Abstract
Purpose of Review
Bone disease is a defining characteristic of multiple myeloma (MM) and the major cause of morbidity. It manifests as lytic lesions or osteopenia and is often associated with severe pain, pathological fracture, spinal cord compression, vertebral collapse, and hypercalcemia. Here, we have reviewed recent data on understanding its biology and treatment.
Recent Findings
The imbalance between bone regeneration and bone resorption underlies the pathogenesis of osteolytic bone disease. Increased osteoclast proliferation and activity accompanied by inhibition of bone-forming osteoblasts leads to progressive bone loss and lytic lesions. Although tremendous progress has been made, MM remains an incurable disease. Novel agents targeting bone disease are under investigation with the goal of not only preventing bone loss and improving bone quality but also harnessing MM tumor growth.
Summary
Current data illustrate that the interactions between MM cells and the tumor-bone microenvironment contribute to the bone disease and continued MM progression. A better understanding of this microenvironment is critical for novel therapeutic treatments of both MM and associated bone disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With more than 30,300 new cases diagnosed in 2016, multiple myeloma (MM) is the second most common hematological cancer and is the 14th leading cause of cancer death in the USA (https://seer.cancer.gov/statfacts/html/mulmy.html). MM is a B cell malignancy characterized by clonal expansion of terminally differentiated, immunoglobulin-producing, transformed plasma cells in the bone marrow (BM) [1, 2]. The evolution of the disease is characterized by an asymptomatic premalignant state, monoclonal gammopathy of undetermined significance (MGUS), and smoldering MM [2]. Smoldering MM is an asymptomatic, precancerous form of myeloma with increased level of plasma cells in bone marrow and/or monoclonal protein but without evidence of end organ involvement. Clinically, MM manifests with hypercalcemia, renal failure, anemia, and/or bone disease (also known as the CRAB criteria). The progression of the disease from a premalignant state into active MM is characterized by the development of osteolytic bone disease (OBD) [3] as one of its disease-defining features. At least 85% of MM patients show some degree of osteopenia [4] at diagnosis, and the severity of bone destruction typically correlates with tumor burden and prognosis [2, 5].
OBD is a consequence of bone homeostasis perturbation. Bone homeostasis is physiologically maintained by the balance between bone formation and bone resorption which is mainly regulated by osteoblasts and osteoclasts, respectively. MM cells are known to secrete factors that enhance osteoclast proliferation and activity while inhibiting osteoblast bone formation [6]. Additionally, the myeloma-driven deregulation of the bone compartment creates a permissive microenvironment for MM cell expansion [7]. Since MM burden and progression tightly depend on the interactions of MM cells with the bone microenvironment, therapies aimed at restoring bone homeostasis targeting osteoblasts, osteoclasts, or both hold promise for the treatment of MM. Animal studies have shown that inhibition of osteoclast function or stimulation of bone formation can reduce tumor burden and prevent OBD [8, 9]. Here, we review bone metabolism in the context of MM disease progression and treatment strategies to mitigate the impact of OBD.
Defining the Cellular Interplay in Myeloma-Derived Bone Disease
Bone Marrow Mesenchymal Stromal Cells
An understanding of the cellular interplay between tumor and the microenvironmental compartment is critical to developing novel therapies for MM (Fig. 1). Bone marrow stromal cells (BMSCs) comprise of cells of hematopoietic (red and white blood cells) and mesenchymal origin (osteoprogenitors, osteoblasts, adipocytes, chondrocytes, endothelial cells, and fibroblasts) [10]. BMSCs indirectly participate in hematopoiesis by secreting cytokines, chemokines, and growth factors such as CXCL12, IL7, LIF, G-CSF, GM-CSF, and IL-6. These factors play a critical role in the maintenance, proliferation, and differentiation of hematopoietic stem and progenitor cells [10,11,12,13]. Mesenchymal cells originate from a mesenchymal stem cell (MSC) [14] via differentiation into lineage-specific cells, which is strictly regulated by physical, chemical, and spatial stimuli. B lymphocytes originate and differentiate in the BM, and studies in mice have reported the crucial role of bone cells during B lymphopoiesis and plasma cell recirculation [13]. For example, induced in vivo cellular deficiency of osteoprogenitors and preosteoblasts depletes the common lymphoid precursor cells and overall B cell populations in the BM [15], while deletion of the α subunit in the G protein arrests B cell differentiation at the pro-B cell stage [16]. Interestingly, deletion of the parathyroid hormone (PTH) receptor, a G-protein-coupled receptor in osteoprogenitors, arrests the differentiation of the pro-B cell into a mature B cell. It also impairs egress of immature B cells from the BM, resulting in increased accumulation of maturing B cells in the BM. This accumulation occurs specifically along the bone surface in part due to overexpression of the vascular cell adhesion molecule 1 (VCAM1) by stromal and bone cells [17].
MM cells influence the behavior of BMSCs through direct cell-cell contact and via secretion of paracrine factors. Adhesion of the myeloma tumor cells to BMSCs stimulates secretion of BMSC-derived factors (such as BAFF) that, in turn, upregulate expression of anti-apoptotic proteins (e.g., MCL-1 and BCL-2) and cell cycle regulating proteins (e.g., serine/threonine kinase Pim-2), which confer chemoresistance to MM cells [18,19,20,21]. Cell-cell interactions between BMSCs and MM cells are mediated via adhesion molecules, such as inter-cellular adhesion molecule 1 (ICAM-1) and VCAM-1 on BMSCs and very late antigen 4 (VLA-4) on MM cells. These interactions promote retention of MM cells within the BM and contribute to increased osteolytic bone lesions [22, 23, 24]. In addition to the MM-BMSC cell-cell interactions, secreted factors such as interleukin 6 (IL-6) are also critical promoters of MM progression [25]. Both MM and BMSCs secrete IL-6 upon stimulation of different signaling pathways including NF-kB and Notch [26,27,28,29]. They also secrete other inflammatory cytokines like TNF-α and IL-1β which can lead to OBD by activating osteoclasts, inhibiting osteoblasts, and increased IL-6 expression [30, 31]. Other factors secreted by both MM cells and BMSCs include vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF-1), IL-1, TGF-β, angiopoietin-1 (Ang-1), platelet-derived growth factor (PDGF), basic-fibroblast growth factor (bFGF), and hepatocyte growth factor (HGF) [32, 33]. These growth factors have been shown to be associated with increased angiogenesis, osteoclastogenesis, and tumor growth in MM [34]. MM patients with advanced bone disease also show higher levels of activin A in the blood [35, 36]. Activin A is an osteoclast-activating factor and inhibitor of osteoblast differentiation but is not secreted by MM cells [36]. Interestingly, MSCs from MM patients, but not healthy donors, were found to secrete activin A, suggesting a possible intrinsic genetic defect in BMSCs of MM patients [36]. Preclinical models showed that targeting activin A reversed osteoblast inhibition and improved MM bone disease. Furthermore, lenalidomide, an immunomodulatory drug commonly used in the treatment of MM, increases activin A secretion from BMSCs, leading to inhibition of osteoblastogenesis. The combination of lenalidomide with activin A-neutralizing antibody could represent a new strategy in managing MM bone disease [37]. BMSCs of MM patients were found to have abnormal gene expression with decreased osteoblastic differentiation potential and aberrantly secrete growth differentiation factor 15 (GDF15), which supports myeloma stem cell survival and self-renewal [38,39,40,41]. Together, these findings highlight the crucial role of the BM microenvironment in the establishment and progression of MM [39, 40, 42, 43].
Drugs currently used in the clinic, like the proteasome inhibitors, can target the cross talk between MM and BMSCs. The proteasome inhibitor bortezomib inhibits MM cell growth by impairing MM cell adhesion to BMSCs and by reducing the secretion of cytokines necessary for MM survival [44]. Bortezomib acts on MSCs directly to increase osteoblast differentiation [45]. Another strategy to decrease MM cell adhesion to stroma involves the inhibition of CXCL-12/CXCR-4 axis, which is reinforced by VLA-4/VCAM-1 interaction, by using CXCR4 inhibitor AMD3100. Mobilization [36] of MM cells was found to enhance their sensitivity to chemotherapy [46].
Osteolineage Cells
Cells of the osteoblast lineage are the main actors in mineral bone apposition and thus primarily affected in MM bone disease. Differentiation of MSCs into bone cells is regulated by activation of two major transcription factors: runt-related transcription factor 2 (RUNX2) and osterix (OSX) [47]. These transcription factors, essential for the maturation of osteoblasts and ossification, rely on the canonical Wnt signaling pathway. The binding of Wnt proteins to frizzled (FZ) family receptors and lipoprotein receptor-related protein (LRP)-5/6 coreceptors forces the release of β-catenin from the cytosolic destruction complex and is internalized in the nucleus, where it acts as coactivator of transcription factors that belong to the TCF/LEF family [48••]. However, Wnt signaling is inhibited by a variety of Wnt antagonists such as dickkopf-1 (DKK1), sclerostin (SOST), and secreted frizzled-related proteins (sFRPs) that compete for the same Wnt coreceptors [48••]. MM cells produce a variety of Wnt antagonists including DKK1, sRFP2, and sRFP3 [49,50,51]. High levels of DKK1 have been found in the BM and blood of MM patients and have been correlated with the presence of focal bone lesions [49]. In vitro and in vivo studies have shown that blocking DKK1 and increasing Wnt signaling restored osteoblast number and trabecular bone while decreasing tumor burden [9, 52,53,54]. A phase IB trial of an anti-DKK1 antibody, BHQ880, in relapsed/refractory MM showed a general trend towards increased BMD, though interpretation is limited given that BHQ880 was given with zoledronic acid [55].
