Abstract
Bone disease is one of the most common complications of multiple myeloma. It is the result of increased osteoclast activity which is not compensated by osteoblast activity and leads to osteolytic lesions characterized by bone pain and increased risk for pathological fracture, spinal cord compression and need for radiotherapy or surgery to the bone. Imaging techniques for the diagnosis of multiple myeloma bone disease include whole-body X-rays, whole-body low-dose CT (WBLDCT), magnetic resonance imaging (MRI), and PET/CT. Bisphosphonates (BPs) are the cornerstone in the treatment of myeloma-related bone disease. Recent studies have revealed novel pathways and molecules that are involved in the biology of myeloma bone disease including the receptor activator of nuclear factor-kappa B ligand/osteoprotegerin pathway, the Wnt signaling inhibitors dickkopf-1 and sclerostin, macrophage inflammatory proteins, activin A, and others. The thorough study of these pathways has provided novel agents that may play a critical role in the management of myeloma-related bone disease in the near future, such as denosumab (anti-RANKL), sotatercept (activin A antagonist), romosozumab (anti-sclerostin), or BHQ-880 (anti-dickkopf-1). In this chapter, we will focus in the imaging techniques used for the diagnosis of multiple myeloma bone disease, as well as the current and future options for its management.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Multiple myeloma (MM) is a common hematological malignancy characterized by the accumulation of abnormal plasma cells in the bone marrow. Despite the improvement in survival after the introduction of novel agents (Kumar et al. 2008; Kastritis et al. 2009), MM remains an incurable plasma-cell malignancy (Jemal et al. 2010; Parker et al. 1998). MM is characterized by osteolytic bone disease due to an elevated function of osteoclasts which is not balanced by a comparable elevation of osteoblast function (Kyle et al. 2003; Terpos and Dimopoulos 2005; Raje and Roodman 2011). Osteolytic lesions are detected in 70–80% of patients at diagnosis and increase the risk for skeletal-related events (SREs: pathologic fractures, spinal cord compression (SCC), requirement for surgery or palliative radiotherapy to bone). SREs have a serious impact on the quality of life (QoL) and survival of MM patients and affect both clinical and economic aspects of their life (Coleman 2007; Roodman 2008; Croucher and Apperley 1998; Cocks et al. 2007; Bruce et al. 1999; McCloskey et al. 1998). The novel International Myeloma Working Group (IMWG) criteria for the diagnosis of symptomatic MM have revealed the value of modern imaging for the management of MM patients, as they include (1) the presence of at least one lytic lesion detected not only by conventional radiography but also by computed tomography (CT), whole-body low-dose CT (WBLDCT), or positron emission tomography/CT (PET/CT) and (2) the presence of >1 focal bone marrow lesions on magnetic resonance imaging (MRI) studies (Rajkumar et al. 2014). Furthermore, novel imaging techniques, such as MRI and PET/CT, provide prognostic information and have been recently proven of value, for the better definition of response to anti-myeloma therapy. Bisphosphonates (BPs) are the cornerstone of therapeutic management of myeloma bone disease, offering considerable benefit in preventing or delaying skeletal-related events and relieving pain (Silbermann and Roodman 2016). This chapter reviews the latest available details of imaging and treatment of myeloma-related bone disease.
7.2 Pathophysiology of Multiple Myeloma Bone Disease
In the adult skeleton, skeletal integrity is coordinated by the synchronized activity of three cell types. Osteoblasts create new bone matrix, osteoclasts are responsible for bone resorption, and osteocytes regulate bone turnover. In MM patients, bone disease is the result of an uncoupling in bone remodeling. It consists of an increase in the osteoclast-mediated bone resorption, which is combined with suppression in the osteoblast, mediated bone mineralization, and defects on osteocyte functions (Bataille et al. 1991). Until today, several direct and indirect interactions between myeloma cells and cells of the bone marrow microenvironment have been recognized. The fact that osteolytic lesions occur close to MM cells suggests that factors secreted by tumor cells lead to direct stimulation of osteoclast-mediated bone resorption and inhibition of osteoblast-mediated bone formation (Terpos and Dimopoulos 2005). In addition to that, the increased bone resorptive progress leads to the release of growth factors that increase the growth of MM cells, leading to a vicious cycle of tumor expansion and bone destruction. Apart from that, interactions via adhesion between MM cells and bone marrow cells result in the production of factors that promote angiogenesis and make the myeloma cells resistant to chemotherapy (Abe et al. 2004; Tanaka et al. 2007). The biologic pathway of the receptor activator of nuclear factor-kappa B (RANK), its ligand (RANKL), and osteoprotegerin (OPG) which is the decoy receptor of RANKL is of major importance for the increased osteoclast activity observed in MM. Myeloma cells disrupt the balance between RANKL and OPG by increasing the expression of RANKL and decreasing the expression of OPG. The resulting increase in RANKL favors the formation and activation of osteoclasts, leading to increased bone resorption (Pearse et al. 2001; Terpos et al. 2003). More recently, activin A has been implicated in MM bone disease, through stimulating RANK expression and inducing osteoclastogenesis (Sugatani et al. 2003; Terpos et al. 2012a). On the other hand, in addition to their stimulatory effect on osteoclasts, myeloma cells have been shown to suppress bone formation (Christoulas et al. 2009). The Wingless-type (Wnt) signaling pathway is one pathway that has been shown to play a key role in osteoblast differentiation and has been implicated in osteoblast suppression in myeloma. The Wnt signaling inhibitors dickkopf-1 (Dkk-1) and sclerostin are secreted by myeloma cells and have been found to be increased in the serum of myeloma patients, leading to the block of osteoblast differentiation and activity (Tian et al. 2003; Colucci et al. 2011; Politou et al. 2006; Terpos et al. 2012b). Soluble frizzled-related protein-2 (sFRP-2), another inhibitor of Wnt signaling, has also been implicated in the suppression of bone formation in myeloma (Oshima et al. 2005). Although the circulating levels of the above molecules and mainly of sclerostin have not been found to be elevated in myeloma patients in all published studies, the importance of Wnt inhibition in the biology of myeloma-related bone disease is undoubted.
