However, protein-based edible films are sensitive to moisture and exhibit poor water vapor barrier (Guilbert et al. 1996; Al-Hassan and Norziah 2012). The permeability of water vapor or gases depends on their movement through a biopolymer film (diffusion)—which is related to the permeant film affinity—and their dissolution through this biopolymer (solubility) (Pavlath and Orts 2009; Miller and Krochta 1997). Thus, the incorporation of components having lower affinity with the diffusing molecule can decrease this rate, for example dispersing lipids in the protein matrix (Bertan et al. 2005b; Yang and Paulson 2000; Limpisophon et al. 2010; Zahedi et al. 2010), since lipids present nonpolar or hydrophobic characteristics. Other strategies can also be employed for the improvement of the water vapor barrier of films, such as applying cross-linking agents (Schmid et al. 2014), applying additional film layers (Cho et al. 2010; Anker et al. 2002), and combining different plasticizers (Wan et al. 2005). Among these techniques, the incorporation of lipids appears to be a promising alternative; since it requires only one step in the manufacturing process, there is high availability of waxes and lipids and some lipids may also exerted additional benefits to the film structure.
Coconut oil is extensively used for food and industrial purposes (Marina et al. 2009a). It shows excellent oxidative stability since more than 90 % of its fatty acids are saturated. Virgin coconut oil (VCO) is known for its important antioxidant activity (Marina et al. 2009b; Seneviratne et al. 2009). Emulsifiers are essential in the formation of protein-based films containing lipid molecules (Zhao 2012). Aiming the formation of a homogeneous film structure, several authors have suggested the use of surfactants such as soy lecithin and yucca extract (Andreuccetti et al. 2011), Tween 40 (Kowalczyk and Baraniak 2014), glycerol monostearate, and Tween 60 and 80 (Bravin et al. 2004). Soy lecithin is an amphoteric surfactant; it is composed of one or two fatty acid groups (hydrophobic) linked to a glycerol chain, a phosphate and alcohol group (hydrophilic) (Stauffer 2005).
Natural biopolymer-based packaging materials have a good potential for enhancing food stability, safety, and quality (Garcia et al. 2010; Chen et al. 2012). Edible pouches and sachets made from heat-sealable biodegradable films can be considered as novel packaging systems, once they permit a controlled release of added active compounds, such as flavors, antioxidants, and antimicrobial agents (McHugh and de Avena-Bustillos 2012).
World production of soybeans is about 315.05 million tons, and Brazil is the second largest producer (94.50 million tons) behind only of the USA (108.01 million tons) (USDA 2015). Due to the significant production of soy protein in Brazil and its recognized ability to form edible films, SPI was chosen as the main matrix in this work. The VCO was incorporated to the film solution in order to improve the water vapor permeability and mechanical properties (elongation) of the SPI edible films. Soy lecithin was chosen as the surfactant agent, since it is a by-product of soybean oil processing. Thus, the objective of this study was to develop, characterize, and study an application of emulsion-based edible films made of soy protein isolate with added virgin coconut oil and soy lecithin. Emulsion-based films were characterized with respect to their mechanical, thermal, and barrier properties. The applicability of the SPI emulsion-based edible films was evaluated in the form of a sealed pack for olive oil.
Material and Methods
Material for Film Production
Commercial SPI (>90 % protein content; Bremil Food Products Industry Ltda., Brazil) was used as the matrix of the film. Anhydrous glycerol (Gly, ≥99.5 % purity; Panreac, Spain) was used as the plasticizer. Distilled water and ethanol (96 % purity; Panreac, Spain) were used as the solvent. VCO (Copra Food Industries, Brazil) was incorporated in the emulsion-based edible films, and soy lecithin (SL; Imcopa Import, Export and Oil Industry S.A., Brazil) was employed as the surfactant.
Preparation of the SPI Emulsion-Based Films
The edible films were prepared using the casting method. The concentration of SPI was fixed in 6.5 % (w/w film-forming solution), and the glycerol content was based on SPI content at 2.5:10 (Gly:SPI, mass basis). Ethanol (20 % w/w film-forming solution) and distilled water (completing 100 % w/w film-forming solution) were applied as the solvent. Ethanol was used in the emulsion-based films to solubilize soy lecithin and also to accelerate the drying process of the film-forming solutions. Table 1 shows the different film formulations studied, varying VCO and SL content. Preliminary experiments were conducted to determine the threshold concentration of SPI, plasticizer (glycerol), surfactant (SL), and VCO in order to produce flexibility on handling films that did not show phase separation and VCO or glycerol exudation, and were also easy to remove from the supports (plates).
Tests were performed with SPI concentrations ranging from 5.0 to 9.0 % (w/w). The forming solution with protein concentrations lower than 6.0 % resulted in films with fragile and brittle texture, while film solutions containing above 7.0 % protein became highly viscous after heating, which hampered their removal from the plates. Thus, the concentration of soy protein isolate was set at 6.5 %. The mass ratio of plasticizer was fixed in 2.5:10 (Gly:SPI), since films produced with concentrations of plasticizer lower than 2.5:10 were fragile and broke easily during their removal from the support. The surfactant was added to the matrix at 1:10, 2:10, and 3:10 (SL:Gly) mass ratios, according to the study performed by Andreuccetti et al. (2009). Higher amounts of soy lecithin were not applied due to the increased difficulty of solubilizing it and to avoid the increasing formation of bubbles in the film-forming solution. In the first tests, the proportion of surfactant was fixed at 3:10 (SL:Gly) and increasing mass ratios of virgin coconut oil (VCO:SPI) were added to the formulation (0.1:10, 0.4:10, 0.7:10, and 1:10). As a result, the film was visually homogeneous for ratios up to 0.7:10 (VCO:SPI). The incorporation of VCO into the SPI matrix was not possible without surfactant. Control films were prepared from solutions containing only SPI, glycerol, water, and ethanol, in order to compare it with those containing VCO and SL.
