Abstract
Edible films and coatings developed through biopolymers have good tensile strength and gaseous barriers but lack moisture barrier properties. To improve the moisture barrier properties of sodium alginate and whey protein-based films, carnauba wax at different concentrations (0.5, 1.0, and 1.5%) was incorporated. Results showed significant variations as the concentration of the carnauba wax increased. The addition of wax at a higher concentration (1.5%) significantly improved the moisture barrier properties (3.12 ± 0.31 g.mm/kPa.h.m2). However, tensile strength reduced (3.92 ± 0.04 to 3.34 ± 0.04 MPa) with the increase in wax concentration. The incorporation of lipid constituents in the emulsion-based films also affects the physicochemical properties including thickness, droplet size, transparency, and microstructure of the films as well. At lower concentrations (0.5%) films showed uniform microstructure and better transparency (81.82 ± 1.63%) but lack in moisture barrier properties. Emulsified edible films with improved moisture barrier and tensile properties are preferable in the food packaging industry. Emulsion-based films and coatings have potential applications in foods, especially in fresh and processed foods, dairy, bakery products, meat, and sausages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most of the packaging in the market is made of synthetic conventional polymers like polyethylene, polypropylene, or polystyrene, which pose health risks due to hazardous ingredient migration into consumables Madera-Santana et al. (2014). Consumer health concerns and solid waste generated Consumer health and solid waste generated cause alarming situations because of the use of non-degradable materials. The use of petrochemical-based packaging materials is gaining popularity. The use of biodegradable and natural polymer-based food packaging materials for food bio-packaging has the potential to be one of the most promising environmental solutions (Sandhu et al. 2017). Synthetic packaging has created serious concerns in humans owing to their interest in safe food with more shelf stability and non-biodegradability (Jafarzadeh et al. 2021). Previously, Ritchie and Roser (2018) reported that only less than 6% of plastics are recycled in the world. After end-use, to degrade these materials into their basic components, approximately 100 years are needed, and their deposal has produced a lot of problems like environmental pollution (Song and Zheng 2014). Furthermore, packaging made from renewable resources has become a driving force in the last 15 years. In the world, around 3.5 billion tons of biopolymers were produced in 2011, while this production reached up to 12 billion tons in 2020 (Crouvisier-Urion et al. 2017).
Carnauba wax originated from the Brazilian palm tree. It is widely used as an edible coating to extend the shelf life of fruits and vegetables. It has a higher melting point and hardness that helps in developing durable coatings (Gupta et al. 2021). Carnauba wax is majorly applied to reduce water loss and gives a shiny appearance as well (Singh et al. 2016). Whey protein is obtained from skim milk while casein is attained through alkali precipitation. These milk-based protein films are flexible, tasteless, and transparent. The tensile and barrier properties of whey protein are gaining attention as it performs well in polysaccharides and other protein-based films. From whey protein by-products, whey protein concentrate and whey protein isolate are mostly used as coating materials. The disadvantages of alginate are the moisture barrier and lower mechanical strength (Kandasamy et al. 2021). Protein-based films also act as a carrier of food additives such as antioxidants, colorants, and antimicrobial agents (Mohamed et al. 2020). The physicochemical properties of proteins are dependent on the arrangement of amino acids, their relative quantity, and side-chain polymers. Different types of globular proteins are gluten, corn zein, and whey protein (Hassan et al. 2018). Protein-based films have better mechanical properties but lower moisture barrier properties owing to their hydrophilic nature. Protein-based coatings also increase the nutritional profile of coated fruits. In edible films and coatings mostly used proteins are casein, whey, gelatin, zein, soy protein isolates, egg albumin, rice, and peanut proteins (Zuhal et al. 2018).
Polysaccharides such as pectin, gellan, chitosan, alginate, and carrageenan have been used as an alternative to plastic-based packaging due to their better antimicrobial, antioxidant properties, and easy extraction (Paidari et al. 2021). Polysaccharides are non-toxic and readily available materials in nature. Alginates are naturally occurring polysaccharides produced from brown algae and water-soluble. It is widely used in packaging films due to its more mechanical strength and environmentally safe biopolymer. However, it shows poor water resistance due to its hydrophobic nature (Jafarzadeh et al. 2021). United States Department of Agriculture and Food and Agricultural Organization has included alginate and its derived compounds are generally recognized as safe (GRAS) product list (Kanikireddy et al. 2019; Parreidt et al. 2018a, b).
Over the last decade, biopolymers from organic sources progressed dramatically for the preparation of biodegradable edible films. Composite films and coatings are systems that blend multiple biopolymers to achieve desirable qualities. Different types of combinations from proteins, polysaccharides, and lipids have been used to make binary and tertiary films and coatings. The use of food-grade biopolymers to make films and coating materials is a new field of study. When used in food systems, edible film and coating systems serve a variety of purposes. These, for example, support the food’s inherent structure and give resistance to moisture loss. They also allow for the controlled release of gases for respiration. Such systems can also be utilized to distribute active ingredients such as vitamins, antibacterial agents, and antioxidants for long-term protection and nutritional profile improvement (Dhumal and Sarkar 2018).