7.3 Imaging for the Diagnosis of Multiple Myeloma Bone Disease
The imaging techniques used for the diagnosis of multiple myeloma bone disease are:
-
1.
Whole-Body X-rays (WBXR).
-
2.
Whole-Body CT (WB-CT).
-
3.
Magnetic resonance imaging (MRI).
-
4.
PET/CT.
7.3.1 Whole-Body X-Rays (WBXR)
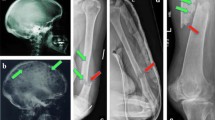
Conventional radiography has been widely used for the identification of osteolytic lesions both at diagnosis and during the course of the disease. The “skeletal survey” (whole-body X-rays, WBXR) at diagnosis should include plain radiographs of the whole skeleton (anteroposterior and lateral views of the skull; posteroanterior view of the chest; anteroposterior and lateral views of the thoracic lumbar and cervical spine (including an open mouth view), humeri and femora and anteroposterior view of the pelvis) (Dimopoulos et al. 2009a). In addition, symptomatic areas should also be specifically visualized. Osteolyses have the typical appearance of “punched-out” lesions with the absence of reactive sclerosis and are more common in the vertebrae, ribs, skull, and pelvis (Terpos et al. 2011). Although the WBXR was the standard of care for many years, it has several limitations:
-
1.
For a lytic lesion to become apparent, >30% loss of trabecular bone must occur.
-
2.
Difficulty of assessment of certain areas, such as the pelvis and the spine.
-
3.
Limitations in the detection of lytic lesion response to anti-myeloma therapy because of delayed evidence of healing.
-
4.
Reduced specificity for the differential diagnosis of myeloma-related versus benign fracture (very important, particularly in cases of new vertebral compression fractures in the absence of other criteria of relapse).
-
5.
Observer dependency (there is very low reproducibility among centers; a higher number of osteolytic lesions detected in academic versus nonacademic centers).
-
6.
Prolonged study length, often not tolerable from patients in severe pain (Dimopoulos et al. 2009a; Terpos et al. 2011).
Thus, the development of novel imaging methods has led to the replacement of WBXR by more advanced techniques, such as the WBLDCT in many European centers or by PET/CT in the USA.
7.3.2 Whole-Body Low-Dose CT (WBLDCT)
WBLDCT was introduced to allow the detection of osteolytic lesions in the whole skeleton with high accuracy, no need for contrast agents and low-radiation dose compared to standard CT (two- to threefold lower-radiation dose versus conventional CT) (Pianko et al. 2014; Ippolito et al. 2013). In several studies, WBLDCT was found to be superior to WBXR for the detection of osteolytic lesions (Pianko et al. 2014; Horger et al. 2005; Kropil et al. 2008; Gleeson et al. 2009; Princewill et al. 2013; Wolf et al. 2014). In one of the largest studies staging myeloma patients, 61% of patients with normal WBXR had more than one osteolytic lesions on WBLDCT (Princewill et al. 2013). According to the latest criteria for symptomatic myeloma, these patients should receive therapy. In the same study, the total number of lesions detected by WBLDCT was 968 versus 248 for WBXR (p < 0.001). The only limitation of this study was its retrospective origin (Princewill et al. 2013). In a more recent prospective study, which included 52 myeloma patients at diagnosis, WBLDCT revealed osteolyses in 12 patients (23%) with negative WBXR and proved to be more sensitive than WBXR mainly in the axial skeleton (p < 0.001). WBLDCT was superior in the detection of lesions in patients with osteopenia and osteoporosis (Wolf et al. 2014).
In total, WBLDCT advantages over WBXR include (1) superior diagnostic sensitivity for depiction of osteolytic lesions, especially in areas where the WBXR detection rate is low, i.e., pelvis and spine; (2) superiority in estimating fracture risk and bone instability; (3) duration of the examination, which is ≤5 min, an important issue for patients in extreme pain; (4) production of higher-quality 3D high-resolution images for planning biopsies and therapeutic interventions; and finally (5) demonstration of unsuspected manifestations of myeloma or other diseases, especially in the lungs and kidneys (33% in the study by Wolf et al.; 37, 31–37). Major disadvantages of WBLDCT include increased length of time required for radiologists to report their findings, lack of availability in several centers (Rajkumar et al. 2014; Pianko et al. 2014), and lack of specificity for the differential diagnosis between malignant and osteoporotic fractures, despite improvements during the last years (Cretti and Perugini 2016). Furthermore, although exposure to radiation is much lower compared to standard CT, it continues to be higher than WBXR: mean dose of WBLDCT is approximately 3.6 and 2.8 mSv for females and males, respectively, versus 1.2 mSv for WBXR (Borggrefe et al. 2015). Nevertheless, the higher diagnostic accuracy of the WBLDCT and patient comfort particularly important for the elderly, often suffering group, renders the dose/quality ratio favorable for WBLDCT. For these reasons, the European Myeloma Network has suggested that WBLDCT should replace conventional radiography as the standard imaging technique for evaluation of bone disease in MM, where available (Terpos et al. 2015a).