MM cells have been reported to also secrete SOST [56]. Although we and others were not able to detect SOST secretion from MM patient cells or MM cell lines [57], the level of circulating SOST in MM patients is significantly higher and correlates with advanced MM disease stage and fractures [58, 43]. Osteocytes are responsible for SOST secretion but the number of osteocytes in MM patients is drastically reduced because of osteolytic lesions. We have recently reported that MSCs of MM patients aberrantly secrete SOST, and this may, at least in part, account for the increased circulating SOST levels in MM patients [43, 57]. In vivo treatment of MM-bearing mice with SOST neutralizing antibody showed reduced bone loss and osteolytic lesions, although no effect was seen on tumor burden [59•]. However, the source of SOST secretion and its effects on MM and tumor microenvironment remain under investigation. Together, these results indicate that rescuing osteoblast activity and increasing bone formation in MM patients can be a potential tool to inhibit MM tumor growth and improve MM bone disease.
Adipocytes
Although BM adipocytes have not been directly implicated in MM bone disease, emerging evidence show that adipocytes support MM growth and survival which negatively correlate with bone mass. Adipocyte and osteoblast lineage commitment, arising from common progenitor MSCs, are tightly regulated events in BM. Wnt signaling is a major promoter of osteoblast lineage while suppressing adipocyte lineage commitment regulated by PPARγ signaling pathway [60, 61]. Moreover, marrow adiposity increases with age, from 30% of the BM volume in young adults to 70% in elderly people [62••]. Conversely, bone formation rates decrease with aging.
Obesity is positively correlated with increased risk of developing MM and is associated with higher levels of BM adiposity [63,64,65]. Compared to patients with MGUS, patients with MM had higher abdominal fat cross-sectional area and higher fat metabolic activity as demonstrated by FDG-PET/computed tomography (CT) [66]. A recent in vivo study demonstrated that diet-induced obesity promoted a MM-like syndrome in mice, suggesting that even an unhealthy diet may be implicated in the initiation of the tumor [67•]. In vitro, BM adipocytes have been shown to support MM growth and survival by secreting adipocyte-specific cytokines (adipokines) (leptin, insulin, adipsin, resistin, etc.) and growth factors [IL-1β, IL-6, IL-10, IL-12, TNFα, monocyte chemoattractant protein 1 (MCP-1), IGF-1, VEGF, SCF, bFGF] [68,69,70,71,72] and protecting MM cells against chemotherapy-induced apoptosis through autophagy activation [73].
BM adipocytes also secrete lipids in the form of free fatty acids that can be used as energy for tumor cell proliferation [74]. Increased levels of free fatty acids were found in the serum of MM patients when compared to healthy donor serum [75]. Although some specific fatty acids resulted in anti-myeloma effects [76, 77], a recent study implicated lysolipids in the origin of monoclonal gammopathies, including MGUS and MM [78••]. They reported that lysolipids act as antigens for plasma cell-derived antibodies and reactivity to this category of lipids accounts for 33% of sporadic human monoclonal gammopathies. The results suggest that extensive immune activation due to lysolipids could potentially represent an initiation factor in sporadic monoclonal gammopathies and could represent novel biomarkers and targets to prevent disease progression. Many lipids also act as ligands for the nuclear receptors involved in PPARγ signaling pathway. Therefore, increased levels of free fatty acids in MM patients likely lead to upregulation of PPARγ signaling, which in turn enhances BM adipogenesis and MM survival while inhibiting osteoblast differentiation. A recent in vitro study demonstrated the direct lipotoxic effect of saturated fatty acids, stearate and palmitate, on human osteoblasts [79, 80]. Data also demonstrated that augmented adipogenesis during obesity and aging impaired bone regeneration [81]. Taken together, BM fat is emerging as an important regulator of MM development and bone disease and, therefore, may represent a new promising therapeutic target.
Osteoclasts
The pathogenesis of MM-induced bone disease underlies primarily in upregulation of osteoclast differentiation and activity that results in unbalanced bone resorption giving rise to characteristic osteolytic lesions [82]. Positive correlations were found among osteoclast numbers, resorptive surface, and disease progression [5, 83, 84]. Several factors mediate osteoclast activation in MM patients. MM cells directly secrete cytokines with osteoclastogenic potential, including IL-1, IL-3, IL-6, TNFα, MIP-1α, MIP-1β, and decoy receptor 3 (DcR3) [85,86,87,88,89,90].
MSCs and bone cells are also a source of osteoclast-activating cytokines. In addition to BAFF, activin A, CXCL12, IL-6, and VEGF, two proteins produced by bone cells play a central role in osteoclast differentiation: the receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegerin (OPG) [91,92,93]. Adhesion of MM cells to BMSCs stimulates RANKL expression by osteoblasts which promote osteoclast differentiation through stimulation of NF-κB and JunN-terminal kinase pathways [94]. In addition, RANKL is able to inhibit osteoclast apoptosis. Denosumab is a human monoclonal antibody that binds to RANKL with high affinity resulting in rapid and extensive bone resorption inhibition but also MM burden modulation [95]. Denosumab is approved for the treatment of bone metastases due to solid tumors (see below). OPG is significantly decreased and correlates with advanced bone disease in MM patients [96]. A high RANKL/OPG ratio is associated with a worse prognosis [5]. Treatments aimed to normalize the RANKL/OPG ratio by increasing OPG and/or reducing RANKL were effective in arresting bone destruction and MM growth in vivo [97,98,99,100].
High levels of the pro-inflammatory cytokine MIP-1α, mainly secreted by MM cells and osteoclasts, correlate with bone disease and poor prognosis [101]. MIP-1α promotes MM cell survival and migration through activation of ERK and AKT signaling, stimulates osteoclastogenesis, and inhibits osteoblastic differentiation by downregulating osterix [102,103,104]. Similar to MIP-1α, high expression of Bruton’s tyrosine kinase (BTK) in MM cells and osteoclasts, fundamental to B-lymphocyte development as well as osteoclast differentiation, has been implicated in MM cell growth and increased bone resorption [105, 106]. The BTK inhibitor ibrutinib demonstrated cytotoxicity on MM cells and was effective in reducing osteolysis through inhibition of the NF-κB pathway [107, 108]. The combination of the Btk inhibitor, CC-292, with the proteasome inhibitor carfilzomib in vivo showed a synergistic effect in reducing the tumor burden and increasing bone volume [109]. In addition to their fundamental role in bone turnover, osteoclasts are implicated in a variety of functions [110, 111]. Osteoclasts are terminally differentiated cells of the monocyte/macrophage lineage and as all cells of the innate immune system, share similar immune receptors and activities. They interact directly with T lymphocytes and activate them in the context of autoimmune disease [112]. Moreover, while T cells produce potent osteoclastogenic cytokines (RANKL and IL-1β), osteoclasts are able to suppress T lymphocyte proliferation in order to maintain a balance in bone remodeling [113]. Cells of the myeloid lineage, including macrophages, dendritic cells, and other myeloid-derived suppressor cells (MDSCs), have been implicated in the establishment of a protective environment for MM cells by suppressing killer T cells and preventing chemotherapy-induced apoptosis [114,115,116]. A recent study demonstrated that osteoclasts protect MM cells against T cell-mediated cytotoxicity via direct inhibition of proliferating T cells [117•]. Because defective T cell-mediated response is a key mechanism for tumor escape from immunological surveillance [118], the immunosuppressive function of osteoclasts and their crucial role in osteolytic disease need to be considered primary targets in the treatment of MM.
Imaging of Multiple Myeloma
Traditionally, imaging using plain films, i.e., the skeletal survey, has been used for the initial assessment of bone disease [119]. Conventional radiography has the advantage of cost and widespread availability. However, a significant limitation is that 30–50% of bone can be destroyed before it can be detected by this modality [120]. Cross-sectional imaging by CT, MRI, or PET-CT have increased sensitivity and are increasingly used for assessing bone disease with the aim of detecting disease at an earlier time point [121].
Whole-Body Low-Dose CT
CT overall has a 4–33% higher detection rate for picking up lytic lesions compared to conventional radiography [122]. To take advantage of the increased sensitivity of CT and to minimize radiation exposure, whole-body low-dose CT (WBLDCT) protocols have been developed [123]. The radiation exposure from WBLDCT is minimal, about double that of the skeletal survey ((4.1 versus 2.4 mSV) [124] versus an estimated 21 mSV for conventional chest, abdomen, and pelvic CT [125]). WBLDCT is more sensitive than conventional radiography for detecting lesions in the spine, pelvis, and thoracic cage, and in one study, led to change in treatment in 18.2% patients [126] and in another study, detected lytic lesions in an additional 23% of patients with plasma cell disorders [127]. In a recent retrospective study by the International Myeloma Working Group, 21.4% of patients who were considered to have smoldering multiple myeloma by skeletal survey were upstaged to active multiple myeloma through WBLDCT [128]. In addition to increased sensitivity, WBLDCT has the advantages of speed and convenience and has replaced the skeletal survey at some European institutions [124].
MRI
MRI plays a key role in the imaging of MM due to its sensitivity for detecting BM involvement in contrast to conventional radiography or CT, which are better suited for detecting lytic lesions [129]. Several patterns of bone marrow involvement have been described, including a normal appearance, focal involvement, diffuse infiltration, and a variegated pattern [130]. MRI is also more sensitive than plain films for detecting lesions; for example, MRI detected lesions in 74% of MM patients compared to 56% by skeletal survey. In the spine, MRI revealed more lesions than the skeletal survey by conventional radiography, 78 versus 16% [131]. This is clinically relevant, as identification of more than one focal lesion by MRI in patients traditionally classified as smoldering MM is associated with a high risk of progression to active disease: 70% progression in 2 years [132]. Furthermore, MRI findings change in response to treatment; there was a strong relationship between response to treatment and changes in diffuse and focal patterns in one study [133]. Due to the prognostic relevance of findings on MRI, the definition for active multiple myeloma has been recently updated to include more than one focal lesion (≥5 mm) seen on MRI [134].