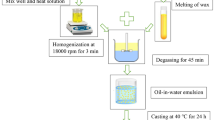
The film-forming solution (FFS) was prepared by dispersing the SPI in distilled water at 25 °C. The pH of the solution was adjusted to 9.00 ± 0.05 (NaOH, 5 N), and the solution was stirred for 60 min. Meanwhile, SL was solubilized in ethanol under magnetic agitation for 90 min at 25 °C. After this, the SL solution, the glycerol, and the VCO were added to the SPI solution, which was heated in a thermostatic bath (Ethik Technology) until the FFS reached 70 ± 3 °C. This temperature was chosen in order to partially disrupt the tertiary and quaternary structure of SPI and to expose its hydrophobic chains. Then, the FFS was homogenized (Polytron PT 3100D) firstly at 13,500 rpm for 1 min, followed by 20,500 rpm for 2 min, according to the methodology described by Rezvani et al. (2013). Fixed volumes of FFS (Table 1) were poured on acrylic plates (14 cm × 14 cm) and dried at 25 ± 3 °C for 24 h. During the step of film drying, the acrylic plates were maintained in a plain table with adjustable feet in order to minimize irregularities in film thickness. After being dried, the films could be easily removed from the plates without using lubricant and without any additional effort. All films were conditioned in desiccators (25 ± 3 °C, 57 ± 3 % relative humidity, maintained using a saturated Mg(NO3)2 solution) during 7 days prior to the analyses so the films could reach moisture equilibrium. This methodology of conditioning was previously reported by some authors (Fakhoury et al. 2012; Galus et al. 2012; Andreuccetti et al. 2009; Ozdemir and Floros 2008; Bertan et al. 2005b), who suggested a minimum of 48 h of film conditioning in these conditions.
Properties of the Emulsion-Based Edible Films
Film Thickness
Film thickness was determined using a digital micrometer with a sensitivity of 1 μm (Mitutoyo, Japan). Measurements of film specimens were taken at ten random points. The results were expressed as the mean of random measurements made in each type of film.
Moisture
Circular film specimens (20 mm in diameter) were weighed and dried at 105 °C for 24 h in a convection oven. The weight loss of each sample was determined, and the moisture content was calculated as the percentage of water removed from the films. Analyses were performed in triplicate.
Opacity
Opacity measurements were performed by spectrophotometry, according to Gontard et al. (1992), Cho and Rhee (2004), Cho et al. (2007), and Zahedi et al. (2010). A spectrum of each film specimen (1 × 4 cm) was recorded using a UV-VIS spectrophotometer (UV-1800; Shimadzu, Japan). The area under the absorbance curve ranging from 360 to 800 nm was calculated as the opacity of the films, which were expressed as absorbance unit (AU) × nanometer/micrometer.
Water Vapor Permeability
The water vapor permeability (WVP) test was performed according to a modification of the Standard Method E96/96M-13 (ASTM Standard E96/E96M 2013). Circular test cells (cups) of 0.06 m (internal diameter) × 0.035 m (depth) with an exposed film area of 0.0028 m2 were used. The cells were filled with anhydrous calcium chloride, and the film specimens were sealed over the circular opening of the test cups. The cups were placed in desiccators containing saturated NaCl solution (25 °C, 75 % relative humidity (RH)). The gain of weight of the cells was monitored every 24 h during 7 days. The experiments were performed in triplicate, and the WVP was calculated using Eq. (1).
where G/t is the rate of humidity absorbed (g s−1), A is the permeation area (m2), x is the average thickness of the film specimen (m), S is the partial pressure of water vapor at the test temperature (Pa), and (R 1 − R 2) is the gradient of relative humidity of the environment containing the calcium chloride (0 % RH) and the saturated NaCl solution (75 %).
Mechanical Properties
The tensile strength (TS, MPa) and elongation at break (ELO, %) of the films were measured on a Brookfield CT3 Texture Analyzer (Brookfield Engineering Laboratories, Inc., USA), according to the Standard Method D882-12 (ASTM Standard D882 2012). Rectangular film strips of 60 mm length and 25 mm width were used as the test specimen. The initial grip separation was set at 20 mm, and the crosshead speed was set at 1 mm s−1. TS was calculated using the equation TS = F / A, where F is the force (N) at maximum load and A is the initial cross-sectional area (m2) of the film. The ELO (%) was determined by dividing the film elongation at rupture by the initial gauge length. Measurements for each film were repeated at least ten times, and the results are expressed as mean values.
Thermogravimetric Analysis
The thermal stability and degradation of films were analyzed by thermogravimetric analysis (TGA) (model 4000; PerkinElmer, USA). Samples weighing about 10–15 mg were scanned from 25 to 600 °C at a heating rate of 10 °C/min. Nitrogen was used as purge gas at (20 mL/min) in order to avoid thermo-oxidative reactions. Thermal analysis was carried out on the control film and on the emulsified edible film that presented the highest concentration of surfactant and coconut oil (formulation A).
Film Pouch Production for Olive Oil Packaging
Pouches were made with the emulsion-based edible films in order to pack olive oil as a condiment that could be easily used in small portions. The methodology was based on the study of Souza et al. (2011), applying some modifications. The films were cut in 100 mm × 40 mm pieces, and two of these were taken and sealed on three sides using a VC999K3/CH9100 sealer (Switzerland), creating a pouch. Samples of olive oil weighing approximately 5.0 ± 1.0 g were used to fill the pouch, which was subsequently sealed to form a four-side sealed pouch, as shown in Fig. 1. A control pack was produced using SPI, Gly, ethanol, water, and SL (1:10, SL:Gly), without VCO, in order to check whether the incorporation of VCO could have some additional effect or negatively influence the stability of the packed olive oil. The control pack was submitted to the same storage conditions of the VCO-containing pouches. The film pouches containing olive oil were stored in desiccators at controlled RH (60 ± 3 %), maintained using a saturated Mg(NO3)2 solution. These desiccators were stored at 30 °C in photoperiod conditions (EL 202, 20-W lamp; EletroLab, Brazil).
The shelf life of the pouched olive oil was monitored for 28 days. The oxidative stability of the olive oil was determined by the peroxide value (POV) according to the AOCS official method Cd 8-53 (AOCS 2001).
Statistical Analysis
Statistical analysis was conducted using the analysis of variance (one-way ANOVA, p < 0.05) procedure available in the STATISTICA® software (StatSoft, Tulsa, USA), version 10. Statistically different means were analyzed using Tukey’s post hoc test. The data were fitted to a second-order equation (Eq. (2)) as a function of the dependent variables.
where b n are constant regression coefficients, Y i is the dependent variable (physical, mechanical, and water barrier properties), and SL and VCO are the independent variables. Response surface graphics were constructed in order to check the influence of independent variables on the properties of emulsion-based films. From the proposed model, we considered only the significant parameters that described the behavior of the dependent variables. The results of Eq. (1) fitting were not presented for those dependent variables where the model was not able to reproduce experimental data.