7.3.3 Magnetic Resonance Imaging
Techniques
Several MRI techniques have been developed for the assessment of the bone marrow involvement in MM. Conventional MRI protocols include T1-weighted, T2-weighted with fat suppression, short time inversion recovery (STIR) and gadolinium T1-weighted with fat suppression (Moulopoulos and Dimopoulos 1997). Myeloma lesions show typically a low signal intensity on T1-weighted images, a high signal intensity on T2-weighted and STIR images, and often enhancement on gadolinium-enhanced images (Libshitz et al. 1992; Weininger et al. 2008).
Limitations of MRI are the prolonged acquisition time, availability issues, the high cost, the exclusion of patients with metal devices in their body, the difficulties in cases of claustrophobic patients, and the limited field of view. To override these restrictions, a WB-MRI methodology, which does not usually require contrast infusion, was developed. The time of WB-MRI is approximately 45 min. Although of interest, this newer technique is not yet widely employed.
All above MRI methods use MRI exquisite contrast and spatial resolution for the depiction of the WB anatomy and specific tissue composition in details.
Novel MRI techniques include diffusion-weighted imaging, dynamic contrast-enhanced MRI, and PET-MRI.
A novel and promising MRI sequence is the diffusion-weighted imaging (DWI-MRI) which derives its contrast mainly from differences in the diffusivity of water molecules in the tissue environment. This functional technique demonstrates alterations in intra- and extracellular water content from disruption of the transmembrane water flux that are visible before identified changes on the morphologic routine sequences (Attariwala and Picker 2013; Muller and Edelman 1995; Wang 2000). DWI-MRI uses the calculation of apparent diffusion coefficient (ADC) values to better evaluate myeloma burden and MRI infiltration patterns (Nonomura et al. 2001; Terpos et al. 2015b). DWI can be used to detect regions with bone marrow infiltration for both diagnosis and monitoring treatment response (Xu et al. 2008), because ADC values are higher in MM patients at diagnosis compared with patients in remission 20 weeks after initiation of treatment (Messiou et al. 2012). In MM patients, the ADC was reproducible (Messiou et al. 2011) and correlated with bone marrow cellularity and microvessel density (MVD) (Hillengass et al. 2011). One disadvantage of DWI is that the ADC is not exclusively influenced by diffusion but also by perfusion. However, improved sequences are under development to differentiate both influences (Lemke et al. 2011). DWI-MRI was found superior to WBXR for the detection of bone involvement in 20 patients with relapsed/refractory MM in all areas of the skeleton except of the skull, where both examinations had equal sensitivity (Giles et al. 2015). In another small study with 24 myeloma patients (both treated and untreated), DWI-MRI was found more sensitive than F18-fluorodeoxyglucose (FDG)-PET in the detection of myeloma lesions (Sachpekidis et al. 2015a). In a recent study, 17 patients were evaluated with DWI-MRI and FDG-PET/CT, and the findings were compared with bone marrow biopsy data. In all studied regions, WB-DWI scores were higher compared to FDG-PET/CT. DWI-MRI was particularly accurate in diagnosing diffuse disease (diffuse disease was observed in 37% of regions imaged on WB-DWI scans versus only 7% on FDG-PET/CT); both techniques were equally sensitive in the detection of focal lesions (Pawlyn et al. 2015). Preliminary reports suggest that DWI-MRI may be used for the better definition of response to therapy, but this has to be confirmed in larger studies and in comparison with PET/CT results (Terpos et al. 2015b; Horger et al. 2011).
The dynamic contrast-enhanced MRI (DCE-MRI) is another MRI technique which evaluates the distribution of a contrast agent inside and outside the blood vessels. Information is assessed by computer-based analysis of repeated images over time. The analysis provides data for blood volume and vessel permeability for the assessment of microcirculation of a specific area (Hillengass et al. 2007; Hillengass and Landgren 2013). More importantly in MM patients, DCE-MRI derived parameters correlated with marrow angiogenesis, microvessel density (MVD) (Huang et al. 2012), as well as in angiogenic response to therapy (Zechmann et al. 2012). Regarding DCE-MRI sampling rate and model, there are two pharmacokinetic models (proposed by Brix and Tofts) that have been applied in the literature. However, a comparison of these models demonstrated that the Brix model is a little bit more robust (Zwick et al. 2010). Since DCE-MRI has not been established in clinical routine, no definite sequence can be recommended.
Positron emission tomography in combination with MRI (PET-MRI) represents a novel imaging modality in which the PET part detects active focal lesions, while the MRI part shows the location of the lesions and gives information on myeloma cell infiltration of the bone marrow. Especially in patients who reach a complete remission (CR), this technique might be able to localize residual sites of disease activity and therefore may help to guide treatment in the future (Fraioli and Punwani 2014). In MM, there is only one prospective study, which compared PET-MRI with PET/CT in 30 myeloma patients with both techniques performed sequentially. There was a high correlation between the two techniques, regarding number of active lesions and average SUV (Sachpekidis et al. 2015b). Further studies with PET-MRI will reveal if there is any value of this technique for MM patients.