PET/CT
Functional imaging using 18fluorodeoxyglucose (18F-FDG) PET/CT has also emerged as a valuable tool for assessing bone disease, particularly assessing the burden of disease and for distinguishing between active and inactive lesions. It already is a standard component for diagnosis and assessment of response in patients with lymphoma and solid tumors. The IMWG has recently described guidelines for its use [135]. An advantage of PET/CT over MRI is the ability to evaluate disease outside the field of view of MRI as well as evaluating extramedullary sites of disease. PET/CT plays an important role in staging plasma cell dyscrasias, for example, confirming a solitary site of disease in solitary plasmacytoma versus active multiple myeloma. Recently, the Intergroupe Francophone du Myélome (IFM) compared PET/CT and MRI in a prospective randomized study in newly diagnosed MM patients [136]. This study found that normalization of PET/CT following induction treatment was associated with significant improvement in progression-free survival. In contrast, normalization of MRI did not have prognostic value. This suggests that PET/CT may play a larger role in the future for assessing response to treatment and be a potential prognostic factor.
Bisphosphonates
Bisphosphonates are a cornerstone of treating and preventing bone disease in MM [137]. Bisphosphonates are related to pyrophosphate and are characterized by a P-C-P backbone. They deposit in bone and reduce osteoclast activity by inhibiting farnesyl pyrophosphate synthase [138]. In MM, the development of higher-potency bisphosphonates such as pamidronate and zoledronic acid, which are given intravenously, was a significant advance for relieving pain due to bone disease and preventing skeletal-related events (SREs) such as pathologic fracture, irradiation or surgery on bone, or spinal cord compression [139,140,141]. Pamidronate was the first bisphosphonate to show a benefit in MM. In patients with at least one osteolytic lesion, pamidronate given every 4 weeks significantly reduced SREs compared to placebo, 24 versus 41% (p < 0.001) after 9 cycles, respectively [139] (Table 1). Importantly, pamidronate also significantly improved quality of life, with decreases in pain scores seen within a month. Zoledronic acid is another bisphosphonate that is also given monthly and is more potent than pamidronate. For treatment of hypercalcemia of malignancy, zoledronic acid was superior to pamidronate [142]. In MM, the efficacy of zoledronic acid in preventing SREs was comparable to pamidronate [143], though zoledronic acid has the main practical advantage of a shorter infusion time compared to pamidronate.
In addition to playing an important supportive role, bisphosphonates may have an anti-MM effect. The MRC Myeloma IX trial is a key trial that compared zoledronic acid to oral clodronate in patients with newly diagnosed MM [144••]. This study found that zoledronic acid reduced mortality by 16% and had superior overall survival, 50 versus 44.5 months with clodronate (p = 0.04). There also was a lower incidence of SREs with zoledronic acid, 27 versus 35% (p = 0.0004) compared to clodronate [145]. Moreover, patients who did not have bone lesions at baseline also had fewer SREs with zoledronic acid [145], though the benefit in survival was seen only in patients with bone disease on study entry [146].
Based on these findings, the National Comprehensive Cancer Network [147] and the International Myeloma Working Group [148] recommend bisphosphonates (pamidronate or zoledronic acid) for all patients with symptomatic MM. The IMWG guidelines note that the optimal duration of bisphosphonate treatment has not been determined for patients who achieve at least a very good partial response; in these patients, bisphosphonates should be given for at least 12 months and up to 24 months. For patients who achieve less than a VGPR, the group recommended treatment until disease progression and further continued at relapse.
Osteonecrosis of the Jaw
Osteonecrosis of the jaw (ONJ) is one of the most serious adverse events of bisphosphonates [149, 150]. ONJ is traditionally defined as exposed, necrotic bone in the jaw that does not heal after 8 weeks, and it is generally painful. The highest risk of ONJ occurs with zoledronic acid due to its increased potency, and initial studies showed an incidence of 4–11% which increases with duration of exposure [151, 152]. In the Myeloma IX trial, the cumulative incidence of ONJ was 3–4% at a median follow-up of 3.7 years [153].
A major risk factor for ONJ is dental extraction [152, 154]. Attention to dental hygiene and minimizing invasive procedures (such as tooth extractions or dental implants) may reduce the risk of ONJ [155]. IMWG guidelines recommend holding bisphosphonate therapy before and after invasive dental procedures for 90 days (though routine dental cleanings and procedures, including root canals, may occur) [148]. Treatment of ONJ is supportive. After healing has occurred, the IMWG recommends resuming bisphosphonate treatment.
Atypical Femur Fracture
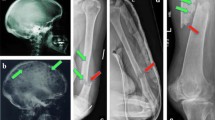
Atypical femur fractures have been better appreciated in patients undergoing oral bisphosphonate therapy for osteoporosis, but are now being seen in MM patients treated with pamidronate or zoledronic acid [156]. These represent isolated cases, as the incidence of atypical femur fracture remains to be determined, as these femur fractures are typically assumed to be pathologic.
Renal Toxicity
Nephrotoxicity is another limiting factor in bisphosphonate use [157]. Bisphosphonates are excreted unchanged in the urine, and impaired renal function can lead to accumulation of the drug. With zoledronic acid, the risk of kidney injury (in this case defined as a rise in creatinine of 0.5 mg/dL) was appreciated in the initial phase III trials, prompting a dose reduction from 8 to 4 mg and increase in the duration of the infusion from 5 to 15 min [140]. Acute tubular necrosis is the main pathology observed. The American Society of Clinical Oncology provides guidelines for the dosing of zoledronic acid in patients with renal impairment [158]. Notably, zoledronic acid is not recommended in patients with severe renal impairment (creatinine clearance <30 mL/min). In the Myeloma IX trial, the rate of acute renal failure was 5–7% in the zoledronic acid arm, similar to 6% in the clodronate arm [144••].
Effect of Myeloma Therapy on Bone Disease
Another key component in managing the bone disease is an effective treatment of the underlying disease. The landscape of MM treatment has changed dramatically in the past decade, with the introduction of two highly effective classes of drugs, immunomodulatory drugs (e.g., lenalidomide, pomalidomide) and proteasome inhibitors (e.g., bortezomib, carfilzomib), along with the recent approval of drugs with novel mechanisms of action, such as the monoclonal antibody targeting CD38 (daratumumab) [159]. The use of these novel agents has been responsible for significant improvements in overall survival [160, 161], and patients diagnosed more recently in 2012 were 25% more likely to survive to 2 years than those diagnosed only 6 years previously [162].
Improvements in treatment have also correlated with improvements in bone-related outcomes. For example, in a subset analysis of the previously described Myeloma IX trial (which randomized patients to zoledronic acid versus pamidronate), the frequency of SREs was lower by 26% in patients randomized to the more effective treatment arm with cyclophosphamide, thalidomide, and dexamethasone compared to the melphalan and prednisone arm [146]. Additionally, the depth of response may influence the impact of bisphosphonate therapy. In a retrospective analysis of the Myeloma IX trial, in patients who have undergone autologous stem cell transplant, the benefit in SRE and overall survival of zoledronic acid versus clodronate was mainly seen in patients who did not achieve a complete response [163].
Moreover, the proteasome inhibitor class has been shown to have bone anabolic effects. Preclinical studies show that proteasome inhibitors such as bortezomib and carfilzomib stimulate osteoblast growth and differentiation and inhibit osteoclast activity [164, 165]. An earlier study showed that patients receiving the combination of bortezomib and dexamethasone (and who also received zoledronic acid) showed an improvement in lumbar spine bone mineral density [166]. In the phase III VISTA study comparing the combination of bortezomib (Velcade) with melphalan and prednisone (VMP) versus melphalan and prednisone (MP) in newly diagnosed transplant-ineligible patients, bone disease and need for radiation therapy was less frequent in the bortezomib arm [167]. There was also radiological evidence of bone healing in some of the patients treated with VMP (versus none of the patients treated with MP) where data was available. Similarly, consolidation with bortezomib, thalidomide, and dexamethasone following autologous stem cell transplant was shown to reduce bone resorption and was associated with a very low incidence of SREs; notably, bisphosphonates were not used [168]. However, in a recent phase II study specifically investigating the effect of consolidation with bortezomib versus observation on bone disease as a primary endpoint in patients who had a partial response or better after autologous stem cell transplant did not see an effect on bone mineral density [169]. Interpretation of these findings is limited as a substantial proportion of patients in both arms previously received bortezomib upfront, and zoledronic acid use was significantly higher in the control arm.
In contrast, the beneficial effect of lenalidomide, a widely used immunomodulatory drug, on bone disease is less certain. A retrospective study of treatment with lenalidomide and dexamethasone in relapsed disease showed that reduction of bone resorption biomarker CTX (carboxy-telopeptide fragments of collagen type-I α1 chains) only occurred in patients responding to treatment, and the treatment did not have any effect on bone formation [170]. On the other hand, the addition of bortezomib to lenalidomide and dexamethasone was associated with increased bone formation markers. The lack of apparent benefit of lenalidomide on bone formation may be mediated by activin A, a member of the TGF-β superfamily that is most commonly associated with embryogenesis and gonadal hormone signaling, but is also involved in bone remodeling and osteoclast formation. This has motivated the study of inhibiting activin A with sotatercept (ACE-011), an activin type IIA receptor fusion protein that binds to activin A, in combination with standard treatment with melphalan, prednisolone, and thalidomide [171] or lenalidomide or pomalidomide in patients with relapsed MM (NCT01562405) [172].
Denosumab
A newer agent for managing bone disease is denosumab. Denosumab is a fully human monoclonal antibody given subcutaneously that targets RANKL. As described above, RANKL is a cytokine produced by osteoblasts that activates the RANK receptor present on osteoclast precursors and osteoclasts and promotes the formation, function, and survival of osteoclasts [173]. Since it is a monoclonal antibody, unlike bisphosphonates, denosumab does not accumulate or persist in bone. It has a circulatory half-life of approximately 26 days, and like other monoclonal antibodies, the clearance of denosumab is through the reticuloendothelial system and importantly does not depend on renal clearance [174].
Like zoledronic acid, denosumab is approved for the prevention of SREs in patients with bone metastases due to solid tumors based on several phase III clinical trials [175]. In these studies, patients were randomized to denosumab 120 mg subcutaneously versus zoledronic acid 4 mg intravenously (or equivalent creatinine clearance adjusted dose) every 4 weeks. In two of the studies, denosumab was superior to zoledronic acid in delaying SREs [176, 177]. Similar to zoledronic acid, the risk of ONJ was seen in about 1.8% of patients [178].