Biopolymer-based packaging films may have better potential to extend the shelf stability, food structure, and end quality maintenance by creating barriers to moisture, oxygen, aroma, and effective transport of functional compounds i.e. antimicrobial, antioxidant, and nutritional components (Jouki et al. 2021). These films have better mechanical strength and gas barrier properties but lack moisture barrier properties. However, lipid-based films have moisture barrier properties but less mechanical strength. Therefore, the main objective of this study is to develop emulsified edible film with properties of lipids and biopolymers together.
Materials and methods
Procurement of raw materials
Food grade carnauba wax was purchased from Kahlwax, Germany. Analytical grade glycerol, tween-20, calcium chloride, and acetic acid were procured from Sigma-Aldrich, (Saint Louis, MO, USA). Whey protein and sodium alginate were purchased from BioChem, (New Jersey, USA). Deionized water was obtained through a milli-Q water purification system (Millipore, Merck, Germany).
Preparation of carnauba wax emulsion
Emulsified edible films and coatings were developed by using different proportions of carnauba wax, whey protein, and sodium alginate according to the protocol given by Kaya et al. (2018). First of all, sodium alginate was dissolved in deionized water using a magnetic stirrer (Model: MS-300HS, Misung Scientific, Korea). Subsequently, whey protein was added to the sodium alginate solution (Table 1). Furthermore, glycerol (1%) as a plasticizer, tween 20 (1%) as an emulsifier, and calcium chloride (1%) as an antimicrobial agent were added to the solution. The mixture was stirred through an ultraturrax (Model: T 18 D, IKA T-25, Germany)) for the preparation of a homogeneous solution. For emulsion preparation, carnauba wax was melted at 84 °C and the aforementioned solution was also heated side by side, separately. After attaining the required temperatures, melted carnauba wax was added to the solution with continuous stirring. Afterward, the solution was homogenized at 18,000 rpm for 3 min. The prepared emulsions were regarded as coating solutions and further used to develop emulsion-based films through the casting method by keeping them at 40 °C for 20 h in a hot air oven (Fig. 1).
Characterization of emulsified edible films and coatings
Emulsified edible films and coatings were characterized for physicochemical (emulsion stability, particle size, thickness, and transparency), rheological (viscosity), microstructural (scanning electron microscopy), mechanical (tensile strength, elongation at breakage), and moisture barrier properties (water vapor permeability).
Emulsion stability
The stability of the emulsions was determined by using the method given by Purwanti et al. (2018). Briefly, freshly prepared samples were poured (6 mL) into a 20 mL test tube with an airtight screwcap and placed at room temperature (25 °C) for 72 h. Initial and final levels of the emulsion were checked and percent phase separation was measured. The stability of the emulsion was calculated by using the following equation:
S = \(\frac{h0-ht}{h0}\) × 100
S = Stability.
h0 = initial level of emulsion.
ht = Final level of the emulsion after 72 h.
Particle size analysis
The emulsion’s particle size was assessed through a laser particle size analyzer (SALD-2300, Laser Particle Size Analyzer, Shimadzu, Japan) as described by Parreidt et al. (2018a, b). For the calculation of the droplet size, a polydispersity distribution model by Schultz was taken as an analysis model. The sample was taken and the obscuration was taken that described the power of laser light lost when it passed through the coating solution. Sample particle size measurement was taken after 2 to 3 min of dispersion and the mean value was calculated through the average value of three readings.
Viscosity
The viscosity of the emulsions was determined through a rheometer (ARES G2 Rheometer, TA Instrument, USA) by using a protocol devised by Jouki et al. (2014). The sample (30 mL) was poured into the concentric cylinder probe and equilibrated for 10 min at 25 ℃. The sample was sheared from 1 to 180 s− 1 and the temperature was maintained at 25 ± 0.1 ℃. The viscosity of the emulsion was checked through the power law model in Pa.s.
Transparency
Transparency of the film samples was assessed by using a UV-visible spectrophotometer (U2020, Serial number: 20 A IRMECO GmbH, Geesthacht/Germany) according to the method of Kaya et al. (2018). At first, films were cut into homogenous strips of spectrophotometer cuvette size and then placed on the side of the cuvette facing light. The wavelength of the UV-visible spectrophotometer was adjusted to 600 nm and calibrated with distilled water. Then, the cuvette containing the film strip was placed into the sampling stage of the UV-visible spectrophotometer.
Scanning electron microscopy (SEM)
Images of emulsified edible films were taken through a scanning electron microscope (S2380N, Hitachi, Japan) to evaluate the microstructure by following the guidelines of Chiumarelli and Hubinger (2014). Film samples of 3 × 3 mm size were cut and placed in a desiccator for 7 days at 0% relative humidity to maintain homogenous moisture content. Samples were coated with a gold layer by using a sputter coater. The images of the film’s surface were observed at a higher voltage (10 kV).
Mechanical properties
Mechanical properties including tensile strength, elongation at breakage, and young’s modulus of emulsified edible films were determined through a texture analyzer (SALD-DS5, Shimadzu Corporation, Japan) according to the protocol proposed by Brzoska et al. (2018). The casted films were cut into 15 mm width and 50 mm long pieces and kept at 25 °C and 65% relative humidity for 12 h. Film samples were clamped in the loading frames and the gauge length was fined at 50 mm and subjected to 50 N force till breakage of the sample. The stretching speed for the films was fixed at 100 mm/min.