MRI Patterns of Marrow Involvement
Five MRI patterns of bone marrow infiltration in myeloma have been reported: (1) normal appearance of the bone marrow, (2) focal involvement (positive focal lesion is considered the lesion of a diameter of at least 5 mm), (3) homogeneous diffuse infiltration, (4) combined diffuse and focal infiltration, and (5) variegated or “salt-and-pepper” pattern with inhomogeneous bone marrow with interposition of fat islands (Baur-Melnyk et al. 2005; Moulopoulos et al. 1992). Low tumor burden is usually associated with a normal MRI pattern, but a high tumor burden is usually suspected when there is diffuse hypointense change on T1-weighted images, diffuse hyperintensity on T2-weighted images, and enhancement with gadolinium injection (Moulopoulos et al. 2005). In several studies, the percentage of symptomatic patients with each of the abnormal MRI bone marrow patterns ranges from 18 to 50% for focal pattern, 25 to 43% for diffuse pattern, and 1 to 5% for variegated pattern (Hillengass and Landgren 2013). The Durie-Salmon PLUS system uses the number of focal lesions (from focal or combined focal/diffuse patterns) for the staging of a myeloma patient and not the diffuse or “salt-and-pepper” patterns (Durie 2006).
MRI Versus Conventional Radiography and Other Imaging Techniques for the Detection of Bone Involvement in Symptomatic Myeloma
MRI is more sensitive compared to WBXR for the detection of bone involvement in MM. In the largest series of patients published to date, MRI was compared to WBXR in 611 patients who received tandem autologous transplantation (ASCT). MRI and WBXR detected focal and osteolytic lesions in 74% and 56% of the imaged anatomic sites, respectively. Furthermore, 52% of 267 patients with normal WBXR had focal lesions on MRI. More precisely, MRI detected more focal lesions compared to lytic lesions in WBXR in the spine (78% vs. 16%, p < 0.001), the pelvis (64% vs. 28%, p < 0.001), and the sternum (24%vs. 3%, p < 0.001). WBXR had better performance than MRI in the ribs (10% vs. 43%, p < 0.001) and the long bones (37% vs. 48%, p = 0.006) and equal results in the skull and the shoulders (Walker et al. 2007). Similar results had been previously reported in smaller studies, where MRI was superior to WBXR for the detection of focal vs. osteolytic lesions in the pelvis (75% vs. 46% of patients) and the spine (76% vs. 42%), especially in the lumbar spine (Ludwig et al. 1987; Ghanem et al. 2006; Lecouvet et al. 1999; Tertti et al. 1995; Narquin et al. 2013). A recent meta-analysis confirmed the superiority of MRI over WBXR regarding the detection of focal lesions and showed that MRI especially outscores WBXR in the axial skeleton but not in the ribs (Regelink et al. 2013).
Although it is clear that MRI can detect bone marrow focal lesions long before the development of osteolytic lesions in the WBXR, other imaging techniques such as PET combined with computed tomography (PET/CT), CT, or WB-CT detect more osteolytic lesions compared to WBXR (Regelink et al. 2013). Is there any evidence that MRI is superior to the other techniques in depicting bone involvement in myeloma? In a study with 41 newly diagnosed MM patients, WB-MRI was found superior to WB-CT in detecting lesions in the skeleton (Baur-Melnyk et al. 2008). In a prospective study, Zamagni et al. compared MRI of the spine and pelvis with WBXR and PET/CT in 46 MM patients at diagnosis. Although PET/CT was superior to WBXR in detecting lytic lesions in 46% of patients (19% had negative WBXR), it failed to reveal abnormal findings in 30% of patients who had abnormal MRI in the same areas, mainly of diffuse pattern. In that study, the combination of spine and pelvic MRI with PET/CT detected both medullary and extramedullary active myeloma sites in almost all patients (92%) (Zamagni et al. 2007). Nevertheless, the Arkansas group was not able to confirm any superiority of MRI over PET/CT in the detection of more focal lesions in a large number of patients (n = 303) within the total of three therapy protocols (Waheed et al. 2013). Still, in 188 patients who had at least 1 focal lesion in MRI, MRI was superior to PET/CT regarding the detection of a higher number of focal lesions (p = 0.032). Furthermore, in this study, the presence of diffuse marrow pattern was not taken into consideration as an abnormal MRI finding (Waheed et al. 2013). Compared to sestamibi technetium-99 m (MIBI) scan, WB-MRI detected more lesions in the vertebrae and the long bones produced similar results in the skull and was inferior in the ribs (Khalafallah et al. 2013). One important question in this point is the value of WB-MRI, which is not available everywhere, over the MRI of the spine and pelvis. In 100 patients with MM and MGUS who underwent WB-MRI, 10% presented with focal lesions merely in the extra-axial skeleton. These lesions would have been ignored if only MRI of the spine and pelvis had been performed (Bauerle et al. 2009).
Other advantages of MRI over WBXR and CT include the discrimination of myeloma from a normal marrow (Moulopoulos and Dimopoulos 1997; Baur et al. 1998); this finding can help in the differential diagnosis between myeloma and benign cause of a vertebral fracture. This is of extreme importance in cases of patients with a vertebral fracture and no other CRAB criteria and no lytic lesions. The MRI can also accurately illustrate the spinal cord and/or nerve root compression for surgical intervention or radiation therapy (Dimopoulos et al. 2009a; Moulopoulos and Dimopoulos 1997). Furthermore, the presence of soft tissue extension of MM and the presence of extramedullary plasmacytomas that are developed in approximately 10–20% of patients during the course of their disease can be precisely visualized by WB-MRI (Moulopoulos et al. 1993; Dimopoulos et al. 2000; Varettoni et al. 2010; Lafforgue et al. 1993). MRI can also help in the better evaluation of avascular necrosis of the femoral head (Lafforgue et al. 1993) and the presence of soft tissue amyloid deposits (Syed et al. 2010). Moreover, the tumor load can be assessed and monitored by MRI even in patients with nonsecretory and oligosecretory MM (Carlson et al. 1995).