Results and Discussion
Properties of the Emulsion-Based Edible Films
Moisture Content
The water content of films is an important parameter, since the water can act as a plasticizer in the polymeric matrix (Sothornvit and Krochta 2005). The moisture content of the control and the emulsified edible films obtained are described in Table 2. The moisture content of control film was 11.65 ± 1.17 %, while for the emulsified films, it ranged from 6.22 ± 0.53 % (formulation D) to 9.86 ± 0.36 % (formulation A). The moisture of the emulsified films was significantly lower than the control film (p < 0.05). Among the emulsified films, it was found that lower water contents were reached in the intermediate SL concentration (2:10, SL:Gly).
Figure 2 presents the response surface of the emulsion-based films’ moisture content as a function of SL and VCO. It was found that the concentration of VCO and SL had a significant effect on the reduction of moisture. However, the moisture was not affected by the interaction of these variables. Thus, the model considering only the significant parameters is given by Eq. (3). It can be observed graphically and by Eq. (3) that the SL content was the most influencing parameter on the reduction of film moisture.
where the errors of the parameters of Eq. (2) are b 0 ± 1.053, b 1 ± 1.118, b 2 ± 2.515, b 11 ± 0.276, and b 22 ± 3.072 with R 2 = 0.715.
In its native state, the polar groups of the SPI are unavailable because the hydrophobic region of the SPI protein is enclosed by a hydrophilic region, maintained by non-covalent forces and disulfide bonds (Qu et al. 2015). The processing conditions applied for the production of SPI emulsion films in this study, namely alkaline conditions (Qu et al. 2015), heating (Keerati-u-rai and Corredig 2010), and the homogenization process (Damodaran 2008), can partially or fully denature the proteins and expose their functional groups, cleave disulfide bonds, and expose hydrophobic and sulfhydryl groups. After this process, probably the tertiary and quaternary structures were disrupted, exposing the hydrophobic side chains of the SPI and making them available to bind to VCO and the nonpolar side of the lecithin molecule. Since soy lecithin is a predominantly lipophilic surfactant (Bueschelberger 2004), the VCO and the hydrophobic chains of the SPI can link to these domains, while the hydrophilic chains of the protein can bind to the polar side of the lecithin (phosphate and alcohol group) and to glycerol. As a result, there are fewer free domains available to bind to the water molecules. Thus, a reduction in the moisture content of SPI emulsion-based films was expected, as also observed by Peng et al. (2013) after the incorporation of lipids and surfactant in films. Indeed, the incorporation of SL and VCO (within the range of this study) resulted in a moisture reduction of the emulsified films when compared to the control one. The moisture reduction can be considered as a positive result, since a decrease in the water content of the structure may increase the stability of the pack during storage. On the other hand, the water can act as a plasticizer and improve the flexibility of edible films. So, the reduction in the moisture content can be considered as a negative or positive result, depending on the purpose for which it is intended for packaging.
Opacity
The opacity values of the emulsion-based films are presented in Table 2. The opacity of control film was 4.53 ± 0.18 (AU × nm/μm). The incorporation of VCO:SPI in the ratio of 0.1:10 at all concentrations of SL showed no significant difference in the opacity of films with respect to the control. Among the emulsified films, there was a linear trend on increasing the opacity, as the concentration of VCO:SPI is increasing, but not with the addition of SL, as also verified in the data fitting (Fig. 3). Since there was no significant influence of SL content on opacity, the model was simplified to a function of VCO only, as shown in Eq. (4).
where the errors of the parameters of Eq. (3) are b 0 ± 0.200, b 2 ± 1.272, and b 22 ± 1.554 with R 2 = 0.896.
This increase in opacity can be explained by the solidification of VCO at room temperature. The VCO has precise melting characteristics: at 21 °C, it is hard and brittle, a typical behavior of oils or fats with a predominance of lauric acid (45.9 to 50.3 % of a total of 90 % saturated fatty acids) (O’Brien 2009). An increased opacity, due to the addition of hydrophobic components, was also observed by Wang et al. (2014) and Bertan et al. (2005b). Thus, it was concluded that VCO (0.7:10, VCO:SPI) had a significant effect on increasing the opacity of SPI films. Opacity is a desired parameter in some packages, as those for foods containing photosensitive compounds, since it can reduce the passage of light.
Water Vapor Permeability
Several properties can influence the permeability of the biopolymer films, such as the chemical structure of the components, the interstitial space between the molecules, the organization of the molecules in the film structure, the orientation or alignment of the protein chains in the structure, and the formation of intermolecular bonds among the chains of the proteins (cross-linking) and biopolymer blends (composite or emulsified films) (Miller and Krochta 1997). Thus, the films of this study were incorporated with VCO and SL to check if the permeability of the control film could be improved and how the components could exert influence on these properties. The results of WVP for the SPI control and SPI emulsion-based films are described in Table 2.
Effect of the Combined Action of SL and VCO
In general, for the emulsified films, WVP values ranged from (5.99 ± 0.71) × 10−11 g m−1 s−1 Pa−1 (formulation C) to (12.0 ± 0.06) × 10−11 g m−1 s−1 Pa−1 (formulation I). The combined incorporation of VCO and SL did not decrease the water permeability of the films when compared to the control. It was expected that the hydrophobic portion of the surfactant should decrease the rate of solubility and diffusion of water molecules through the film structures (Rhim et al. 2002). Similarly, the use of VCO should also decrease the water permeability due to their hydrophobic nature. However, this effect was not found. According to Gontard et al. (1994), if materials with high steric dimensions (such as SL) are employed at the composite film formulations and if these components are not associated with the protein chains, the structural matrix of the films could stretch. So, a small degree of irregularity in a film can exponentially increase the diffusion rate (Pavlath and Orts 2009) since there is an increase of interstitial spaces between the protein chains, allowing water molecules to move more easily through the structure.
Effect of the Addition of SL
The effect of the addition of SL was checked by comparing the control film to the emulsified films using fixed proportions of VCO. The first assays involved the application of the lower proportion of VCO (0.1:10, VCO:SPI) and three concentrations of SL (1:10, 2:10, and 3:10, SL:Gly), formulations I, F and C, respectively. It was found that the three formulations evaluated showed higher WVP than the control film (5.57 ± 0.30 × 10−11 g−1 m−1 s−1 Pa−1). However, in formulation C, these values were significantly equal to the control (p < 0.05). For these systems containing 0.1:10 (VCO:SPI), the WVP of the emulsified films decreased with the increasing lecithin content.