Moisture barrier properties
Emulsified edible films were assessed for their moisture barrier properties by checking the water vapor permeability of the film samples following the official method of ASTM (2016). Purposely, films were applied on the payne permeability cups (Elcometer 5100/1, USA) containing 10 mL of distilled water and kept at 25 °C and 65% relative humidity. Weight changes were measured for 10 h for every 1 h interval. The water vapor permeability of the films was calculated by using the equation:
WVP = Slope × L.
A ×\(\varDelta \text{P}\)
Where;
L = Average film thickness (m).
A = Moisture transfer area of the cup (m2).
\(\varDelta \text{P}\) = Partial water vapor pressure difference
Slope = Constant representing weight change vs. time calculated via linear regression.
Statistical analysis
All the experiments were carried out in triplicate and the results are presented in means ± SD after statistical analysis. The statistical analysis was performed through OriginPro 2021b 9.85 (Origin Lab Co., USA) Difference among treatments was measured to check the level of significance through one-way ANOVA for physicochemical, mechanical, and moisture barrier properties. Moreover, the post-hoc comparison was performed through Tucky’s HSD test at P˂0.05.
Results and discussion
Emulsion stability
Table 2 shows the emulsion stability of carnauba wax-based emulsion in the sodium alginate and whey protein. Emulsion stability increased significantly as the concentration of the dispersed particles from both bees and carnauba wax increased in the treatments. For stabilizing emulsion solution, its composition, formulation, type, and concentration of emulsifier play a pivotal role (Purwanti et al. 2018). Emulsifiers help in stabilizing emulsion by reducing interfacial forces among immiscible phases i.e. in oil and water phases. In this way, they assist to develop a film with oil in water emulsion that inhibits the coalescence of dispersed lipid particles (Rousseau 2013). Low molecular weight surface-active agents, amphiphilic biopolymers, and active ingredients provide stabilization of liquid but depend on their concentration level, storage conditions, and reacting forces (electrostatic and steric repulsions) among them (Rees et al. 2012).
Earlier, Syarifuddin et al. (2019) developed two lipid-type oil in water emulsions and studied their effect on emulsion stability. Emulsions were developed with 10, 20, 30, and 40% sunflower or hydrogenated palm kernel oil in whey protein isolates and sodium caseinate. Furthermore, 0–40% sucrose was added and the pH of the water phase was maintained at pH 7. The continuous and dispersed phase behavior of the different phases was checked by using differential scanning colorimetry (DSC). Rheological properties and emulsion stability were derived from the DSC data for gravitation phase separation and lipid droplet size analysis. Results depicted that crystallization in lipid before the water phase results in destabilization of the emulsion even at low concentrations of sucrose (0, 2.5, and 5%). However, in the opposite case stability of the emulsion increased if water crystallization occurred before lipid crystallization. Emulsions with a double concentration of whey protein isolates and 0% sucrose considerably improved emulsion stability. Besides, lipid destabilization, thickening, and flocculation also caused instability in the emulsion systems.
In general, the results are in agreement with one of the previous studies conducted by Cerimedo et al. (2010) who developed and evaluated the emulsion stability of sodium caseinate in different lipid-based compounds. Results described that the mechanism of destabilization mainly depends on the lipid-to-protein ratio. Similarly, smaller protein contents in the emulsion are accompanied by the lower surface coverage leading to coalescence. Moreover, higher protein contents in the film cause partial re-stabilization by forming a stronger gelling network. Additionally, the findings of Day et al. (2007) illustrated the behavior of sodium caseinate-based emulsified edible coatings. They concluded that the concentration of protein-to-lipid contents had a significant effect on the stability of sodium caseinate-based emulsions.
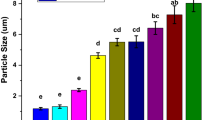
Particle size
Figure 2 for particle size of carnauba wax-based emulsion showed a substantial effect among treatments. Particle size is directly influenced by lipid compound concentration and speed of homogenization during emulsion preparation. Mohammadi et al. (2020) developed emulsion and checked the effect of higher lipid concentration, particle size, and distribution. They observed that particle size and their distribution in emulsion solution are concerned with the concentration of lipid. Furthermore, emulsifiers i.e., tween-20 or tween-80 also influence the lipid particle size and their distribution in the film-forming matrix. The higher homogenization speed and the concentration of the emulsifier results in smaller particle size. This is due to the emulsifying property of the surfactant that caused a reduction in surface tension that increased their solubility in water. In the opposite case, emulsifiers also prevent negative mechanisms in film-forming solutions such as lipid aggregation and coalescence. Mostly, the distribution of lipids in an aqueous phase depends on different conditions including homogenization speed, type of homogenizer, time, and temperature of the process. Moreover, lipids with lower melting points result in smaller size droplets that improve the emulsion system as well.
Earlier, a research study regarding the particle size of an oil-in-water emulsion having sodium caseinate (2–6% w/w) was investigated by Lian et al. (2017). Heat treatment was also applied during emulsion development. In the recombined sodium caseinate emulsion, at low and medium concentrations the attraction forces were low but with the increase in concentration at 6% attraction forces enhanced. When attraction forces strengthened, the particle size of the dispersed particles increased. The described results are similar to current study findings but the particle size of emulsion solutions varies according to the nature of the ingredient, the concentration used and processing conditions applied.