In conclusion, according to the latest IMWG guidelines, MRI is the gold standard imaging technique for the detection of bone marrow involvement in MM (grade A). MRI detects bone marrow involvement and not bone destruction. MRI of the spine and pelvis can detect approximately 90% of focal lesions in MM, and thus it can be used in cases where WB-MRI is not available (grade B). MRI is the procedure of choice to evaluate a painful lesion in myeloma patients, mainly in the axial skeleton, and to detect spinal cord compression (grade A). MRI is particularly useful in the evaluation of collapsed vertebrae, especially when myeloma is not active, where the possibility of osteoporotic fracture is high (grade B) (Dimopoulos et al. 2015).
Prognostic Value of MRI
The prognostic significance of MRI findings in symptomatic myeloma has been evaluated. The largest study in the literature included 611 patients who received tandem ASCT-based protocols. Focal lesions are detected by spinal MRI and not seen on WBXR independently correlated with overall survival (OS). Resolution of the focal lesions on MRI posttreatment occurred in 60% of the patients who had superior survival. At disease progression after complete response (CR), MRI revealed new focal lesions in 26% of patients, enlargement of previous focal lesions in 28%, and both features in 15% of patients (Walker et al. 2007). In a more recent analysis of the same group on 429 patients, patients who had >7 focal lesions in MRI (n = 147) had a 73% probability of 3-year OS vs. 86% for those who had 0–7 focal lesions (n = 235) and 81% for those who had diffuse pattern of marrow infiltration (n = 47; p = 0.04). PET/CT and WBXR also produced similar results in the univariate analysis. In the multivariate analysis, from the imaging variables, only the presence of >2 osteolytic lesions in WBXR at diagnosis and the presence of >3 focal lesions in the PET/CT, 7 days post-ASCT had independent prognostic value for inferior OS (p = 0.01 and 0.03, respectively). However, we have to mention the high percentage of patients (232/429, 54%) who had no detectable osteolytic lesions by WBXR and the absence of evaluation of diffuse MRI pattern in this study (Usmani et al. 2013).
The MRI pattern of marrow infiltration has also reported to have a prognostic significance in newly diagnosed patients with symptomatic disease (Moulopoulos et al. 2005; Lecouvet et al. 1998; Moulopoulos et al. 2012). In the conventional chemotherapy (CC) era, Moulopoulos et al. published that the median OS of newly diagnosed MM patients was 24 months if they had diffuse MRI pattern versus 51, 52, and 56 months for those with focal, variegated, and normal patterns, respectively (p = 0.001) (Moulopoulos et al. 2005). This is possibly because diffuse MRI marrow pattern correlates with increased angiogenesis and advanced disease features (Moulopoulos et al. 2010; Song et al. 2014). The same group also reported the prognostic value of MRI patterns in 228 symptomatic MM patients who received upfront regimens based on novel agents. Patients with diffuse pattern had inferior survival compared to patients with other MRI patterns; moreover, the combination of diffuse MRI pattern, ISS-3 stage, and high-risk cytogenetics could identify a group of patients with very poor survival: median of 21 months and a probability of 3-year OS of only 35% (Moulopoulos et al. 2012). Another study in 126 patients with newly diagnosed symptomatic myeloma who underwent an ASCT showed that the diffuse and the variegated MRI patterns had an independent predictive value for disease progression (HR: 1.922, p = 0.008) (Song et al. 2014). Finally, in patients with progressive or relapsed MM, an increased signal of DCE-MRI offered shorter PFS, possibly due to its association with higher MVD (Hillengass et al. 2007).
MRI and Response to Anti-myeloma Therapy
An interesting finding is that a change in MRI pattern correlates with response to therapy. Moulopoulos et al. firstly reported in the era of CC that CR is characterized by complete resolution of the preceding marrow abnormality, while partial response (PR) is characterized by changeover of diffuse pattern to variegated or focal patterns (Moulopoulos et al. 1994). In a retrospective study that was conducted in the era of novel agents, response to treatment was compared with changes in infiltration patterns of WB-MRI before and after ASCT (n = 100). There was a strong correlation between response to anti-myeloma therapies and changes in both diffuse (p = 0.004) and focal (p = 0.01) MRI patterns. Furthermore, the number of focal lesions at second MRI was of prognostic significance for OS (p = 0.001) (Hillengass et al. 2012). Another study in 33 patients who underwent an ASCT showed that WB-MRI data demonstrated progressive disease in ten patients (30%) and response to high-dose therapy in 23 (70%). Eight (80%) of the ten patients with progressive disease revealed intramedullary lesions, and two patients (20%) had intra- and extramedullary lesions. WB-MRI had a sensitivity of 64%, specificity of 86%, positive predictive value of 70%, negative predictive value of 83%, and accuracy of 79% for detection of remission (Bannas et al. 2012). This study supports that one of the disadvantages of MRI is that it often provides false-positive results because of persistent nonviable lesions. Thus, PET/CT might be more suitable than MRI for determination of remission status (Derlin et al. 2013). Indeed in a large study of 191 patients, PET/CT revealed faster change of imaging findings than MRI in patients who responded to therapy (Spinnato et al. 2012). It seems that the PET/CT normalization after treatment can offer more information compared to MRI for the better definition of CR (Bartel et al. 2009).