When keeping the proportion of VCO in 0.4:10 or 0.7:10 (VCO:SPI), there was an increase in the WVP for higher amounts of lecithin. As the emulsion formed in this work is an oil-water type (due to lecithin characteristics), this may have led to an inhomogeneous distribution of lecithin and VCO in the polymer matrix. Soy lecithin is a hydrophobic surfactant type, and because of this, it is less soluble in the continuous hydrophilic phase of the film-forming emulsion. Maybe for these reasons, inconsistent results of WVP were obtained. The expected tendency for these data was the decrease in WVP with increasing SL and VCO. Andreuccetti et al. (2011) evaluated the effect of the surfactants SL and yucca extract (Yucca schidigera) on the WVP of gelatin-based edible films. Films containing yucca extract presented lower values of WVP when compared to the lecithin-based ones. The authors attributed this behavior to the low solubility of lecithin in water, which, in turn, may have generated a non-homogeneous distribution of the surfactant in the matrix.
Effect of VCO Addition
The increasing addition of VCO only showed a positive effect on reducing the films’ permeability at the lecithin ratio of 1:10 (SL:Gly). For the 3:10 (SL:Gly) ratio, the best WVP value was obtained for the lowest concentration of VCO applied, 0.1:10 (VCO:SPI). Bravin et al. (2004) concluded that the concentration and type of lipid exert different effects on the water vapor barrier of film composites of corn starch and methylcellulose. They found that the WVP of these edible films was significantly reduced by the addition of soybean oil (10 to 20 %) but was not reduced when using cocoa butter. To this behavior, it was attributed to the formation of discontinuous zones, which were probably crystallization related. The water vapor transmission through emulsion-based or composite films still depends on the nature (crystal arrangement and chain length) and dispersion of the lipid in the matrix (Gallo et al. 2000; Bertan et al. 2005b; Bravin et al. 2004) and on the type of surfactant applied (Peng et al. 2013; Andreuccetti et al. 2011; Bravin et al. 2004).
Although the methodology proposed did not lead to a decrease in the WVP of the emulsion-based films when compared to the control, the WVP values obtained in this study were slightly lower than those from other studies involving soy protein films (Denavi et al. 2009) and soy protein isolate films incorporated with beeswax, span 20, and glycerol (Chao et al. 2010), for example. Other studies involving different protein-based films showed similar trends, such as films produced with whey protein and glycerol (Ramos et al. 2013) as well as gelatin, triacetin, lauric acid, and a blend of stearic and palmitic acid (Bertan et al. 2005a). However, the WVP values of this study were higher than those obtained in films involving other matrices, for example quinoa protein-chitosan-sunflower oil edible film (Valenzuela et al. 2013), chitosan-virgin coconut oil film (Binsi et al. 2013), and synthetic films, such as polyethylene films (low-density polyethylene (LDPE)) (Peychès-Bach et al. 2009, 0.14 × 10−11 to 0.28 × 10−11 g m−1 s−1 Pa−1).
The addition of VCO and/or SL had no positive effect on reducing the film WVP when compared to the control one. This behavior probably occurred due to the difference in the structural and chemical nature of the components used in the formulations. The incompatibility of the components may have limited the incorporation of VCO and SL to the protein matrix, leading to the formation of heterogeneous zones. These zones may have been formed by the concentration of lipids in a region or by the remaining hydrophilic ends of the surfactant or glycerol, which have affinity to humidity. The structural volume of SL and the lipophilic nature of the surfactant in an aqueous medium may also had led to the formation of cavities, facilitating the permeation of water in the structure of the films. The second-order model did not reproduce adequately the experimental data of WVP for the emulsified films.
Mechanical Properties
The investigated mechanical properties—ELO (%) and TS (MPa)—of the control and emulsion-based edible films are shown in Table 3. Regardless of the concentration of SL used, by increasing the proportion of VCO from 0.1:10 to 0.7:10 (VCO:SPI), there was also an increase in the elongation of the films, comparing to the control one (103.75 ± 16.78 %). Monedero et al. (2009) reported an increase in elongation of SPI films added with oleic acid and attributed this behavior to the plasticizing effect of the acid in the matrix.
From the adjusted second-order model, it was found that the SL and VCO content had significant effect on increasing the elongation of the emulsion-based films, as shown in Fig. 4. The model considering the significant parameters is described by Eq. (5).
where the errors of the parameters of Eq. (4) are b 0 ± 17.80, b 1 ± 19.54, b 12 ± 9.91, b 11 ± 4.73, and b 22 ± 26.15 with R 2 = 0.815.
The TS of the films ranged from 7.65 ± 0.48 MPa (formulation D) to 12.19 ± 0.43 MPa (formulation B). For the higher and lower ratios of VCO (0.1:10 and 0.7:10, VCO:SPI), the tensile strength showed the opposite behavior of elongation; that is, the incorporation of VCO into the SPI matrix caused a decrease in TS values. This mechanism can be explained by the partial replacement of polymers by lipids in the matrix, since the interactions of only polar polymer molecules are stronger than those involving only lipid molecules and also stronger than those between lipids and polar polymer molecules (Yang and Paulson 2000). The addition of lipids in the matrix by means of an emulsion may induce the development of discontinuities in the polymer network of the structures (preferential break points), leading to a decrease in TS (Bravin et al. 2004). The decrease in TS due to the addition of lipids into the matrix was also observed by Valenzuela et al. (2013), Atarés et al. (2010), and Yang and Paulson (2000). The second-order model did not reproduce adequately the experimental data of TS for the emulsified films.
A positive effect on the elongation of the films was found due to the incorporation of VCO and SL in SPI films. These components must have acted as plasticizers or lubricants in the protein structure of the films, resulting in the increase of flexibility and the reduction of tensile strength.
Thermogravimetric Analysis
Thermogravimetric analysis and derivative thermogravimetric (DTG) curves of the control film and SPI emulsion-based edible film (formulation A) are illustrated in Fig. 5.