Viscosity
Figure 3 for carnauba wax-based emulsion viscosity showed significant variations. The viscosity of the coating solutions improved significantly as the concentration of the dispersed beeswax and carnauba wax increased in the treatments. There are several reasons behind the higher viscosity of the emulsion is the presence of biopolymers that helps in the development of the jelly-type structure. Higher viscosity is better for the film formation as the proper thickness of the films is obtained. Low viscous emulsions lose their moisture quickly during drying and proper thickness of the film is not attained. The viscosity of the different treatments increased linearly as the concentration of beeswax increased due to the formation of larger particle size in the emulsion causing obstruction in the flow.
Earlier one of the peers, Fabra et al. (2011) conducted a study to check the influence of lipid self-association and homogenization conditions on the physical properties of sodium caseinate-based films. They have developed oleic and stearic acids based sodium caseinate films by using the casting method. The film-forming emulsion was prepared by using different concentrations of lipid compounds and homogenization speed. The prepared films were analyzed for particle size and rheological analysis. Oleic and stearic acids were homogenized well in the film solution. The addition of oleic and stearic acids significantly affected the film flexibility, and more surface roughness with an increased concentration of lipid compound. However, the opposite effect was observed for viscosity and particle size. More homogenization speed and time produce smaller size particles and less viscous solutions.
Fabra et al. (2009) checked the effect of beeswax and fatty acid addition on the properties of sodium caseinate films and dispersions. Beeswax and different fatty acids were used during sodium caseinate films preparation. The film-forming emulsion was characterized by particle size, viscoelastic properties, and surface tension. The emulsions with more concentration of beeswax showed larger particle size and more viscous solution. The lipid concentration, homogenization speed, and time directly influenced the viscosity of the film-forming solutions.
Moisture content
Figure 4 moisture content for carnauba wax-based film demonstrated the substantial variations among different treatments. Moisture contents increased significantly as the concentration of the dispersed beeswax increased in the treatments. Retention of moisture content in the films is directly related to the concentration of lipid and protein contents in the film structures.
Previously, Syarifuddin et al. (2019) developed and characterized whey protein isolates or pectin-based films in beeswax and butter aroma. They have evaluated the influence of beeswax concentration and butter aroma on the physicochemical properties of whey protein or pectin-based films. Results described that films with beeswax and butter aroma expressed better physical and moisture barrier properties. Moisture content values attained for whey protein: beeswax and whey protein: beeswax: butter aroma films were 23.17% and 35.35%, respectively. Similarly, in the current study moisture content for beeswax and carnauba wax films values ranged from 18.13 ± 0.82–45.83 ± 1.39% and 22.14 ± 0.7-49.26 ± 1.83%, respectively is at par with the previous study findings. Films with more beeswax concentration showed a better response in retaining the moisture content of the film. The findings of the current study also showed a significant response when the concentration of whey to pectin increased in the beeswax-based emulsion films.
Previous research, conducted by Zahedi et al. (2019), described that the moisture contents of carboxymethyl cellulose-based nanocomposite films with zinc oxide were found to be in the range of 27.75 ± 4.7 to 32.88 ± 4.6% among different treatments. The obtained values in the current study are similar to those given values but the difference in values is due to different ingredients, processing conditions, and concentrations used. Moisture content plays a major role in the retention of fruits and vegetable shelf life. Low moisture is preferred to extend the shelf life as at higher moisture contents microbial growth occurs resulting in deterioration of the food commodities other than fresh produce. In emulsified edible films high moisture contents reduce the flexibility of the films and cause degradation owing to microbial attack. However, antimicrobial agents are added to encounter microbial growth in such films.
Transparency
Table 2 represents the carnauba wax-based film’s transparency, which showed the significant effect among different treatments. Transparency is directly affected by the concentration of materials used during film development as some compounds are opaque, while some of them have thick colors that affect the transparency of the films. The opacity of the films increased substantially as a function of the wax type and concentration which can reduce the application of emulsion films with higher transparency. Different types of waxes showed varying levels of opacity in the developed films e.g., carnauba wax-based films are more opaque than candelilla wax, however, their differences are statistically alike. However, the presence of an emulsifier resulted in the reduction of the opacity which is related to the smaller particle size of the wax. Furthermore, keeping in mind that the better arrangement of lipid particles in the structure of biopolymer-based film expressed an effective barrier against the visible spectrum of UV-VIS light at 600 nm. This arrangement of lipid particles in the film structure plays a vital role while analyzing materials for transparency checks for specific food items. During film forming structure development, different droplet size arrangements occurred that may cause mechanisms such as creaming, flocculation, coalescence or aggregation, etc. These mechanisms can influence the homogeneity and light scattering properties of the films thus resulting in limited film transparency. Moreover, several studies have reported a decrease in film transparency of biopolymer films as a result of the addition of waxes (Haruna et al. 2019; Kowalzyck et al., 2016; Kowalzyck et al., 2014).