However, in a third study, the 244 study, about 10% of the population were MM patients, and an ad hoc analysis showed that survival was unexpectedly worse in the MM cohort treated with denosumab compared to zoledronic acid [179]. Consequently, denosumab is not approved in MM (at the time of this writing). However, conclusions from the study about the MM cohort are limited [180]. Given the small number of MM patients, there were imbalances between the study arms that favored the zoledronic acid arm; the denosumab arm had more patients with renal dysfunction, and the zoledronic acid arm patients were treated more often with novel agents and autologous stem cell transplantation. In addition, there was unequal early withdrawal censoring that may have favored the zoledronic acid arm. These findings confounded the interpretation of outcomes in the MM cohort.
To address these limitations in the 244 study, a larger phase III study enrolling only patients with MM was recently conducted, and presented at the International Myeloma Workshop [181••]. This is the largest international study in MM, randomizing 1718 newly diagnosed patients with at least one bone lesion to denosumab versus zoledronic acid, both given according to standard q4 week schedules. Patients were stratified by type of therapy, intent for autologous stem cell transplant, ISS stage, and region. The primary endpoint was time to SRE, defined as pathologic fracture (vertebral or non-vertebral), need for radiation therapy or bone surgery, or spinal cord compression. In this study, denosumab was non-inferior to zoledronic acid (22.83 versus 23.98 months); overall survival was similar in both arms. Interestingly, progression-free survival, an exploratory endpoint, was longer in the denosumab arm (46.09 months) compared to the zoledronic acid arm (35.38 months), p = 0.036. Adverse events of interest include ONJ, which was 4.1% in the denosumab arm compared to 2.8% in the zoledronic acid arm. Importantly, the incidence of renal toxicity was significantly lower with denosumab, 10 versus 17.1% (p < 0.001), especially in patients with renal insufficiency at baseline (creatinine clearance ≤60 mL/min), 12.9 versus 26.4%, respectively. Additionally, there were fewer acute phase reactions in the denosumab group, 5.4 versus 8.7%. Overall, these findings may change practice, given that kidney dysfunction is common in MM and often presents a major barrier to effective use of osteoclast-targeted therapy. Indeed, denosumab may be able to play a key role for managing and preventing bone disease in this challenging patient population. The improved PFS observed in the denosumab arm warrants further evaluation.
Vertebroplasty and Kyphoplasty
Vertebroplasty (injection of methyl methacrylate or bone cement) and kyphoplasty (use of an inflatable balloon followed by instillation of bone cement) are percutaneous procedures for treating compression fractures, which are common in MM [182, 183]. The Cancer Patient Fracture Evaluation study randomized 134 patients with malignancy and at least one vertebral compression fracture to balloon kyphoplasty versus non-surgical management [184]. Forty percent of the patients in the study had MM, and at 1 month, there was significant improvement in back pain and quality of life. A recent retrospective series showed durable improvement in disability which persisted at 1 year and also noted better outcomes in patients after treatment was initiated [185]. These procedures can play a key adjunctive role for managing bone-related pain.
Palliative Radiation Therapy
Radiation also plays an important role for palliation of painful bony lesions in MM. An estimated 38% of patients are expected to receive radiation over the course of their illness [186], and this figure remains similar in the era of novel treatments [187]. The primary indication for the use of radiation therapy is treating bone pain, and other indications include impeding fracture, cord compression, or relief of symptoms associated with a mass (i.e., cranial nerve palsies or organ or joint dysfunction). Minimal doses of radiation can be used, given the radiosensitivity of plasma cell lesion [188]. Doses of 20–35 Gy can be used, but it is essential to consider ability to retreat when designing treatment fields, particularly of the spine. In our experience, we have found that 20 Gy delivered in either five or ten fractions provide adequate symptom relief [189]. A retrospective series found that 76.4% of patients reporting complete relief of pain with palliative radiation with a median dose of 30 Gy [187].
Ongoing Areas of Study
An ongoing question is the amount of bisphosphonate necessary for improving bone disease. This is especially true given that current MM therapies are increasingly more effective than when the initial studies with bisphosphonates were conducted as well as concerns for adverse events such as ONJ with chronic exposure.
A lower dose of pamidronate was evaluated by the Nordic Myeloma Study group, which compared a 30 mg dose of pamidronate versus the standard dose of 90 mg in a double-blind, randomized study [190]. The rates of SREs were similar, and there was a trend towards less adverse events in the 30 mg dose group with fewer episodes of ONJ and nephrotoxicity.
With zoledronic acid, its monthly dosing schedule is based on suppression of urine N-telopeptide of type I collagen (NTX), a biomarker for bone resorption, for 4 to 8 weeks after a single dose of zoledronic acid [191, 192]. To further understand the duration of bone resorption suppression, a study measured urine NTX in 29 patients who achieved at least a partial response and who previously received bisphosphonate therapy. In these patients, urine NTX (uNTX) continued to be suppressed in nearly all these patients for 6 months after a single dose of zoledronic acid, and this correlated with freedom from SRE [193], suggesting that the zoledronic acid could be given less frequently.
These findings were further supported in the Z-MARK study, which evaluated less frequent zoledronic acid dosing based on urine NTX levels in patients who had already received at least 1 year of bisphosphonate therapy [194]. For patients where uNTX <50 nmol/mmol creatinine, zoledronic acid was given every 12 months; otherwise, it was given every 4 weeks. This study enrolled 121 patients, and nearly all patients, 117 patients, were assigned to the q12 week arm. The SRE rate was low, 5.8% in the first year and 4.9% in the second year, and the 2-year incidence of ONJ was 3.3%. These findings suggest that less frequent dosing is feasible with low risk of SRE.
Evaluation of less frequent dosing has been evaluated in a phase III study, CALGB 70604, which randomized bisphosphonate-naïve metastatic breast cancer and prostate cancer, and MM patients with at least one bone lesion to every 12 week dosing of zoledronic acid versus every 4 week dosing [195]. In the MM cohort, which comprised 15.3% of the study, the SRE rate was similar; 55% of patients had an SRE in the every 12 week arm versus 60% in the every 4 week arm. In the overall group, the rate of kidney dysfunction, defined as increase ≥0.5 mg/dL over baseline if baseline creatinine ≤1.4 or ≥1 mg/dL if baseline >1.4 mg/dL, was higher in the every 4 week arm, 19.9 versus 15.5% (p = 0.02). The ONJ rate in the overall cohort trended towards higher in the every 4 week arm, 2 versus 1% (p = 0.08). A limitation of the study is the high dropout rate due to patient withdrawal, with only 47% of patients with two or more years of treatment. Overall, this study adds support for every 12 week dosing, though the effect of this approach on overall survival remains to be determined.
Finally, patients with severe kidney dysfunction (creatinine clearance <30 mL/min) have an unmet need for managing bone disease, as current guidelines do not recommend use of zoledronic acid. Denosumab, given its lack of nephrotoxicity and lack of dependence on renal clearance, is currently being evaluated in a phase II study in this patient population (NCT02833610).
Conclusions
Here, we discuss the complexity of the cross talk between MM and the surrounding BM stromal microenvironment. Although the overall survival and quality of life of MM patients have significantly improved due to the introduction of novel therapeutic strategies, MM remains an incurable disease. MM progression and resistance tightly depend on the interactions with the stromal compartment; hence, treatments targeting this interplay are fundamental for the cure of MM and osteolytic bone disease. Osteoclast-targeted therapy with bisphosphonates has become an integral part of supportive therapy and plays a significant role in addressing the bone disease. Newer drugs, such as denosumab and other bone anabolic agents, are currently under investigation and may represent promising tools in the treatment of MM-derived bone disease. Further understanding of the MM stromal microenvironment and the development of novel agents targeting them are therefore needed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351(18):1860–73. doi:10.1056/NEJMra041875.
Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–60. doi:10.1056/NEJMra1011442.
Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. J Clin Oncol Off J Am Soc Clin Oncol. 2005;23(15):3412–20. doi:10.1200/JCO.2005.04.242.
Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. doi:10.4065/78.1.21.
Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J, et al. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood. 2003;102(3):1064–9. doi:10.1182/blood-2003-02-0380.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37. doi:10.1038/nm.3394.
Podar K, Chauhan D, Anderson KC. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2009;23(1):10–24. doi:10.1038/leu.2008.259.
Choi SJ, Oba Y, Gazitt Y, Alsina M, Cruz J, Anderson J, et al. Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. J Clin Invest. 2001;108(12):1833–41. doi:10.1172/JCI13116.
Edwards CM, Edwards JR, Lwin ST, Esparza J, Oyajobi BO, McCluskey B, et al. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111(5):2833–42. doi:10.1182/blood-2007-03-077685.
Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20(8):833–46. doi:10.1038/nm.3647.
Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–34. doi:10.1038/nature12984.
Panaroni C, Tzeng YS, Saeed H, Wu JY. Mesenchymal progenitors and the osteoblast lineage in bone marrow hematopoietic niches. Curr Osteoporos Rep. 2014;12(1):22–32. doi:10.1007/s11914-014-0190-7.
Panaroni C, Wu JY. Interactions between B lymphocytes and the osteoblast lineage in bone marrow. Calcif Tissue Int. 2013;93(3):261–8. doi:10.1007/s00223-013-9753-3.
Fridenshtein A, Petrakova KV, Kuralesova AI, Frolova GI. Precursor cells for osteogenic and hemopoietic tissues. Analysis of heterotopic transplants of bone marrow. Tsitologiia. 1968;10(5):557–67.
Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103(9):3258–64.
Wu JY, Purton LE, Rodda SJ, Chen M, Weinstein LS, McMahon AP, et al. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105(44):16976–81.
Panaroni C, Fulzele K, Saini V, Chubb R, Pajevic PD, Wu JY. PTH signaling in osteoprogenitors is essential for B-lymphocyte differentiation and mobilization. J Bone Miner Res. 2015;30(12):2273–86. doi:10.1002/jbmr.2581.
Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC. Advances in biology of multiple myeloma: clinical applications. Blood. 2004;104(3):607–18. doi:10.1182/blood-2004-01-0037.