To improve the results of MRI for the most accurate detection of remission, the DW-MRI has been recently used. In the first preliminary report, ADC values in active myeloma were significantly higher than marrow in remission (Messiou et al. 2012). Furthermore, the mean ADC increased in 95% of responding patients and decreased in all (n = 5) non-responders (p = 0.002). An increase of ADC by 3.3% was associated with a positive response, having a sensitivity of 90% and specificity of 100%. Furthermore, there was a negative correlation between changes of ADC and changes of biochemical markers of response (r = −0.614, p = 0.001) (Giles et al. 2014). Large prospective clinical studies are definitely justified by these results.
The Value of MRI in the Definition of Smoldering/Asymptomatic Myeloma
The presence of lytic lesions by WBXR is included in the definition of symptomatic myeloma, based on studies showing that patients with at least one lytic lesion in WBXR have a median time to progression (TTP) of 10 months (Dimopoulos et al. 1993). However, in patients with no osteolytic lesions in WBXR, the MRI reveals abnormal marrow appearance in 20–50% of them (Moulopoulos et al. 1992; Moulopoulos et al. 2005; Moulopoulos et al. 1995; Hillengass et al. 2010; Kastritis et al. 2013); these patients are at a higher risk for progression. Moulopoulos et al. reported that patients with SMM and abnormal MRI studies required therapy after a median of 16 vs. 43 months for those with normal MRI (p < 0.01) (Moulopoulos et al. 1995). Hillengass and colleagues evaluated WB-MRI in 149 SMM patients. Focal lesions were detected in 42 (28%) patients, while >1 focal lesion was present in 23 patients (15%) who had high risk of progression (HR = 4.05, p < 0.001). The median TTP was 13 months, and the progression rate at 2 years was 70%. On multivariate analysis, the presence of >1 focal lesion remained a significant predictor of progression after adjusting for other risk factors including bone marrow plasmacytosis, serum and urine M protein levels, and suppression of uninvolved immunoglobulins. In the same study, the diffuse marrow infiltration on MRI was also associated with increased risk for progression (HR = 3.5, p < 0.001) (Hillengass et al. 2010). Kastritis and colleagues also showed in 98 SMM patients that abnormal marrow pattern in the MRI of the spine, which was present in 21% of patients, was associated with high risk of progression with a median TTP to symptomatic myeloma of 15 months (p = 0.001) (Kastritis et al. 2013).
An important issue is whether patients who have two or more small focal lesions (<5 mm) should be considered as patients with symptomatic myeloma and how to manage them. The Heidelberg group analyzed very recently data of 63 SMM patients who had at least two WB-MRIs performed for follow-up before progression into symptomatic disease. The definition of radiological progression according to MRI findings included one of the following: (1) development of a new focal lesion, (2) increase of the diameter of an existing focal lesion, and (3) detection of novel or progressive diffuse MRI pattern. The second MRI was performed 3–6 months after the performance of the first MRI. Evaluation of response according to IMWG criteria was also performed. Progressive disease according to MRI was observed in approximately 50% of patients, while 40% of patients developed symptomatic MM based on the CRAB criteria. In the multivariate analysis, MRI-PD was an independent prognostic factor for progression. Patients with stable MRI findings had no higher risk of progression, even when focal lesions were present at the initial MRI (Merz et al. 2014). Prospective clinical trials should be conducted to confirm the above findings.
MRI Findings in Monoclonal Gammopathy of Undetermined Significance (MGUS)
MGUS by definition is characterized by the absence of osteolytic lesions. However, MGUS patients have higher incidence of osteoporosis and vertebral fractures compared to normal population (Pepe et al. 2006; Van de Donk et al. 2014). In a small study which included 37 patients with MGUS or SMM, MRI abnormalities were detected in 20% of them. These patients had a higher time to progression (TTP) to symptomatic myeloma compared to patients with a normal MRI who did not progress after a median follow-up of 30 months (Vande Berg et al. 1997). A prospective study in 331 patients with MGUS or SMM revealed that the detection of multiple (>1) focal lesions by MRI conferred an increased risk of progression (Dhodapkar et al. 2014). In another large study, which included only MGUS patients (n = 137) who underwent a WB-MRI at diagnosis, a focal infiltration pattern was detected in 23% of them. Independent prognostic factors for progression to symptomatic myeloma included the presence and number of focal lesions and the value of M protein (Hillengass et al. 2014).
MRI and Solitary Plasmacytoma of the Bone (SPB)
The diagnosis of SBP includes the presence of a solitary bone lesion, with a confirmed infiltration by plasma cells in the biopsy of the lesion, absence of clonal plasma cells in the trephine bone marrow biopsy, and no CRAB criteria. Although definitive radiotherapy usually eradicates the local disease, the majority of patients will develop MM because of the growth of previously occult lesions which have not been detected by WBXR (Dimopoulos et al. 2000). Moulopoulos et al. published that spinal MRI revealed additional focal lesions in 4/12 SBP patients. After treatment with radiotherapy to the painful lesion, three patients developed systemic disease within 18 months from diagnosis (Moulopoulos et al. 1993). Furthermore, Liebross et al. observed that among SBP patients with spinal disease, 7/8 staged by WBXR alone developed MM compared to only 1/7 patients who also had spinal MRI (Liebross et al. 1998).