Santos et al. (2017) conducted a study to observe the influence of different concentrations of carnauba wax on the physicochemical properties of chitosan-based films. Chitosan films were prepared and evaluated by taking different concentrations (0, 15, 30, 40, and 50% w/w) of carnauba wax for optical and barrier properties. Results depicted that the opacity of the films increased significantly as the concentration of carnauba wax increased. Higher concentration (50%) showed opacity of around 10.5% more as compared to the control sample. Results described that as the concentration of carnauba wax increased in the film-forming solution the transparency of the film decreased momentously. Study outcomes are similar to the current study results as transparency of the film significantly decreased when the concentration of lipid compound increased in the film-forming mixture.
Scanning electron microscopy (SEM)
The carnauba wax-based film microstructure was analyzed qualitatively through the scanning electron microscopy technique to check the presence of lipids, biopolymers, and also the presence of surface-active agent tween-20. Figure 5 shows images of scanning electron micrographs for beeswax-based films for different treatments. Owing to variation in the composition of films structure different internal arrangements were observed. The films prepared with the addition of waxes showed discontinuities and roughness in structure owing to the hydrophobic nature and immiscibility of phases. The irregular structure of prepared films revealed the solid-state and the distribution of the droplet size in the film matrix. However, smaller particles of wax were noticed in the presence of emulsifier tween-20. Besides, the flotation mechanism explains the coalescence and aggregation of higher concentrations of lipid particles at the solvent evaporation surface. Floatation phenomena depend on the lipid concentration, particle size, viscosity of the emulsion solution, and the interfacial forces among the surface of the wax droplets. The film roughness is related to different mechanisms such as creaming, aggregation, or coalescence during film drying. Results of different research described that more irregularity in the film structure is directly related to the increased concentration of waxes in the film structure. Moreover, Jiménez et al. (2010) described that during the drying of the films solvent evaporation causes changes in the concentration, viscosity, aggregation, and creaming that affect the inner structure and physical properties of the films. Additionally, the increase in wax concentration enhances the discontinuities in the dried film matrix. The rough surface of the film is connected with the aggregation of wax and lipid recrystallization during film drying. However, the presence of wax crystals results in increased surface roughness. Similarly, the same observations have been noticed in the film structure prepared with the addition of waxes (Zhang et al. 2018; Chiumarelli and Hubinger 2014; Muscat et al. 2013).
Earlier, one of the peers, Galus et al. (2020) studied the effect of carnauba and candelilla wax incorporation on the physicochemical properties of sodium caseinate-based films. During film development, plasticizer (glycerol) and emulsifier (Tween-80) was also added. The results depicted that sample images showed heterogeneous structure even while lipid particle distribution was regular in the film-forming emulsion. Among treatments, different internal arrangement was observed due to change in the composition of the films. Films prepared with carnauba wax showed more irregular lipid organization as compared to candelilla wax in the film structure. The presence of emulsifiers and plasticizers in the films resulted in better dispersion of lipid particles in the film-forming emulsions.
Jiménez et al. (2012) reported that the coarse surface of the upper part of the film was due to the presence of larger droplet size. Likewise, if the droplet size in the film emulsion is smaller, then it would help to develop a fine surface film depending upon the nature of the constituents used for film formation. Furthermore, Atarés et al. (2010) described that the particle size of the emulsion impacts the microstructural characteristics and mechanical properties of the emulsified edible films. Small particles showed a droplet size of less than 1 μm. Sometimes the small particles aggregate and larger size droplets are formed. Likewise, it was also reported that a low concentration of lipids resulted in smaller droplet size and vice versa.
Mechanical Properties
Table 3 shows tensile strength, elongation at breakage, and young’s modulus. Results showed substantial variations among treatments. the tensile strength of lipid-based compounds decreased significantly as the concentration increased. Tensile strength is affected by the concentration of biopolymers as well because these interact with each other and helped to improve mechanical strength whereas the lipid-based component is decreasing the strength of the films.
Generally, lipid addition in film structure results in weaker mechanical properties and higher moisture barrier properties. The results of the current investigation are in accordance with the outcomes of Galus and Kadzińska (2015). They studied the mechanical strength of emulsified edible films as compared to the pure lipid film. Mostly, vegetable oils and some essential oils are used as a lipid-based covering of the hydrocolloids in the form of layering or a composite film. Waxes from lipid-based compounds are a good source for the development of emulsified edible films and coatings. The emulsification process allows providing good mechanical properties to the films by adding different types of emulsifiers.
Recently, a research experiment was conducted by Vieira et al. (2021). They developed chitosan and sodium alginate-based emulsion films. About, 2% w/w chitosan solution was dissolved in 1% sodium alginate solution. Lipids in different concentrations were used (25%, 50%, and 100% with biopolymers. To improve mechanical properties glycerol was also added up to 25% w/w. The effect of oil and lecithin agents was checked for mechanical and moisture barrier properties after drying the films. Oil concentration was maintained for a more plasticizing effect and increased elasticity, flexibility, and stretchability while with beeswax elasticity and flexibility decreased with respect to sodium alginate films. Pereda et al. (2012) explored the mechanical properties and moisture barrier properties of composite films developed from chitosan and olive oil. Different films were prepared from varying concentrations of lipid constituent and composite bilayer plasticized with 25% sorbitol on a dry basis. Mechanical properties were investigated at different relative humidity levels. Results depicted that increasing the concentration of chitosan and lipids decreases the young’s modulus and tensile strength while increasing elongation at breakage (%).