For both films, four well-defined stages of weight loss were observed. In the first stage, similar weight losses (Dw1) were found, corresponding to 5.6 and 5.3 % at temperatures (Td1) of 70.80 and 72.04 °C, for the control and formulation A films, respectively. Guerrero et al. (2011) and Su et al. (2010) observed that up to 100 °C, the weight loss at the first stage is related to the moisture loss of films. The second stage was verified at temperatures (Td2) of 266.95 and 257.01 °C for the control and formulation A films, respectively. In this case, formulation A showed the lower weight loss (Dw2) (22.4 %) than the control film (30.5 %). This stage was associated with beginning of SPI film degradation, whereas the main loss occurs at the range of 270–380 °C (Song et al. 2013). For film A, Td2 value can be attributed to the thermal decomposition of coconut oil. The onset temperature of pure coconut oil is 257 °C and involves the rupture of oxygenated hydrocarbons present in the oil into volatile lower molecular hydrocarbons, carbon dioxide, and carbon monoxide (Jayadas and Nair 2006).
The third stage (306.83 and 333.12 °C) and fourth stage (318.14 and 364.8 °C) are probably associated to the degradation of soy protein. Kumar et al. (2010) attributed the decomposition of soy protein and loss of glycerol in the range of 300–400 °C. Tongnuanchan et al. (2014) associated a thermal degradation in the range of 301.29–334.48 °C to the protein fractions in fish gelatin film incorporated with essential oils and surfactants.
Film Pouch Production for Olive Oil Packaging
The storage conditions of the sealed pouches were chosen in order to estimate the susceptibility or stability of the packaged olive oil: accelerated conditions (30 °C, 60 % RH) and using photoperiod (12 h light and 12 h darkness), simulating a real storage condition. This application was performed to preliminarily and qualitatively provide an indicative of the oxygen barrier capability of the emulsion-based films, once oxygen is the main responsible for altering the quality of oils. If the quality of olive oil could be maintained, we would indirectly have an indicative of the oxygen permeability of the packaging. Olive oil oxidative stability was monitored up to 28 days through peroxide analysis, and the results are shown in Table 4.
The peroxide value of the olive oil stored at 30 °C increased with storage time regardless of the formulation of the film. After 7 days of storage, the POV of the olive oil increased rapidly, approximately 42.09, 33.70, and 27.82 % for control pack, formulation G, and formulation I, respectively. According to Cho et al. (2010), the lipid oxidation in the early stages of storage occurs due to the consumption of oxygen present in the headspace of the pouch. After the first 7 days, the level of hydroperoxides in the olive oil increased relatively slow up to 28 days of the storage. It is known that SPI films show low oxygen permeability values (Rhim et al. 2006; Denavi et al. 2009; Cho et al. 2010) at low and intermediate relative humidity. Cho et al. (2010) verified that the SPI films had better oxygen barrier properties than the NY/mLLDPE (nylon/metallocene-catalyzed linear low-density polyethylene), the material usually applied for packing some condiments. Other plastics, such as LDPE and high-density polyethylene (HDPE) showed lower oxygen barrier than SPI films, as concluded by Rhim et al. (2006). Thus, in the present work, most likely the oxidation reactions ceased due to the complete consumption of oxygen in the headspace of the pouch.
There was no significant difference in the stability of the olive oil samples as a result of the different packaging formulations. In other words, the addition of VCO to the emulsion-based edible films did not exert an additional effect in reducing the oxidative rancidity when compared to the control pack containing only lecithin. Cho et al. (2010) evaluated the use of bilayer film pouches made of corn zein and soy protein isolate to pack olive oil at 50 °C and 30 to 50 % RH. These authors concluded that after 30 days of storage, the POV of the olive oil was approximately 50 mEq/kg of oil and that these pouches were able to control lipid rancidification at dry and intermediate RH ranges. According to the Codex Alimentarius (CODEX STAN 33-1981, Rev. 1-1989, 2001), the maximum limit of POV to maintain the quality of extra virgin olive oil is 20 mEq O2/kg. After 28 days of storage, the POV of packaged olive oil in the emulsion-based edible films did not reach the maximum limit regulated by the Codex Alimentarius.
We concluded that the SPI-based edible films could be applied as pouch packaging since the integrity of the pouch can be maintained during storage, even though studies should be made in the sense of improving their tensile strength. The peroxide values of the olive oil increased with the time of storage, independent of film formulation. However, this behavior was only pronounced in the first days of storage (first week), probably due to the oxygen present in the pouch headspace. The methodology proposed in this work was applied as a means to obtain an indicative of oxygen permeation, since this gas is the main responsible for affecting the quality of oils. Additionally, direct oxygen measurements should be made to investigate the oxygen barrier properties of these packages in order to fully understand the parameters influencing the permeation of the gas. Other strategies could be employed to maintain or prolong the stability of these films as well, such as packing the oil under vacuum or inert atmospheres.
Conclusions
In the present work, the incorporation of VCO was proposed to improve the water vapor barrier and mechanical properties of films made of SPI. In order to homogenize the system and disperse the VCO evenly, SL was used as a surfactant in the film-forming solution. Although it was not possible to improve the WVP of the films with the addition of coconut oil and surfactant, their values were, in some cases, lower than those from other SPI films found in the literature and similar to films made of some other proteins. All emulsified film formulations resulted in films with improved flexibility, which is an important property for making flexible packages. Although this increase in elongation has led to a decrease in the tensile strength, the flexibility of these films allows them to be applicable in a range of products, since the package can be adapted to the surface of the products without damaging their structure. The reduction in moisture content and increased opacity was also observed in emulsion-based films, but their effect may be considered positive or negative, depending on the destination of the packages. Thus, a most appropriate formulation must be adjusted according to the characteristics of the food product to be packaged. The integrity of the SPI emulsion pouches was maintained during the storage period, which is an interesting attribute for future applications of these films as flexible packages. We believe that this work can contribute to the area of emulsified films, since there are few studies in which the influence of the surfactant is studied at various concentrations in a more detailed depth.
Abbreviations
- SPI:
-
Soy protein isolate
- VCO:
-
Virgin coconut oil
- SL:
-
Soy lecithin
- Gly:
-
Glycerol
- FFS:
-
Film-forming solution
- TS:
-
Tensile strength
- ELO:
-
Elongation at break
- WVP:
-
Water vapor permeability
- TGA:
-
Thermogravimetric analysis
References
Al-Hassan, A. A., & Norziah, M. H. (2012). Starch-gelatin edible films: water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocolloids, 26(1), 108–117.
Andreuccetti, C., Carvalho, R. A., & Grosso, C. R. F. (2009). Effect of hydrophobic plasticizers on functional properties of gelatin-based films. Food Research International, 42(8), 1113–1121.