Neri P, Kumar S, Fulciniti MT, Vallet S, Chhetri S, Mukherjee S, et al. Neutralizing B-cell activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin Cancer Res. 2007;13(19):5903–9. doi:10.1158/1078-0432.CCR-07-0753.
Tai YT, Li XF, Breitkreutz I, Song W, Neri P, Catley L, et al. Role of B-cell-activating factor in adhesion and growth of human multiple myeloma cells in the bone marrow microenvironment. Cancer Res. 2006;66(13):6675–82. doi:10.1158/0008-5472.CAN-06-0190.
Asano J, Nakano A, Oda A, Amou H, Hiasa M, Takeuchi K, et al. The serine/threonine kinase Pim-2 is a novel anti-apoptotic mediator in myeloma cells. Leukemia. 2011;25(7):1182–8. doi:10.1038/leu.2011.60.
Michigami T, Shimizu N, Williams PJ, Niewolna M, Dallas SL, Mundy GR, et al. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood. 2000;96(5):1953–60.
Markovina S, Callander NS, O’Connor SL, Xu G, Shi Y, Leith CP, et al. Bone marrow stromal cells from multiple myeloma patients uniquely induce bortezomib resistant NF-kappaB activity in myeloma cells. Mol Cancer. 2010;9:176. doi:10.1186/1476-4598-9-176.
Hao M, Zhang L, An G, Meng H, Han Y, Xie Z, et al. Bone marrow stromal cells protect myeloma cells from bortezomib induced apoptosis by suppressing microRNA-15a expression. Leuk Lymphoma. 2011;52(9):1787–94. doi:10.3109/10428194.2011.576791.
Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7(8):585–98. doi:10.1038/nrc2189.
Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood. 1996;87(3):1104–12.
Urashima M, Chauhan D, Uchiyama H, Freeman GJ, Anderson KC. CD40 ligand triggered interleukin-6 secretion in multiple myeloma. Blood. 1995;85(7):1903–12.
Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3(10):756–67. doi:10.1038/nrc1186.
Nefedova Y, Cheng P, Alsina M, Dalton WS, Gabrilovich DI. Involvement of Notch-1 signaling in bone marrow stroma-mediated de novo drug resistance of myeloma and other malignant lymphoid cell lines. Blood. 2004;103(9):3503–10. doi:10.1182/blood-2003-07-2340.
Galson DL, Silbermann R, Roodman GD. Mechanisms of multiple myeloma bone disease. Bonekey Rep. 2012;1:135. doi:10.1038/bonekey.2012.135.
Lee C, Oh JI, Park J, Choi JH, Bae EK, Lee HJ, et al. TNF alpha mediated IL-6 secretion is regulated by JAK/STAT pathway but not by MEK phosphorylation and AKT phosphorylation in U266 multiple myeloma cells. Biomed Res Int. 2013;2013:580135. doi:10.1155/2013/580135.
Hideshima T, Podar K, Chauhan D, Anderson KC. Cytokines and signal transduction. Best Pract Res Clin Haematol. 2005;18(4):509–24. doi:10.1016/j.beha.2005.01.003.
Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15(12):1950–61.
Giuliani N, Storti P, Bolzoni M, Palma BD, Bonomini S. Angiogenesis and multiple myeloma. Cancer Microenviron. 2011;4(3):325–37. doi:10.1007/s12307-011-0072-9.
Terpos E, Kastritis E, Christoulas D, Gkotzamanidou M, Eleutherakis-Papaiakovou E, Kanellias N, et al. Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy. Ann Oncol. 2012;23(10):2681–6. doi:10.1093/annonc/mds068.
Vallet S, Mukherjee S, Vaghela N, Hideshima T, Fulciniti M, Pozzi S, et al. Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc Natl Acad Sci U S A. 2010;107(11):5124–9. doi:10.1073/pnas.0911929107.
Scullen T, Santo L, Vallet S, Fulciniti M, Eda H, Cirstea D, et al. Lenalidomide in combination with an activin A-neutralizing antibody: preclinical rationale for a novel anti-myeloma strategy. Leukemia. 2013;27(8):1715–21. doi:10.1038/leu.2013.50.
Corre J, Mahtouk K, Attal M, Gadelorge M, Huynh A, Fleury-Cappellesso S, et al. Bone marrow mesenchymal stem cells are abnormal in multiple myeloma. Leukemia. 2007;21(5):1079–88. doi:10.1038/sj.leu.2404621.
Corre J, Labat E, Espagnolle N, Hebraud B, Avet-Loiseau H, Roussel M, et al. Bioactivity and prognostic significance of growth differentiation factor GDF15 secreted by bone marrow mesenchymal stem cells in multiple myeloma. Cancer Res. 2012;72(6):1395–406. doi:10.1158/0008-5472.CAN-11-0188.
Tanno T, Lim Y, Wang Q, Chesi M, Bergsagel PL, Matthews G, et al. Growth differentiating factor 15 enhances the tumor-initiating and self-renewal potential of multiple myeloma cells. Blood. 2014;123(5):725–33. doi:10.1182/blood-2013-08-524025.
Reagan MR, Mishima Y, Glavey SV, Zhang Y, Manier S, Lu ZN, et al. Investigating osteogenic differentiation in multiple myeloma using a novel 3D bone marrow niche model. Blood. 2014;124(22):3250–9. doi:10.1182/blood-2014-02-558007.
Feng Y, Wen J, Mike P, Choi DS, Eshoa C, Shi ZZ, et al. Bone marrow stromal cells from myeloma patients support the growth of myeloma stem cells. Stem Cells Dev. 2010;19(9):1289–96. doi:10.1089/scd.2010.0010.
Eda H, Santo L, Wein MN, Hu DZ, Cirstea DD, Nemani N, et al. Regulation of sclerostin expression in multiple myeloma by Dkk-1: a potential therapeutic strategy for myeloma bone disease. J Bone Miner Res Off J Am Soc Bone Miner Res. 2016;31(6):1225–34. doi:10.1002/jbmr.2789.
Hideshima T, Chauhan D, Podar K, Schlossman RL, Richardson P, Anderson KC. Novel therapies targeting the myeloma cell and its bone marrow microenvironment. Semin Oncol. 2001;28(6):607–12.
Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, et al. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest. 2008;118(2):491–504. doi:10.1172/JCI33102.
Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113(18):4341–51. doi:10.1182/blood-2008-10-186668.
Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13(7):791–801.
•• Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92. This paper thoroughly reviews the current understanding of WNT signaling regulation of bone homeostasis and evaluate the importance of therapeutically target this pathway in patients with bone-related diseases.
Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–94. doi:10.1056/NEJMoa030847.
Oshima T, Abe M, Asano J, Hara T, Kitazoe K, Sekimoto E, et al. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106(9):3160–5. doi:10.1182/blood-2004-12-4940.
Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Donofrio G, Bonomini S, et al. Production of Wnt inhibitors by myeloma cells: potential effects on canonical Wnt pathway in the bone microenvironment. Cancer Res. 2007;67(16):7665–74. doi:10.1158/0008-5472.CAN-06-4666.
Yaccoby S, Ling W, Zhan F, Walker R, Barlogie B, Shaughnessy JD Jr. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109(5):2106–11. doi:10.1182/blood-2006-09-047712.
Fulciniti M, Tassone P, Hideshima T, Vallet S, Nanjappa P, Ettenberg SA, et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114(2):371–9. doi:10.1182/blood-2008-11-191577.
Pozzi S, Fulciniti M, Yan H, Vallet S, Eda H, Patel K, et al. In vivo and in vitro effects of a novel anti-Dkk1 neutralizing antibody in multiple myeloma. Bone. 2013;53(2):487–96. doi:10.1016/j.bone.2013.01.012.
Iyer SP, Beck JT, Stewart AK, Shah J, Kelly KR, Isaacs R, et al. A phase IB multicentre dose-determination study of BHQ880 in combination with anti-myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events. Br J Haematol. 2014;167(3):366–75. doi:10.1111/bjh.13056.
Colucci S, Brunetti G, Oranger A, Mori G, Sardone F, Specchia G, et al. Myeloma cells suppress osteoblasts through sclerostin secretion. Blood Cancer J. 2011;1(6):e27. doi:10.1038/bcj.2011.22.
Delgado-Calle J, Bellido T, Roodman GD. Role of osteocytes in multiple myeloma bone disease. Curr Opin Support Palliat Care. 2014;8(4):407–13. doi:10.1097/SPC.0000000000000090.
Terpos E, Christoulas D, Katodritou E, Bratengeier C, Gkotzamanidou M, Michalis E, et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int J Cancer. 2012;131(6):1466–71. doi:10.1002/ijc.27342.
• McDonald MM, Reagan MR, Youlten SE, Mohanty ST, Seckinger A, Terry RL, et al. Inhibiting the osteocyte specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood. 2017; doi:10.1182/blood-2017-03-773341. In this study anti-sclerostin treatment prevented myeloma-induced bone loss and reduced osteolytic bone lesions. Combination treatment with anti-sclerostin and zoledronic acid increased bone mass and fracture resistance when compared to zoledronic acid treatment alone. This study defines a therapeutic strategy superior to the current standard of care.
Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282(19):14515–24.
Fairfield H, Falank C, Harris E, Demambro V, McDonald M, Pettitt JA, et al. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol. 2017; doi:10.1002/jcp.25976.
•• Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, et al. Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat Commun. 2015;6:7808. doi:10.1038/ncomms8808. This study defines spatially and functionally different types of bone marrow adipocytes. Consideration of these marrow adipose subpopulations may be important for future studies linking marrow fat to bone biology, haematopoiesis and MM.
Landgren O, Rajkumar SV, Pfeiffer RM, Kyle RA, Katzmann JA, Dispenzieri A, et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance among black and white women. Blood. 2010;116(7):1056–9. doi:10.1182/blood-2010-01-262394.
Islam R, Altundag K, Kurt M, Altundag O, Turen S. Association between obesity and multiple myeloma in postmenopausal women may be attributed to increased aromatization of androgen in adipose tissue. Med Hypotheses. 2005;65(5):1001–2. doi:10.1016/j.mehy.2005.05.014.