Moisture barrier properties
Water vapor permeability
Table 2 described that the water vapor permeability of carnauba wax-based films showed a momentous effect among different treatments. The addition of lipids in the film-forming solutions significantly reduced the water vapor permeability of biopolymer-based films. Nevertheless, biopolymer-based films containing lipids have lower water vapor permeability as compared to the films developed from the monolayer of the wax. This is just because monolayer wax-based film is more effective as compared to the heterogeneous biopolymer-based films with wax addition. Likewise, moisture transfer occurred only through the continuous phase which is the protein layer rather than lipids. At lower concentrations around 0.5 to 1%, there is no significant difference in moisture transfer of wax-based films. The significant differences can be noticed easily at higher concentrations of wax in the film-forming structure (Muscat et al. 2013). On the contrary, Zhang et al. (2018) noted a substantial resistance in the moisture barrier of gelatin-based carnauba wax at lower concentrations (0.5 and 0.75%). However, the better efficiency in water vapor permeability of the film is due to the presence of large droplet size and rough surface, respectively. The main purpose for the inclusion of lipids into biopolymer-based films is their lower moisture barrier properties due to their hydrophilic nature (Galus and Kadzińska 2015). Therefore, several research studies have been conducted to check the moisture barrier properties of different fats and oils, waxes, and resins based compounds in combination with biopolymers including whey protein isolates, pea starch, gelatin, chitosan, and cassava starch (Galus et al. 2020; Janjarasskul et al. 2014).
Previously, Fabra et al. (2010) checked the effect of moisture barrier properties of sodium caseinate films as influenced by the moisture content, composition, and concentration of lipid constituents. Prepared films were also influenced by the action of relative humidity and moisture content of the films. Results described that the lipid effect on moisture barrier properties depends on both ingredients and the moisture content in the films. The addition of lipid contents in the film increased moisture barrier properties as compared to the treatment without lipid contents. Films with more moisture contents resulted in lower water vapor permeability. Results also described that films with more moisture and lower lipid contents had lower moisture barrier properties.
The outcomes of Khwaldia (2010) described the moisture barrier and mechanical properties of the sodium caseinate and sodium caseinate-paraffin wax. The prepared films were applied on paper and their mechanical and moisture barrier properties were analyzed. The moisture barrier properties increased with an increase in the coating solution. A higher concentration of wax in the coating solution resulted in lower water vapor permeability of films. The concentration of the wax is inversely related to the moisture barrier properties of the films due to its hydrophobic nature.
Conclusion
This study has shown that both lipid and biopolymers have the potential to use in emulsion-based packaging. Emulsion-based films were developed by taking carnauba wax (0.5, 1.0 and 1.5%) and varying concentration of whey protein, and sodium alginate. Results depicted that the addition of carnauba wax improved the moisture barrier properties and water solubility of the films and 1.5% wax concentration showed the lowest value for WVP (3.12 ± 0.31 g.mm/kPa.h.m2) and WS (26.76 ± 1.01%). The concentration of wax affects the mechanical properties of the films and the maximum value for TS (3.92 ± 0.04 MPa), EB (13.11 ± 0.37%), and YM (363.33 ± 5.73 MPa), respectively. Nevertheless, the incorporation of appropriate concentration positively influenced the mechanical and moisture barrier properties. If emulsion-based packaging is prepared with suitable ingredients it will not only improve the mechanical and moisture barrier properties but also have the potential to enhance the shelf life of foods especially mangoes, bananas, and citrus. It will also help to launch the concept of eco-friendly, biodegradable, and cost-effective packaging. Change in wax concentration significantly (P < 0.05) influenced the film’s mechanical properties, so there is a need to work with different combinations and recent compounds to improve tensile strength and elongation at breakage properties with moisture barrier properties of films.