7.3.4 PET-CT
PET/CT Detection of Bone Involvement in Myeloma
FDG-PET/CT is a functional imaging method, which combines demonstration of hypermetabolic activity in intramedullary and extramedullary sites (PET) with evidence of osteolysis (CT). Several studies have shown that PET/CT is more sensitive compared to WBXR for the detection of osteolytic lesions in MM (Zamagni et al. 2007; Bredella et al. 2005; Lütje et al. 2009; Breyer et al. 2006). This has been confirmed by the largest meta-analysis in the field (Regelink et al. 2013). The higher detection rate of PET/CT over WBXR for the presence of osteolytic lesions is especially important for patients with SMM. In one study with 120 patients with SMM based on the previous IMWG criteria (Zamagni et al. 2007), 16% of patients with normal WBXR had positive PET/CT results. The median time to progression (TTP) for PET/CT-positive patients was 1.1 years versus 4.5 for patients with negative PET/CT, while the probability of progression at 2 years for PET/CT-positive patients was 58% (Zamagni et al. 2015a). The largest study in the field involved 188 with suspected SMM examined with PET/CT. PET/CT was positive in 39% of patients. The probability of progression to symptomatic MM within 2 years was 75% for patients with a positive PET/CT under observation versus only 30% for patients with a negative PET/CT. This probability was higher if hypermetabolic activity was combined with underlying osteolysis (2-year progression rate: 87%). The median TTP was 21 versus 60 months for PET/CT-positive and negative patients, respectively (Siontis et al. 2015). The results of these two studies support the integration of changes in imaging requirements in the new IMWG diagnostic criteria for MM; detection of osteolytic lesions by PET/CT is a criterion for symptomatic MM (Rajkumar et al. 2014).
Compared to MRI, as mentioned previously, PET/CT performs equally well in detecting focal lesions, but MRI is better in detecting diffuse disease (Baur-Melnyk et al. 2008; Zamagni et al. 2007; Breyer et al. 2006).
Value of PET/CT for Better Definition of Complete Response to Anti-myeloma Therapy
Data obtained from PET/CT in 40 MM patients, including average SUV and FDG kinetic parameters K1, influx, and fractal dimension, correlated significantly with the percentage of bone marrow infiltration on trephine biopsies (PC %) (Sachpekidis et al. 2015c). Furthermore, PET/CT efficiently detected extramedullary disease in patients both at diagnosis and at relapse (Tirumani et al. 2016). Consequently, PET/CT was tested for better definition of CR in 282 MM patients. It was performed at diagnosis and every 12–18 months afterward. At diagnosis, 42% of MM patients had >3 focal lesions; in 50% of these patients, SUV max was >4.2. After treatment, PET/CT was negative in 70% of patients, while 53% of patients achieved CR according to IMWG criteria. Approximately 30% of patients at CR had positive PET/CT. More importantly, PET/CT negativity was an independent predictor for prolonged PFS and OS in CR patients; median PFS was 50 months for PET/CT-positive and 90 months for PET/CT-negative CR patients (Zamagni et al. 2015b). PET/CT, therefore, provides more accurate definition of CR, and it has been suggested that it should be incorporated to the CR criteria (Paiva et al. 2015).
Prognostic Significance of PET/CT
Several studies have confirmed the value of PET/CT as an independent factor for survival in MM patients both at diagnosis and posttreatment (Bartel et al. 2009; Zamagni et al. 2011; Patriarca et al. 2015; Fonti et al. 2015; Lapa et al. 2014; Cascini et al. 2013). In 192 newly diagnosed patients who underwent ASCT, the presence of extramedullary disease and SUV max >4.2 on PET/CT performed at diagnosis and the persistence of FDG uptake post-ASCT were independent variables, adversely affecting PFS (Zamagni et al. 2011). In the largest study in the field, 429 patients who were treated with total therapy protocols in Arkansas were evaluated with both MRI and PET/CT at diagnosis and 7 days post-ASCT. From the imaging variables, in the multivariate analysis, only the detection of >2 osteolytic lesions by WBXR at diagnosis and the detection of >3 focal lesions by PET/CT, 7 days post-ASCT, were independent prognostic factors for inferior OS. Limitation of this study was the exclusion of diffuse MRI pattern from the analysis (Usmani et al. 2013). Despite this limitation, studies reported to date support the role of PET/CT after therapy, deeming it the best imaging technique for the follow-up of myeloma patients. Indeed, in a recent study which has been reported only in an abstract form, 134 patients who were eligible for treatment with ASCT were randomized to receive eight cycles of bortezomib-lenalidomide-dexamethasone (VRD) followed by 1-year maintenance with lenalidomide or three cycles of VRD followed by ASCT plus two cycles of VRD consolidation and 1-year lenalidomide maintenance. PET/CT and WB-MRI were performed after induction and before maintenance. Both techniques were positive at diagnosis in more than 90% of patients. After induction therapy and before maintenance, more patients continued to have positive MRI than PET/CT (93% versus 55% and 83% versus 21%, respectively), possibly due to earlier reduction of activity of PET/CT lesions. Both after induction and before maintenance, normalization of PET/CT and not of MRI could predict for PFS, while only normalization of PET/CT before maintenance could predict for OS (30-month OS rate: 70% in PET/CT-positive patients versus 94.6% in patients with negative PET/CT, p = 0.01) (Moreau et al. 2015).