Andreuccetti, C., Carvalho, R. A., Galicia-García, T., Martínez-Bustos, F., & Grosso, C. R. F. (2011). Effect of surfactants on the functional properties of gelatin-based edible films. Journal of Food Engineering, 103(2), 129–136.
Anker, M., Berntsen, J., Hermansson, A.-M., & Stading, M. (2002). Improved water vapor barrier of whey protein films by addition of an acetylated monoglyceride. Innovative Food Science & Emerging Technologies, 3(1), 81–92.
Antoniou, J., Liu, F., Majeed, H., Qazi, H. J., & Zhong, F. (2014). Physicochemical and thermomechanical characterization of tara gum edible films: effect of polyols as plasticizers. Carbohydrate Polymers, 111, 359–365.
AOCS. (2001). Official methods and recommended practices of the American Oil Chemists’ Society (5th ed.). Champaign: AOCS.
ASTM Standard D882 (2012). Standard test method for tensile properties of thin plastic sheeting. ASTM International, West Conshohocken, PA, doi: 10.1520/D0882-12. www.astm.org.
ASTM Standard E96/E96M (2013). Standard test methods for water vapor transmission of materials. ASTM International, West Conshohocken, PA. doi: 10.1520/E0096_E0096M. www.astm.org.
Atarés, L., de Jesús, C., Talens, P., & Chiralt, A. (2010). Characterization of SPI-based edible films incorporated with cinnamon or ginger essential oils. Journal of Food Engineering, 99(3), 384–391.
Bertan, L. C., Fakhouri, F. M., Siani, A. C., & Grosso, C. R. F. (2005a). Influence of the addition of lauric acid to films made from gelatin, triacetin and a blend of stearic and palmitic acids. Macromolecular Symposium, 229(1), 143–149.
Bertan, L. C., Tanada-Palmu, P. S., Siani, A. C., & Grosso, C. R. F. (2005b). Effect of fatty acids and ‘Brazilian elemi’ on composite films based on gelatin. Food Hydrocolloids, 19(1), 73–82.
Binsi, P. K., Ravishankar, C. N., & Gopal, S. (2013). Development and characterization of and edible composite film based on chitosan and virgin coconut oil with improved moisture sorption properties. Journal of Food Science, 78(4), E526–E534.
Blanco-Pascual, N., Fernández-Martín, F., & Montero, P. (2014). Jumbo squid (Dosidicus gigas) myofibrillar protein concentrate for edible packaging films and storage stability. LWT- Food Science and Technology, 55(2), 543–550.
Bravin, B., Peressini, D., & Sensidoni, A. (2004). Influence of emulsifier type and content on functional properties of polysaccharide lipid-based edible films. Journal of Agricultural and Food Chemistry, 52(21), 6448–6455.
Bueschelberger, H.-G. (2004). Lecithins. In R. J. Whitehurst (Ed.), Emulsifiers in food technology (pp. 3–39). Oxford: Blackwell.
Chang, C., & Nickerson, M. T. (2014). Effect of plasticizer-type and genipin on the mechanical, optical, and water vapor barrier properties of canola protein isolate-based edible films. European Food Research and Technology, 238(1), 35–46.
Chao, Z., Yue, M., Xiaoyan, Z., & Dan, M. (2010). Development of soybean protein-isolate edible films incorporated with beeswax, Span 20 and glycerol. Journal of Food Science, 75(6), C493–C497.
Chen, C.-H., Kuo, W.-S., & Lai, L.-S. (2012). Development of tapioca starch/decolorized hsian-tsao leaf gum-based antimicrobial films: physical characterization and evaluation against Listeria monocytogenes. Food and Bioprocess Technology, 6(6), 1516–1525.
Chiumarelli, M., & Hubinger, M. D. (2014). Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocolloids, 38, 20–27.
Cho, S. Y., & Rhee, C. (2004). Mechanical properties and water vapor permeability of edible films made from fractionated soy proteins with ultrafiltration. LWT- Food Science and Technology, 37(8), 833–839.
Cho, S. Y., Park, J.-W., Batt, H., & Thomas, R. L. (2007). Edible films made from membrane processed soy protein concentrates. LWT- Food Science and Technology, 40(3), 418–423.
Cho, S. Y., Lee, S. Y., & Rhee, C. (2010). Edible oxygen barrier bilayer film pouches from corn zein and soy protein isolate for olive oil packaging. LWT- Food Science and Technology, 43(8), 1234–1239.
CODEX STAN 33-1981 (Rev. 1-1989) (2001). Codex Alimentarius. Fats, oils and related products. Rome: Food and Agricultural Organization of the United Nations.
Damodaran, S. (2008). Aminoácidos, Peptídeos e Proteínas. In S. Damodaran, K. L. Parkin, & O. R. Fennema (Eds.), Fennema’s food chemistry (4th ed., pp. 217–330). Boca Raton: CRC.
Dangaran, K., Tomasula, P. M., & Qi, P. (2009). Structure and function of protein-based edible films and coatings. In M. Embuscado & K. C. Huber (Eds.), Edible film and coatings for food applications (pp. 25–56). New York: Springer.
Denavi, G., Tapia-Blácido, D. R., Añón, M. C., Sobral, P. J. A., Mauri, A. N., & Menegalli, F. C. (2009). Effects of drying conditions on some physical properties of soy protein films. Journal of Food Engineering, 90(3), 341–349.
Fakhoury, F. M., Martelli, S. M., Bertan, L. C., Yamashita, F., Mei, L. H. I., & Queiroz, F. P. C. (2012). Edible films made from blends of manioc starch and gelatin—influence of different types of plasticizer and different levels of macromolecules on their properties. Food Science & Technology, 49(1), 149–154.
Friesen, K., Chang, C., & Nickerson, M. (2015). Incorporation of phenolic compounds, rutin and epicatechin, into soy protein isolate films: mechanical, barrier and cross-linking properties. Food Chemistry, 172, 18–23.
Gallo, J.-A. Q., Debeaufort, F., Callegarin, F., & Voilley, A. (2000). Lipid hydrophobicity, physical state and distribution effects on the properties of emulsion-based edible films. Journal of Membrane Science, 180, 37–46.
Galus, S., Mathieu, H., Lenart, A., & Debeaufort, F. (2012). Effect of modified starch or maltodextrin incorporation on the barrier and mechanical properties, moisture sensitivity and appearance of soy protein isolate-based edible films. Innovative Food Science & Emerging Technologies, 16, 148–154.