References
ASTM. 2016. Standard test methods for water vapor transmission of materials (E96/ E96M). Annual book of ASTM standards. Philadelphia: American Society for Testing and Materials
L. Atarés, J. Bonilla, A. Chiralt, Characterization of sodium caseinate-based edible films incorporated with cinnamon or ginger essential oils. J. Food Eng. 100, 678–687 (2010)
N. Brzoska, M. Mueller, L. Nasui, M. Schmid, Effects of film constituents on packaging-relevant properties of sodium caseinate-based emulsion films. Progress in Organic Coatings. 114, 250–258 (2018)
M.S.Á Cerimedo, C.H. Iriart, R.J. Candal, M.L. Herrera, Stability of emulsions formulated with high concentrations of sodium caseinate and trehalose. Food Res. Int. 43, 1482–1493 (2010)
M. Chiumarelli, M.D. Hubinger, Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocoll. 38, 20–27 (2014)
K. Crouvisier-Urion, A. Lagorce-Tachon, C. Lauquin, P. Winckler, W. Tongdeesoontorn, S. Domenek, F. Debeaufort, T. Karbowiak, Impact of the homogenization process on the structure and antioxidant properties of chitosan-lignin composite films. Food Chem. 236, 120–126 (2017)
L. Day, M. Xu, P. Hoobin, I. Burgar, M.A. Augustin, Characterisation of fish oil emulsions stabilised by sodium caseinate. Food Chem. 105, 469–479 (2007)
C.V. Dhumal, P. Sarkar, Composite edible films and coatings from food-grade biopolymers. J. Food Sci. Technol. 55, 4369–4383 (2018)
M. Fabra, A. Jiménez, L. Atarés, P. Talens, A. Chiralt, Effect of fatty acids and beeswax addition on properties of sodium caseinate dispersions and films. Biomacromolecules. 10, 1500–1507 (2009)
M. Fabra, R. Pérez-Masiá, P. Talens, A. Chiralt, Influence of the homogenization conditions and lipid self-association on properties of sodium caseinate-based films containing oleic and stearic acids. Food Hydrocoll. 25, 1112–1121 (2011)
M.J. Fabra, P. Talens, A. Chiralt, Influence of calcium on tensile, optical and water vapour permeability properties of sodium caseinate edible films. J. Food Eng. 96, 356–364 (2010)
S. Galus, J. Kadzińska, Food applications of emulsion-based edible films and coatings. Trends in Food Science and Technology 45, 273–283 (2015)
S. Galus, E.A. Arik Kibar, M. Gniewosz, K. Kraśniewska, Novel materials in the preparation of edible films and coatings-A review. Coatings. 10, 674–688 (2020)
S. Gupta, J. Ivvala, H. Grewal, Development of natural wax based durable superhydrophobic coatings. Industrial Crops and Products. 171, e113871 (2021)
M.H. Haruna, Y. Wang, J. Pang, Konjac glucomannan-based composite films fabricated in the presence of carnauba wax emulsion: Hydrophobicity, mechanical and microstructural properties evaluation. J. Food Sci. Technol. 56, 5138–5145 (2019)
B. Hassan, S.A.S. Chatha, A.I. Hussain, K.M. Zia, N. Akhtar, Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 109, 1095–1107 (2018)
S. Jafarzadeh, A. Salehabadi, A.M. Nafchi, N. Oladzadabbasabadi, S.M. Jafari, Cheese packaging by edible coatings and biodegradable nanocomposites; improvement in shelf life, physicochemical and sensory properties. Trends in Food Science & Technology. 116, 218–231 (2021)
T. Janjarasskul, D.J. Rauch, K.L. Mccarthy, J.M. Krochta, Barrier and tensile properties of whey protein–candelilla wax film/sheet. LWT-Food Sci. Technol. 56, 377–382 (2014)
M. Jouki, N. Khazaei, A. Jouki, Fabrication and characterization of an active biodegradable edible packaging film based on sesame seed gum (Sesamum indicum L.). J. Food Meas. Charact. 15, 4748–4757 (2021)
M. Jouki, F.T. Yazdi, S.A. Mortazavi, A. Koocheki, Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocoll. 36, 9–19 (2014)
A. Jiménez, M. Fabra, P. Talens, A. Chiralt, Effect of lipid self-association on the microstructure and physical properties of hydroxypropyl-methylcellulose edible films containing fatty acids. Carbohydr. Polym. 82, 585–593 (2010)
A. Jiménez, M.J. Fabra, P. Talens, A. Chiralt, Effect of re-crystallization on tensile, optical and water vapour barrier properties of corn starch films containing fatty acids. Food Hydrocoll. 26, 302–310 (2012)
S. Kandasamy, J. Yoo, J. Yun, H.B. Kang, K.H. Seol, H.W. Kim, J.S. Ham, Application of whey protein-based edible films and coatings in food industries: An updated overview. Coatings. 11, e1056 (2021)
V. Kanikireddy, K. Kanny, Y. Padma, R. Velchuri, G. Ravi, B. Jagan Mohan Reddy, M. Vithal, Development of alginate-gum acacia‐Ag0 nanocomposites via green process for inactivation of foodborne bacteria and impact on shelf life of black grapes (Vitis vinifera). J. Appl. Polym. Sci. 136, 47331 (2019)
M. Kaya, P. Ravikumar, S. Ilk, M. Mujtaba, L. Akyuz, J. Labidi, A.M. Salaberria, Y.S. Cakmak, S.K. Erkul, Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innovative Food Science and Emerging Technologies 45, 287–297 (2018)
K. Khwaldia, Water vapor barrier and mechanical properties of paper-sodium caseinate and paper‐sodium caseinate‐paraffin wax films. J. Food Biochem. 34, 998–1013 (2010)