Wallin A, Larsson SC. Body mass index and risk of multiple myeloma: a meta-analysis of prospective studies. Eur J Cancer. 2011;47(11):1606–15. doi:10.1016/j.ejca.2011.01.020.
Veld J, O’Donnell EK, Reagan MR, Yee AJ, Torriani M, Rosen CJ, et al. Abdominal adipose tissue in MGUS and multiple myeloma. Skelet Radiol. 2016;45(9):1277–83. doi:10.1007/s00256-016-2425-4.
• Lwin ST, Olechnowicz SW, Fowler JA, Edwards CM. Diet-induced obesity promotes a myeloma-like condition in vivo. Leukemia. 2015;29(2):507–10. doi:10.1038/leu.2014.295. This work demonstrates that diet-induced obesity creates a permissive environment for the development of an MGUS-like condition, associated with myeloma cell accumulation in bone marrow, an increase in serum paraprotein and mild bone loss.
Caers J, Deleu S, Belaid Z, De Raeve H, Van Valckenborgh E, De Bruyne E, et al. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia. 2007;21(7):1580–4. doi:10.1038/sj.leu.2404658.
Sprynski AC, Hose D, Kassambara A, Vincent L, Jourdan M, Rossi JF, et al. Insulin is a potent myeloma cell growth factor through insulin/IGF-1 hybrid receptor activation. Leukemia. 2010;24(11):1940–50. doi:10.1038/leu.2010.192.
Sprynski AC, Hose D, Caillot L, Reme T, Shaughnessy JD Jr, Barlogie B, et al. The role of IGF-1 as a major growth factor for myeloma cell lines and the prognostic relevance of the expression of its receptor. Blood. 2009;113(19):4614–26. doi:10.1182/blood-2008-07-170464.
Greco EA, Lenzi A, Migliaccio S. The obesity of bone. Ther Adv Endocrinol Metab. 2015;6(6):273–86. doi:10.1177/2042018815611004.
Sakurai T, Ogasawara J, Kizaki T, Sato S, Ishibashi Y, Takahashi M, et al. The effects of exercise training on obesity-induced dysregulated expression of adipokines in white adipose tissue. Int J Endocrinol. 2013;2013:801743. doi:10.1155/2013/801743.
Liu Z, Xu J, He J, Liu H, Lin P, Wan X, et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget. 2015;6(33):34329–41. doi:10.18632/oncotarget.6020.
Zub KA, Sousa MM, Sarno A, Sharma A, Demirovic A, Rao S, et al. Modulation of cell metabolic pathways and oxidative stress signaling contribute to acquired melphalan resistance in multiple myeloma cells. PLoS One. 2015;10(3):e0119857. doi:10.1371/journal.pone.0119857.
Jurczyszyn A, Czepiel J, Gdula-Argasinska J, Pasko P, Czapkiewicz A, Librowski T, et al. Plasma fatty acid profile in multiple myeloma patients. Leuk Res. 2015;39(4):400–5. doi:10.1016/j.leukres.2014.12.010.
Nagata Y, Ishizaki I, Waki M, Ide Y, Hossen MA, Ohnishi K, et al. Palmitic acid, verified by lipid profiling using secondary ion mass spectrometry, demonstrates anti-multiple myeloma activity. Leuk Res. 2015;39(6):638–45. doi:10.1016/j.leukres.2015.02.011.
Abdi J, Garssen J, Faber J, Redegeld FA. Omega-3 fatty acids, EPA and DHA induce apoptosis and enhance drug sensitivity in multiple myeloma cells but not in normal peripheral mononuclear cells. J Nutr Biochem. 2014;25(12):1254–62. doi:10.1016/j.jnutbio.2014.06.013.
•• Nair S, Branagan AR, Liu J, Boddupalli CS, Mistry PK, Dhodapkar MV. Clonal immunoglobulin against lysolipids in the origin of myeloma. N Engl J Med. 2016;374(6):555–61. doi:10.1056/NEJMoa1508808. This study shows for the first time that clonal immunoglobulins specifically react against a subgroup of lysolipids which might be responsible for at least 33% of sporadic monoclonal gammopathies.
Wang D, Haile A, Jones LC. Dexamethasone-induced lipolysis increases the adverse effect of adipocytes on osteoblasts using cells derived from human mesenchymal stem cells. Bone. 2013;53(2):520–30. doi:10.1016/j.bone.2013.01.009.
Gunaratnam K, Vidal C, Gimble JM, Duque G. Mechanisms of palmitate-induced lipotoxicity in human osteoblasts. Endocrinology. 2014;155(1):108–16. doi:10.1210/en.2013-1712.
Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017; doi:10.1016/j.stem.2017.02.009.
Bataille R, Chappard D, Marcelli C, Dessauw P, Sany J, Baldet P, et al. Mechanisms of bone destruction in multiple myeloma: the importance of an unbalanced process in determining the severity of lytic bone disease. J Clin Oncol Off J Am Soc Clin Oncol. 1989;7(12):1909–14. doi:10.1200/JCO.1989.7.12.1909.
Valentin-Opran A, Charhon SA, Meunier PJ, Edouard CM, Arlot ME. Quantitative histology of myeloma-induced bone changes. Br J Haematol. 1982;52(4):601–10.
Taube T, Beneton MN, McCloskey EV, Rogers S, Greaves M, Kanis JA. Abnormal bone remodelling in patients with myelomatosis and normal biochemical indices of bone resorption. Eur J Haematol. 1992;49(4):192–8.
Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23(3):435–41. doi:10.1038/leu.2008.336.
Lee JW, Chung HY, Ehrlich LA, Jelinek DF, Callander NS, Roodman GD, et al. IL-3 expression by myeloma cells increases both osteoclast formation and growth of myeloma cells. Blood. 2004;103(6):2308–15. doi:10.1182/blood-2003-06-1992.
Nanes MS. Tumor necrosis factor-alpha: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15.
Kitaura H, Sands MS, Aya K, Zhou P, Hirayama T, Uthgenannt B, et al. Marrow stromal cells and osteoclast precursors differentially contribute to TNF-alpha-induced osteoclastogenesis in vivo. J Immunol. 2004;173(8):4838–46.
Abe M, Hiura K, Wilde J, Moriyama K, Hashimoto T, Ozaki S, et al. Role for macrophage inflammatory protein (MIP)-1alpha and MIP-1beta in the development of osteolytic lesions in multiple myeloma. Blood. 2002;100(6):2195–202.
Colucci S, Brunetti G, Mori G, Oranger A, Centonze M, Mori C, et al. Soluble decoy receptor 3 modulates the survival and formation of osteoclasts from multiple myeloma bone disease patients. Leukemia. 2009;23(11):2139–46. doi:10.1038/leu.2009.136.
Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barille S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 2001;98(13):3527–33.
Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, et al. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci U S A. 2001;98(20):11581–6. doi:10.1073/pnas.201394498.
Roux S, Amazit L, Meduri G, Guiochon-Mantel A, Milgrom E, Mariette X. RANK (receptor activator of nuclear factor kappa B) and RANK ligand are expressed in giant cell tumors of bone. Am J Clin Pathol. 2002;117(2):210–6. doi:10.1309/BPET-F2PE-P2BD-J3P3.
Ehrlich LA, Roodman GD. The role of immune cells and inflammatory cytokines in Paget’s disease and multiple myeloma. Immunol Rev. 2005;208:252–66. doi:10.1111/j.0105-2896.2005.00323.x.
Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12(4):1221–8. doi:10.1158/1078-0432.CCR-05-1933.
Seidel C, Hjertner O, Abildgaard N, Heickendorff L, Hjorth M, Westin J, et al. Serum osteoprotegerin levels are reduced in patients with multiple myeloma with lytic bone disease. Blood. 2001;98(7):2269–71.
Croucher PI, Shipman CM, Lippitt J, Perry M, Asosingh K, Hijzen A, et al. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 2001;98(13):3534–40.
Terpos E, Politou M, Szydlo R, Nadal E, Avery S, Olavarria E, et al. Autologous stem cell transplantation normalizes abnormal bone remodeling and sRANKL/osteoprotegerin ratio in patients with multiple myeloma. Leukemia. 2004;18(8):1420–6. doi:10.1038/sj.leu.2403423.
Terpos E, Mihou D, Szydlo R, Tsimirika K, Karkantaris C, Politou M, et al. The combination of intermediate doses of thalidomide with dexamethasone is an effective treatment for patients with refractory/relapsed multiple myeloma and normalizes abnormal bone remodeling, through the reduction of sRANKL/osteoprotegerin ratio. Leukemia. 2005;19(11):1969–76. doi:10.1038/sj.leu.2403890.
Heath DJ, Vanderkerken K, Cheng X, Gallagher O, Prideaux M, Murali R, et al. An osteoprotegerin-like peptidomimetic inhibits osteoclastic bone resorption and osteolytic bone disease in myeloma. Cancer Res. 2007;67(1):202–8. doi:10.1158/0008-5472.CAN-06-1287.
Uneda S, Hata H, Matsuno F, Harada N, Mitsuya Y, Kawano F, et al. Macrophage inflammatory protein-1 alpha is produced by human multiple myeloma (MM) cells and its expression correlates with bone lesions in patients with MM. Br J Haematol. 2003;120(1):53–5.
Han JH, Choi SJ, Kurihara N, Koide M, Oba Y, Roodman GD. Macrophage inflammatory protein-1alpha is an osteoclastogenic factor in myeloma that is independent of receptor activator of nuclear factor kappaB ligand. Blood. 2001;97(11):3349–53.
Lentzsch S, Gries M, Janz M, Bargou R, Dorken B, Mapara MY. Macrophage inflammatory protein 1-alpha (MIP-1 alpha ) triggers migration and signaling cascades mediating survival and proliferation in multiple myeloma (MM) cells. Blood. 2003;101(9):3568–73. doi:10.1182/blood-2002-08-2383.