Garcia, L. C., Pereira, L. M., de Luca Sarantópoulos, C. I. G., & Hubinger, M. D. (2010). Selection of an edible starch coating for minimally processed strawberry. Food and Bioprocess Technology, 3(6), 834–842.
Gofferje G, Schmid M, Stäbler A. Characterization of Jatropha curcas L. protein cast films with respect to packaging relevant properties. Int J Polym Sci. 2014; ID 630585.
Gontard, N., Guilbert, S., & Cuq, J.-L. (1992). Edible wheat gluten films: influence of the main process variables on film properties using response surface methodology. Journal of Food Science, 57(1), 190–195.
Gontard, N., Duchez, C., Cuq, J.-L., & Guilbert, S. (1994). Edible blended biofilms of wheat gluten and lipids: water vapor permeability and other physical properties. International Journal of Food Science and Technology, 29(1), 39–50.
González, A., Strumia, M. C., & Igarzabal, C. I. (2011). Cross-linked soy protein as material for biodegradable films: synthesis, characterization and biodegradation. Journal of Food Engineering, 106(4), 331–338.
Guerrero, P., Hanani, Z. A. N., Kerry, J. P., & de la Caba, K. (2011). Characterization of soy protein-based films prepared with acids and oils by compression. Journal of Food Engineering, 107(1), 41–49.
Guilbert, S., Gontard, N., & Gorris, L. G. M. (1996). Prolongation of the shelf-life of perishable food products using biodegradable films and coatings. LWT- Food Science and Technology, 29(1-2), 10–17.
Hammann, F., & Schmid, M. (2014). Determination and quantification of molecular interactions in protein films: a review. Materials, 7(12), 7975–7996.
Hopkins, E. J., Chang, C., Lam, R. S. H., & Nickerson, M. T. (2015). Effects of flaxseed oil concentration on the performance of a soy protein isolate-based emulsion-type film. Food Research International, 67, 418–425.
Jayadas, N. H., & Nair, K. P. (2006). Coconut oil as base oil for industrial lubricants—evaluation and modification of thermal, oxidative and low temperature properties. Tribology International, 39(9), 873–878.
Jost, V., Kobsik, K., Schmid, M., & Noller, K. (2014). Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydrate Polymers, 110, 309–319.
Keerati-U-Rai, M., & Corredig, M. (2010). Heat-induced changes occurring in oil/water emulsions stabilized by soy glycinin and β-conglycinin. Journal of Agricultural and Food Chemistry, 58(16), 9171–9180.
Kokoszka, S., Debeaufort, F., Hambleton, A., Lenart, A., & Voilley, A. (2010). Protein and glycerol contents affect physico-chemical properties of soy protein isolate-based edible films. Innovative Food Science and Emerging, 11(3), 503–510.
Kowalczyk, D., & Baraniak, B. (2014). Effect of candelilla wax on functional properties of biopolymer emulsion films—a comparative study. Food Hydrocolloids, 41, 195–209.
Krochta, J. M., & Miller, K. S. (1997). Oxygen and aroma barrier properties of edible films: a review. Trends in Food Science and Technology, 8(7), 228–237.
Kumar, P., Sandeep, K. P., Alavi, S., Truong, V. D., & Gorga, R. E. (2010). Preparation and characterization of bio-nanocomposite films based on soy protein isolate and montmorillonite using melt extrusion. Journal of Food Engineering, 100(3), 480–489.
Limpisophon, K., Tanaka, M., & Osako, K. (2010). Characterisation of gelatin-fatty acid emulsion films based on blue shark (Prionace glauca) skin gelatin. Food Chemistry, 122(4), 1095–1101.
Marina, A. M., Che Man, Y. B., & Amin, I. (2009a). Virgin coconut oil: emerging functional food oil. Trends in Food Science and Technology, 20(10), 481–487.
Marina, A. M., Che Man, Y. B., Nazimah, S. A. H., & Amin, I. (2009b). Chemical properties of virgin coconut oil. Journal of American Oil Chemists’ Society, 86(4), 301–307.
Marquez, G. R., Di Pierro, P., Esposito, M., Mariniello, L., & Porta, R. (2014). Application of transglutaminase-crosslinked whey protein/pectin films as water barrier coatings in fried and baked foods. Food and Bioprocess Technology, 7(2), 447–455.
McHugh, T. H., & de Avena-Bustillos, R. J. (2012). Applications of edible films and coatings to processed foods. In E. A. Baldwin, R. Hagenmaier, & J. Bai (Eds.), Edible coatings and films to improve food quality (2nd ed., pp. 291–318). Boca Raton: CRC.
Miller, K. S., & Krochta, J. M. (1997). Oxygen and aroma barrier properties of edible films: a review. Trends in Food Science and Technology, 8(7), 228–237.
Monedero, F. M., Fabra, M. J., Talens, P., & Chiralt, A. (2009). Effect of oleic acid-beeswax mixtures on mechanical, optical and water barrier properties of soy protein isolate based films. Journal of Food Engineering, 91(4), 509–515.
Nishinari, K., Fang, Y., Guo, S., & Phillips, G. O. (2014). Soy proteins: a review on composition, aggregation and emulsification. Food Hydrocolloids, 39(301–318), 2014.
O’Brien, R. D. (2009). Raw materials. In: Fats and oils: formulating and processing for applications, 3rd ed. (3rd, pp. 1-72). Boca Raton: CRC.
Ou, S., Wang, Y., Tang, S., Huang, C., & Jackson, M. G. (2005). Role of ferulic acid in preparing edible films from soy protein isolate. Journal of Food Engineering, 70(2), 205–210.
Ozdemir, M., & Floros, J. D. (2008). Optimization of edible whey protein films containing preservatives for mechanical and optical properties. Journal of Food Engineering, 84(1), 116–123.
Pan, H., Jiang, B., Chen, J., & Jin, Z. (2014a). Assessment of the physical, mechanical, and moisture-retention properties of pullulan-based ternary co-blended films. Carbohydrate Polymers, 112, 94–101.
Pan, H., Jiang, B., Chen, J., & Jin, Z. (2014b). Blend-modification of soy protein/lauric acid edible films using polysaccharides. Food Chemistry, 151, 1–6.