D. Kowalczyk, B. Baraniak, Effect of candelilla wax on functional properties of biopolymer emulsion films–a comparative study. Food Hydrocoll. 41, 195–209 (2014)
D. Kowalczyk, W. Gustaw, E. Zięba, S. Lisiecki, J. Stadnik, B. Baraniak, Microstructure and functional properties of sorbitol-plasticized pea protein isolate emulsion films: Effect of lipid type and concentration. Food Hydrocoll. 60, 353–363 (2016)
X. Lian, Y. Xue, Z. Zhao, G. Xu, S. Han, H. Yu, Progress on upgrading methods of bio-oil: A review. Int. J. Energy Res. 41, 1798–1816 (2017)
T. Madera-Santana, Y. Freile-Pelegrín, J. Azamar-Barrios, Physicochemical and morphological properties of plasticized poly (vinyl alcohol)–agar biodegradable films. Int. J. Biol. Macromol. 69, 176–184 (2014)
S.A. Mohamed, M. El-Sakhawy, M.A.M. El-Sakhawy, Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 238, e116178 (2020)
M. Mohammadi, S. Mirabzadeh, R. Shahvalizadeh, H. Hamishehkar, Development of novel active packaging films based on whey protein isolate incorporated with chitosan nanofiber and nano-formulated cinnamon oil. Int. J. Biol. Macromol. 149, 11–20 (2020)
D. Muscat, R. Adhikari, S. Mcknight, Q. Guo, B. Adhikari, The physicochemical characteristics and hydrophobicity of high amylose starch-glycerol films in the presence of three natural waxes. J. Food Eng. 119, 205–219 (2013)
S. Paidari, N. Zamindar, R. Tahergorabi, M. Kargar, S. Ezzati, S.H. Musavi, Edible coating and films as promising packaging: A mini review. J. Food Meas. Charact. 15, 4205–4214 (2021)
S. Parreidt, T.K. Müller, M. Schmid, Alginate-based edible films and coatings for food packaging applications. Foods. 7, 170–208 (2018a)
S. Parreidt, T.M. Schott, M. Schmid, K. Müller, Effect of presence and concentration of plasticizers, vegetable oils, and surfactants on the properties of sodium-alginate-based edible coatings. Int. J. Mol. Sci. 19, 742 (2018b)
M. Pereda, G. Amica, N.E. Marcovich, Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 87, 1318–1325 (2012)
N. Purwanti, A.S. Zehn, E.D. Pusfitasari, N. Khalid, E.Y. Febrianto, S.S. Mardjan, Andreas, I. Kobayashi, Emulsion stability of clove oil in chitosan and sodium alginate matrix. Int. J. Food Prop. 21, 566–581 (2018)
D. Rees, G. Farrell, J. Orchard, Crop post-harvest: science and technology, vol. 3 (Perishables, John Wiley and Sons, 2012), pp. 1–464
H. Ritchie, M. Roser. 2018. Plastic pollution. Our World in Data. https://ourworldindata.org/plastic-pollution?utm_source=newsletter
D. Rousseau, Trends in structuring edible emulsions with Pickering fat crystals. Curr. Opin. Colloid Interface Sci. 18, 283–291 (2013)
K.S. Sandhu, L. Sharma, C. Singh, A.K. Siroha. 2017. Recent advances in biodegradable films, coatings and their applications. Plant biotechnology: Recent advancements and developments. Springer. pp. 271–296
T.M. Santos, A.M. Pinto, A.V. De Oliveira, H.L. Ribeiro, C.A. Caceres, E.N. Ito, H.M. Azeredo, Physical properties of cassava starch–carnauba wax emulsion films as affected by component proportions. Int. J. Food Sci. Technol. 49, 2045–2051 (2014)
Santos, F.K.G.D., K.N.D.O. Silva, T.D.N. Xavier, R.H.D.L. Leite and E.M.M. Aroucha. 2017. Effect of the addition of carnauba wax on physicochemical properties of chitosan films. Materials Research. 20:479-484
S. Singh, P. Khemariya, A. Rai, A.C. Rai, T.K. Koley, B. Singh, Carnauba wax-based edible coating enhances shelf-life and retain quality of eggplant (Solanum melongena) fruits. LWT-Food Sci. Technol. 74, 420–426 (2016)
Y. Song, Q. Zheng, Ecomaterials based on food proteins and polysaccharides. Polym. Reviews 54, 514–571 (2014)
A. Syarifuddin, P. Hamsiohan, M. Bilang. 2019. Characterization of edible film from dangke whey/pectin, beeswax, and butter aroma. AIP Conference Proceedings, 2019. AIP Publishing LLC, 020021. pp. 1–7
T.M. Vieira, M. Moldão-Martins, V.D. Alves, Design of chitosan and alginate emulsion-based formulations for the production of monolayer crosslinked edible films and coatings. Foods. 10, e1654 (2021)
S.M. Zahedi, M.S. Hosseini, M. Karimi, A. Ebrahimzadeh, Effects of postharvest polyamine application and edible coating on maintaining quality of mango (Mangifera indica L.) cv. Langra during cold storage. Food Sci. Nutr. 7, 433–441 (2019)
Y. Zhang, B.K. Simpson, M.J. Dumont. 2018. Effect of beeswax and carnauba wax addition on properties of gelatin fi Zahedi, S.M.,M.S. Hosseini, M. Karimi, A. Ebrahimzadeh. 2019. Effects of postharvest polyamine application and edible coating on maintaining quality of mango (Mangifera indica L.) cv. Langra during cold storage. Food Science and Nutrition. 7:433–441
O. Zuhal, Y. Yavuz, S. Kerse, Edible film and coating applications in fruits and vegetables. Alinteri J. Agric. Sci. 33, 221–226 (2018)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Butt, M.S., Akhtar, M., Maan, A.A. et al. Fabrication and characterization of carnauba wax-based films incorporated with sodium alginate/whey protein. Food Measure 17, 694–705 (2023). https://doi.org/10.1007/s11694-022-01636-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01636-3