Vallet S, Pozzi S, Patel K, Vaghela N, Fulciniti MT, Veiby P, et al. A novel role for CCL3 (MIP-1alpha) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia. 2011;25(7):1174–81. doi:10.1038/leu.2011.43.
de Weers M, Mensink RG, Kraakman ME, Schuurman RK, Hendriks RW. Mutation analysis of the Bruton’s tyrosine kinase gene in X-linked agammaglobulinemia: identification of a mutation which affects the same codon as is altered in immunodeficient xid mice. Hum Mol Genet. 1994;3(1):161–6.
Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132(5):794–806. doi:10.1016/j.cell.2007.12.037.
Rushworth SA, Bowles KM, Barrera LN, Murray MY, Zaitseva L, MacEwan DJ. BTK inhibitor ibrutinib is cytotoxic to myeloma and potently enhances bortezomib and lenalidomide activities through NF-kappaB. Cell Signal. 2013;25(1):106–12. doi:10.1016/j.cellsig.2012.09.008.
Tai YT, Chang BY, Kong SY, Fulciniti M, Yang G, Calle Y, et al. Bruton tyrosine kinase inhibition is a novel therapeutic strategy targeting tumor in the bone marrow microenvironment in multiple myeloma. Blood. 2012;120(9):1877–87. doi:10.1182/blood-2011-12-396853.
Eda H, Santo L, Cirstea DD, Yee AJ, Scullen TA, Nemani N, et al. A novel Bruton’s tyrosine kinase inhibitor CC-292 in combination with the proteasome inhibitor carfilzomib impacts the bone microenvironment in a multiple myeloma model with resultant antimyeloma activity. Leukemia. 2014;28(9):1892–901. doi:10.1038/leu.2014.69.
Charles JF, Aliprantis AO. Osteoclasts: more than “bone eaters”. Trends Mol Med. 2014;20(8):449–59. doi:10.1016/j.molmed.2014.06.001.
Wu Y, Humphrey MB, Nakamura MC. Osteoclasts—the innate immune cells of the bone. Autoimmunity. 2008;41(3):183–94. doi:10.1080/08916930701693180.
Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009;5(12):667–76. doi:10.1038/nrrheum.2009.217.
Grassi F, Manferdini C, Cattini L, Piacentini A, Gabusi E, Facchini A, et al. T cell suppression by osteoclasts in vitro. J Cell Physiol. 2011;226(4):982–90. doi:10.1002/jcp.22411.
Gorgun GT, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, et al. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121(15):2975–87. doi:10.1182/blood-2012-08-448548.
Leone P, Berardi S, Frassanito MA, Ria R, De Re V, Cicco S, et al. Dendritic cells accumulate in the bone marrow of myeloma patients where they protect tumor plasma cells from CD8+ T-cell killing. Blood. 2015;126(12):1443–51. doi:10.1182/blood-2015-01-623975.
Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114(17):3625–8. doi:10.1182/blood-2009-05-220285.
• An G, Acharya C, Feng X, Wen K, Zhong M, Zhang L, et al. Osteoclasts promote immune suppressive microenvironment in multiple myeloma: therapeutic implication. Blood. 2016;128(12):1590–603. doi:10.1182/blood-2016-03-707547. This work shows that osteoclasts significantly protect MM cells against T-cell-mediated cytotoxicity via direct inhibition of proliferating CD4(+) and CD8(+) T cells. The results support the rationale of targeting immune proteins and cytokines secreted by osteoclasts to improve anti-MM immunity.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–61. doi:10.1016/j.ccell.2015.03.001.
Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple myeloma. Leukemia. 2009;23(9):1545–56. doi:10.1038/leu.2009.89.
Edelstyn GA, Gillespie PJ, Grebbell FS. The radiological demonstration of osseous metastases. Experimental observations. Clin Radiol. 1967;18(2):158–62.
Terpos E, Moulopoulos LA, Dimopoulos MA. Advances in imaging and the management of myeloma bone disease. J Clin Oncol. 2011;29(14):1907–15. doi:10.1200/JCO.2010.32.5449.
Regelink JC, Minnema MC, Terpos E, Kamphuis MH, Raijmakers PG, Pieters-van den Bos IC, et al. Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review. Br J Haematol. 2013;162(1):50–61. doi:10.1111/bjh.12346.
Pianko MJ, Terpos E, Roodman GD, Divgi CR, Zweegman S, Hillengass J, et al. Whole-body low-dose computed tomography and advanced imaging techniques for multiple myeloma bone disease. Clin Cancer Res. 2014;20(23):5888–97. doi:10.1158/1078-0432.CCR-14-1692.
Mangiacavalli S, Pezzatti S, Rossini F, Doni E, Cocito F, Bolis S, et al. Implemented myeloma management with whole-body low-dose CT scan: a real life experience. Leuk Lymphoma. 2016;57(7):1539–45. doi:10.3109/10428194.2015.1129535.
McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc. 2015;90(10):1380–92. doi:10.1016/j.mayocp.2015.07.011.
Kropil P, Fenk R, Fritz LB, Blondin D, Kobbe G, Modder U, et al. Comparison of whole-body 64-slice multidetector computed tomography and conventional radiography in staging of multiple myeloma. Eur Radiol. 2008;18(1):51–8. doi:10.1007/s00330-007-0738-3.
Wolf MB, Murray F, Kilk K, Hillengass J, Delorme S, Heiss C, et al. Sensitivity of whole-body CT and MRI versus projection radiography in the detection of osteolyses in patients with monoclonal plasma cell disease. Eur J Radiol. 2014;83(7):1222–30. doi:10.1016/j.ejrad.2014.02.008.
Hillengass J, Moulopoulos LA, Delorme S, Koutoulidis V, Hielscher T, Engelhart J, et al. Findings of whole body computed tomography compared to conventional skeletal survey in patients with monoclonal plasma cell disorders—a study of the International Myeloma Working Group. Blood. 2016;128(22):4468.
Dimopoulos MA, Hillengass J, Usmani S, Zamagni E, Lentzsch S, Davies FE, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol. 2015;33(6):657–64. doi:10.1200/JCO.2014.57.9961.
Moulopoulos LA, Varma DG, Dimopoulos MA, Leeds NE, Kim EE, Johnston DA, et al. Multiple myeloma: spinal MR imaging in patients with untreated newly diagnosed disease. Radiology. 1992;185(3):833–40. doi:10.1148/radiology.185.3.1438772.
Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD Jr, et al. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol. 2007;25(9):1121–8. doi:10.1200/JCO.2006.08.5803.
Hillengass J, Fechtner K, Weber MA, Bauerle T, Ayyaz S, Heiss C, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010;28(9):1606–10. doi:10.1200/JCO.2009.25.5356.
Hillengass J, Ayyaz S, Kilk K, Weber MA, Hielscher T, Shah R, et al. Changes in magnetic resonance imaging before and after autologous stem cell transplantation correlate with response and survival in multiple myeloma. Haematologica. 2012;97(11):1757–60. doi:10.3324/haematol.2012.065359.
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e48. doi:10.1016/S1470-2045(14)70442-5.
Cavo M, Terpos E, Nanni C, Moreau P, Lentzsch S, Zweegman S, et al. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017;18(4):e206–e17. doi:10.1016/S1470-2045(17)30189-4.
Moreau P, Attal M, Karlin L, Garderet L, Facon T, Macro M, et al. Prospective evaluation of MRI and PET-CT at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/DFCI 2009 trial. Blood. 2015;126(23):395.
Raje NS, Yee AJ, Roodman GD. Advances in supportive care for multiple myeloma. J Natl Compr Cancer Netw. 2014;12(4):502–11.
Favus MJ. Bisphosphonates for osteoporosis. N Engl J Med. 2010;363(21):2027–35. doi:10.1056/NEJMct1004903.
Berenson JR, Lichtenstein A, Porter L, Dimopoulos MA, Bordoni R, George S, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334(8):488–93. doi:10.1056/NEJM199602223340802.
Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J. 2001;7(5):377–87.
Terpos E, Sezer O, Croucher PI, Garcia-Sanz R, Boccadoro M, San Miguel J, et al. The use of bisphosphonates in multiple myeloma: recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol. 2009;20(8):1303–17. doi:10.1093/annonc/mdn796.
Major P, Lortholary A, Hon J, Abdi E, Mills G, Menssen HD, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol. 2001;19(2):558–67.
Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98(8):1735–44. doi:10.1002/cncr.11701.
•• Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376(9757):1989–99. doi:10.1016/S0140-6736(10)62051-X. This trial demonstrated an improvement in overall survival in multiple myeloma with the use of a more potent bisphosphonate, zoledronic acid, when compared to clodronate.
Morgan GJ, Child JA, Gregory WM, Szubert AJ, Cocks K, Bell SE, et al. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): secondary outcomes from a randomised controlled trial. Lancet Oncol. 2011;12(8):743–52. doi:10.1016/S1470-2045(11)70157-7.
Morgan GJ, Davies FE, Gregory WM, Szubert AJ, Bell SE, Drayson MT, et al. Effects of induction and maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: the Medical Research Council myeloma IX trial. Blood. 2012;119(23):5374–83. doi:10.1182/blood-2011-11-392522.
Kumar SK, Callander NS, Alsina M, Atanackovic D, Biermann JS, Chandler JC, et al. Multiple myeloma, version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15(2):230–69.
Terpos E, Morgan G, Dimopoulos MA, Drake MT, Lentzsch S, Raje N, et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol. 2013;31(18):2347–57. doi:10.1200/JCO.2012.47.7901.
Raje N, Woo SB, Hande K, Yap JT, Richardson PG, Vallet S, et al. Clinical, radiographic, and biochemical characterization of multiple myeloma patients with osteonecrosis of the jaw. Clin Cancer Res. 2008;14(8):2387–95. doi:10.1158/1078-0432.CCR-07-1430.
Woo SB, Hellstein JW, Kalmar JR. Systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144(10):753–61.
Dimopoulos MA, Kastritis E, Anagnostopoulos A, Melakopoulos I, Gika D, Moulopoulos LA, et al. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica. 2006;91(7):968–71.