Pavlath, A. E., & Orts, W. (2009). Edible films and coatings: why, what, and how? In M. E. Embuscado & K. C. Huber (Eds.), Edible films and coatings for food applications (pp. 1–24). New York: Springer.
Peng, Y., Yin, L., & Yunfei, L. (2013). Combined effects of lemon essential oil and surfactants on physical and structural properties of chitosan films. International Journal of Food Science and Technology, 48(1), 44–50.
Peychès-Bach, A., Moutounet, M., Peyron, S., & Chalier, P. (2009). Factors determining the transport coefficients of aroma compounds through polyethylene films. Journal of Food Engineering, 95(1), 45–53.
Qu, P., Huang, H., Wu, G., Sun, E., Chang, Z. (2015). Effects of hydrolysis degree of soy protein isolate on the structure and performance of hydrolyzed soy protein isolate/urea/formaldehyde copolymer resin. Journal of Applied Polymer Science, 132(7), doi: 10.1002/APP.41469.
Ramos, O. L., Reinas, I., Silva, S., Fernandes, J. C., Cerqueira, M. A., Pereira, R. N., Vicente, A. A., Poças, M. F., Pintado, M. E., & Malcata, F. X. (2013). Effect of whey protein purity and glycerol content upon physical properties of edible films manufactured therefrom. Food Hydrocolloids, 30(1), 110–122.
Rezvani, E., Schleining, G., Sümen, G., & Taherian, A. R. (2013). Assessment of physical and mechanical properties of sodium caseinate and stearic acid based film-forming emulsions and edible films. Journal of Food Engineering, 116(2), 598–605.
Rhim, J. W., Gennadios, A., Weller, C. L., & Hanna, M. A. (2002). Sodium dodecyl sulfate treatment improves properties of cast films from soy protein isolate. Industrial Crops and Products, 15(3), 199–205.
Rhim, J.-W., Mohanty, K. A., Singh, S. P., & Ng, P. K. W. (2006). Preparation and properties of biodegradable multilayer films based on soy protein isolate and poly(lactide). Industrial and Engineering Chemistry Research, 45(9), 3059–3066.
Schmid, M., Sängerlaub, S., Wege, L., & Stäble, A. (2014). Properties of transglutaminase crosslinked whey protein isolate coatings and cast films. Packaging Technology and Science, 27(10), 799–817.
Seneviratne, K. N., Hapuarachchl, C. D., & Ekanayake, S. (2009). Comparison of the phenolic-dependent antioxidant properties of coconut oil extracted under cold and hot conditions. Food Chemistry, 114(4), 1444–1449.
Song, X., Zhou, C., Fu, F., Chen, Z., & Wu, Q. (2013). Effect of high-pressure homogenization on particle size and film properties of soy protein isolate. Industrial Crops and Products, 43, 538–544.
Sothornvit, R., & Krochta, J. M. (2005). Plasticizers in edible films and coatings. In J. H. Han (Ed.), Innovations in food packaging (pp. 403–433). London: Elsevier Academic.
Souza, C. O., Silva, L. T., Silva, J. R., López, J. A., Veiga-Santos, P., & Druzian, J. I. (2011). Mango and acerola pulp as antioxidant additives in cassava starch bio-based film. Journal of Agricultural and Food Chemistry, 59(6), 2248–2254.
Stauffer, C. E. (2005). Emulsifiers for the food industry. In F. Sahidi (Ed.), Bailey’s industrial oil and fat products: edible oil and fat products: products and applications (vol. 4) (6th ed., pp. 229–268). Hoboken: Wiley.
Su, J.-F., Huang, Z., Yuan, X.-Y., Wang, X.-Y., & Li, M. (2010). Structure and properties of carboxymethyl cellulose/soy protein isolated blend edible films crosslinked by Maillard reactions. Carbohydrate Polymers, 79(1), 145–153.
Tongnuanchan, P., Benjakul, S., & Prodpran, T. (2014). Structural, morphological and thermal behavior characterisations of fish gelatin incorporated with basil and citronella essential oils as affected by surfactants. Food Hydrocolloids, 41, 33–43.
USDA (2015). United States Department of Agriculture. Data and statistics: soybeans: world supply and distribution. Available at: http://www.usda.gov/wps/portal/usda/usdahome?navid=DATA_STATISTICS. Accessed 24 March 2015.
Valenzuela, C., Abugoch, L., & Tapia, C. (2013). Quinoa protein-chitosan-sunflower oil edible film: mechanical, barrier and structural properties. LWT- Food Science and Technology, 50(2), 531–537.
Wan, V. C.-H., Kim, M. S., & Lee, S.-Y. (2005). Water vapor permeability and mechanical properties of soy protein isolate edible films composed of different plasticizer combinations. Journal of Food Science, 70(6), 387–391.
Wang, Z., Zhou, J., Wang, X.-X., Zhang, N., Sun, X.-X., & Ma, Z.-S. (2014). The effects of ultrasonic/microwave assisted treatment on the water vapor barrier properties of soybean protein isolate-based oleic acid/stearic acid blend edible films. Food Hydrocolloids, 35, 51–58.
Winkler, H., Vorwerg, W., & Schmid, M. (2015). Synthesis of hydrophobic whey protein isolate by acylation with fatty acids. European Polymer Journal, 62, 10–18.
Yang, L., & Paulson, A. T. (2000). Effects of lipids on mechanical and moisture barrier properties of edible gellan film. Food Research International, 33(7), 571–578.
Zahedi, Y., Ghanbarzadeh, B., & Sedaghat, N. (2010). Physical properties of edible emulsified films based on pistachio globulin protein and fatty acids. Journal of Food Engineering, 100(1), 102–108.
Zhao, Y. (2012). Application of commercial coatings. In E. A. Baldwin, R. Hagenmaier, & J. Bai (Eds.), Edible coatings and films to improve food quality (2nd ed., pp. 319–332). Boca Raton: CRC.
Acknowledgments
The authors thank CAPES (Coordination for the Improvement of Higher Education Personnel), the Post-Graduation Program in Food Engineering (UFPR), and IMCOPA for supplying the soy lecithin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carpiné, D., Dagostin, J.L.A., Bertan, L.C. et al. Development and Characterization of Soy Protein Isolate Emulsion-Based Edible Films with Added Coconut Oil for Olive Oil Packaging: Barrier, Mechanical, and Thermal Properties. Food Bioprocess Technol 8, 1811–1823 (2015). https://doi.org/10.1007/s11947-015-1538-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-015-1538-4