Zervas K, Verrou E, Teleioudis Z, Vahtsevanos K, Banti A, Mihou D, et al. Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: a single-centre experience in 303 patients. Br J Haematol. 2006;134(6):620–3. doi:10.1111/j.1365-2141.2006.06230.x.
Morgan GJ. Further analyses of the myeloma IX study. Lancet. 2011;378(9793):768–9. doi:10.1016/S0140-6736(11)61374-3.
Badros A, Terpos E, Katodritou E, Goloubeva O, Kastritis E, Verrou E, et al. Natural history of osteonecrosis of the jaw in patients with multiple myeloma. J Clin Oncol. 2008;26(36):5904–9. doi:10.1200/JCO.2008.16.9300.
Dimopoulos MA, Kastritis E, Bamia C, Melakopoulos I, Gika D, Roussou M, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20(1):117–20. doi:10.1093/annonc/mdn554.
Chang ST, Tenforde AS, Grimsrud CD, O’Ryan FS, Gonzalez JR, Baer DM, et al. Atypical femur fractures among breast cancer and multiple myeloma patients receiving intravenous bisphosphonate therapy. Bone. 2012;51(3):524–7. doi:10.1016/j.bone.2012.05.010.
Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int. 2008;74(11):1385–93. doi:10.1038/ki.2008.356.
Kyle RA, Yee GC, Somerfield MR, Flynn PJ, Halabi S, Jagannath S, et al. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25(17):2464–72. doi:10.1200/JCO.2007.12.1269.
Yee AJ, Raje NS. Sequencing of nontransplant treatments in multiple myeloma patients with active disease. Hematol Educ Program Am Soc Hematol Am Soc Hematol Educ Program. 2016;2016(1):495–503. doi:10.1182/asheducation-2016.1.495.
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111(5):2516–20. doi:10.1182/blood-2007-10-116129.
Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–8. doi:10.1038/leu.2013.313.
Fonseca R, Abouzaid S, Bonafede M, Cai Q, Parikh K, Cosler L, et al. Trends in overall survival and costs of multiple myeloma, 2000–2014. Leukemia. 2017; doi:10.1038/leu.2016.380.
Larocca A, Child JA, Cook G, Jackson GH, Russell N, Szubert A, et al. The impact of response on bone-directed therapy in patients with multiple myeloma. Blood. 2013;122(17):2974–7. doi:10.1182/blood-2013-04-498139.
Mohty M, Malard F, Mohty B, Savani B, Moreau P, Terpos E. The effects of bortezomib on bone disease in patients with multiple myeloma. Cancer. 2014;120(5):618–23. doi:10.1002/cncr.28481.
Zangari M, Suva LJ. The effects of proteasome inhibitors on bone remodeling in multiple myeloma. Bone. 2016;86:131–8. doi:10.1016/j.bone.2016.02.019.
Terpos E, Christoulas D, Kokkoris P, Anargyrou K, Gavriatopoulou M, Migkou M, et al. Increased bone mineral density in a subset of patients with relapsed multiple myeloma who received the combination of bortezomib, dexamethasone and zoledronic acid. Ann Oncol. 2010;21(7):1561–2. doi:10.1093/annonc/mdq259.
Delforge M, Terpos E, Richardson PG, Shpilberg O, Khuageva NK, Schlag R, et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur J Haematol. 2011;86(5):372–84. doi:10.1111/j.1600-0609.2011.01599.x.
Terpos E, Christoulas D, Kastritis E, Roussou M, Migkou M, Eleutherakis-Papaiakovou E, et al. VTD consolidation, without bisphosphonates, reduces bone resorption and is associated with a very low incidence of skeletal-related events in myeloma patients post ASCT. Leukemia. 2014;28(4):928–34. doi:10.1038/leu.2013.267.
Sezer O, Beksac M, Hajek R, Sucak G, Cagirgan S, Linkesch W, et al. Effects of single-agent bortezomib as post-transplant consolidation therapy on multiple myeloma-related bone disease: a randomized phase II study. Br J Haematol. 2017; doi:10.1111/bjh.14637.
Terpos E, Christoulas D, Kastritis E, Katodritou E, Papatheodorou A, Pouli A, et al. The combination of lenalidomide and dexamethasone reduces bone resorption in responding patients with relapsed/refractory multiple myeloma but has no effect on bone formation: final results on 205 patients of the Greek myeloma study group. Am J Hematol. 2014;89(1):34–40. doi:10.1002/ajh.23577.
Abdulkadyrov KM, Salogub GN, Khuazheva NK, Sherman ML, Laadem A, Barger R, et al. Sotatercept in patients with osteolytic lesions of multiple myeloma. Br J Haematol. 2014;165(6):814–23. doi:10.1111/bjh.12835.
Yee AJ, Laubach JP, Nooka AK, O’Donnell EK, Weller EA, Couture NR, et al. Phase 1 dose-escalation study of sotatercept (ACE-011) in combination with lenalidomide and dexamethasone in patients with relapsed and/or refractory multiple myeloma. Blood. 2015;126:4241.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. doi:10.1038/nature01658.
Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48(4):677–92. doi:10.1016/j.bone.2010.11.020.
Yee AJ, Raje NS. Denosumab, a RANK ligand inhibitor, for the management of bone loss in cancer patients. Clin Interv Aging. 2012;7:331–8. doi:10.2147/CIA.S14566.
Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813–22. doi:10.1016/S0140-6736(10)62344-6.
Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–9. doi:10.1200/JCO.2010.29.7101.
Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341–7. doi:10.1093/annonc/mdr435.
Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29(9):1125–32. doi:10.1200/JCO.2010.31.3304.
Raje N, Vadhan-Raj S, Willenbacher W, Terpos E, Hungria V, Spencer A, et al. Evaluating results from the multiple myeloma patient subset treated with denosumab or zoledronic acid in a randomized phase 3 trial. Blood Cancer J. 2016;6:e378. doi:10.1038/bcj.2015.96.
•• Raje N, Terpos E, Willenbacher W, Shimizu K, Garcia-Sanz R, Durie B et al. An international randomized, double blind trial comparing denosumab with zoledronic acid for the treatment of bone disease in patients with newly diagnosed multiple myeloma. 16th International Myeloma Workshop, March 1–4, New Delhi 2017 | Abstract OP-046, p. e39–e40. https://show.jspargo.com/IMW/files/2017IMWAbstractBook.pdf. This is the largest international trial in multiple myeloma and establishes comparable efficacy of denosumab to zoledronic acid with less adverse renal events. This is an important finding given the frequency of kidney disease in MM.
Dudeney S, Lieberman IH, Reinhardt MK, Hussein M. Kyphoplasty in the treatment of osteolytic vertebral compression fractures as a result of multiple myeloma. J Clin Oncol. 2002;20(9):2382–7.
Fourney DR, Schomer DF, Nader R, Chlan-Fourney J, Suki D, Ahrar K, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(1 Suppl):21–30.
Berenson J, Pflugmacher R, Jarzem P, Zonder J, Schechtman K, Tillman JB, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12(3):225–35. doi:10.1016/S1470-2045(11)70008-0.
McDonald RJ, McDonald JS, Kallmes DF, Lehman VT, Diehn FE, Wald JT, et al. Effect of systemic therapies on outcomes following vertebroplasty among patients with multiple myeloma. AJNR Am J Neuroradiol. 2016;37(12):2400–6. doi:10.3174/ajnr.A4925.
Featherstone C, Delaney G, Jacob S, Barton M. Estimating the optimal utilization rates of radiotherapy for hematologic malignancies from a review of the evidence: part II—leukemia and myeloma. Cancer. 2005;103(2):393–401. doi:10.1002/cncr.20755.
Talamo G, Dimaio C, Abbi KK, Pandey MK, Malysz J, Creer MH, et al. Current role of radiation therapy for multiple myeloma. Front Oncol. 2015;5:40. doi:10.3389/fonc.2015.00040.
Leigh BR, Kurtts TA, Mack CF, Matzner MB, Shimm DS. Radiation therapy for the palliation of multiple myeloma. Int J Radiat Oncol Biol Phys. 1993;25(5):801–4.
Yee AJ, Winkfield KM, Raje NS. Plasma cell dyscrasias. In: Tomblyn M, Winkfield KM, Dabaja B, editors. Radiation medicine rounds: hematologic malignancies. New York: Demos Medical Publishing; 2012. p. 473–88.
Gimsing P, Carlson K, Turesson I, Fayers P, Waage A, Vangsted A, et al. Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): a double-blind, randomised controlled trial. Lancet Oncol. 2010;11(10):973–82. doi:10.1016/S1470-2045(10)70198-4.
Berenson JR, Vescio RA, Rosen LS, VonTeichert JM, Woo M, Swift R, et al. A phase I dose-ranging trial of monthly infusions of zoledronic acid for the treatment of osteolytic bone metastases. Clin Cancer Res. 2001;7(3):478–85.
Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, et al. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002;42(11):1228–36.
Patel CG, Yee AJ, Scullen TA, Nemani N, Santo L, Richardson PG, et al. Biomarkers of bone remodeling in multiple myeloma patients to tailor bisphosphonate therapy. Clin Cancer Res. 2014;20(15):3955–61. doi:10.1158/1078-0432.CCR-14-0434.
Raje N, Vescio R, Montgomery CW, Badros A, Munshi N, Orlowski R, et al. Bone marker-directed dosing of zoledronic acid for the prevention of skeletal complications in patients with multiple myeloma: results of the Z-MARK study. Clin Cancer Res. 2016;22(6):1378–84. doi:10.1158/1078-0432.CCR-15-1864.
Himelstein AL, Foster JC, Khatcheressian JL, Roberts JD, Seisler DK, Novotny PJ, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48–58. doi:10.1001/jama.2016.19425.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Cristina Panaroni and Andrew Yee declare no conflict of interest.
Noopur Raje reports payment for work as a consultant for Amgen and Novartis, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cancer-induced Musculoskeletal Diseases
Rights and permissions
About this article
Cite this article
Panaroni, C., Yee, A.J. & Raje, N.S. Myeloma and Bone Disease. Curr Osteoporos Rep 15, 483–498 (2017). https://doi.org/10.1007/s11914-017-0397-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-017-0397-5