At this point, it is crucial to mention that one of the major limitations of PET/CT is the lack of standardization and the controversies regarding SUV level of positivity. Recently, an Italian panel of experts introduced novel criteria for the interpretation of PET/CT images (Nanni et al. 2016). Large, multicenter, studies with prospective evaluation of these new criteria will reveal their clinical impact.
Other PET/CT Indications and Limitations
PET/CT may be used for the work-up of patients with SBP at diagnosis (Fouquet et al. 2014). However, it is not clear whether PET/CT or MRI is more suitable in this setting, since restaging PET/CT after radiotherapy has a number of false-positive findings (Alongi et al. 2015). PET/CT also has a role in patients with nonsecretory or oligosecretory myeloma for the detection of active lesions in the body (Lonial and Kaufman 2013). Major limitations of PET/CT include high cost, lack of availability in many centers and countries, and false-positive results due to inflammation of other underlying pathology.
7.4 Management of Multiple Myeloma Bone Disease
Bisphosphonates (BPs) are the mainstay in the management of MM bone disease. They are artificial analogues of pyrophosphates. In comparison with natural pyrophosphates, bisphosphonates are resistant to phosphatase-induced hydrolysis (Rogers et al. 2000). Bisphosphonates cause osteoclast suppression. They bind to calcium-containing molecules such as hydroxyapatite (Terpos et al. 2009). Osteoclast-induced bone resorption causes exposure of hydroxyapatite. Bisphosphonates bind to the exposed molecules of hydroxyapatite. This fact leads to increased concentration of bisphosphonates within the lytic lesions (Terpos et al. 2009; Boonekamp et al. 1986; Rowe et al. 1999). There are two main groups of bisphosphonates, each with a differently proposed mechanism of action (Terpos et al. 2009). Nonnitrogen-containing bisphosphonates induce osteoclast apoptosis via their cytotoxic ATP analogues. On the other hand, nitrogen-containing bisphosphonates downregulate osteoclast activity by inhibiting the HMG-CoA reductase pathway. Etidronate and clodronate (CLO) are nonnitrogen-containing bisphosphonates. Zoledronic acid (ZOL), ibandronate, pamidronate (PAM), and risedronate are nitrogen-containing bisphosphonates. All bisphosphonates have similar physicochemical properties; however, their anti-resorbing activity is different. Their activity is drastically increased when an amino group is entered into the aliphatic carbon chain. Thus, pamidronate is 100- and 700-fold more potent than etidronate, both in vitro and in vivo, while zoledronic acid and ibandronate show 10,000- to 100,000-fold greater potency than etidronate (Terpos et al. 2014). Bisphosphonates also appear to affect the microenvironment in which tumor cells grow and may have direct anti-tumor activity (Mundy and Yoneda 1998; Yin et al. 1999; Diel et al. 1998; Aparicio et al. 1998; Shipman et al. 1997; Dhodapkar et al. 1998). Possible mechanisms include the reduction of IL-6 secretion by bone marrow stromal cells or the expansion of gamma/delta T cells with possible anti-MM activity. The aim of bisphosphonates use is the reduction of SREs in patients with myeloma bone disease (Christoulas et al. 2009).
According to the latest IMWG Guidelines, bisphosphonates should be initiated in MM patients, with (grade A) or without (grade B) detectable osteolytic bone lesions in conventional radiography, who are receiving anti-myeloma therapy, as well as patients with osteoporosis (grade A) or osteopenia (grade C) due to myeloma. The beneficial effect of zoledronic acid in patients without detectable bone disease by MRI or PET/CT is not known. Oral clodronate, intravenous pamidronate, and intravenous zoledronic acid have been licensed for the management of myeloma bone disease. Etidronate and ibandronate were found to be ineffective for the treatment of bone disease in myeloma patients (Daragon et al. 1993; Menssen et al. 2002). Several studies have evaluated the effects of bisphosphonates (BPs) on SREs and bone pain in patients with MM (Terpos et al. 2013a).
7.4.1 Etidronate
Etidronate was found to be ineffective in two placebo-controlled studies in myeloma patients (Daragon et al. 1993; Belch et al. 1991).
7.4.2 Ibandronate
Ibandronate is ineffective in reducing SREs or improving bone pain in patients with MM (Menssen et al. 2002).
7.4.3 Clodronate
The oral BP, clodronate, reduced the proportion of patients with MM who experienced progression of osteolytic lesions by 50% compared with placebo (24% vs. 12%, p = 0.026) and reduced the time to first SRE and the rate of nonvertebral fracture (6.8% vs. 13.2% for placebo, p = 0.04) in patients with newly diagnosed MM (McCloskey et al. 1998). Two major, placebo-controlled, randomized trials have been performed in MM. Lahtinen et al. reported reduction of the development of new osteolytic lesions by 50% in myeloma patients who received oral CLO for 2 years that was independent of the presence of lytic lesions at baseline (Lahtinen et al. 1992). In the other study, although there was no difference in overall survival (OS) between CLO and placebo patients, patients who received CLO and did not have vertebral fractures at baseline appeared to have a survival advantage (59 vs. 37 months). Both vertebral and nonvertebral fractures as well as the time to first nonvertebral fracture and severe hypercalcemia were reduced in the CLO group after 1 year of follow-up, and at 2 years, the patients who received CLO had better performance status and less myeloma-related pain than patients treated with placebo (McCloskey et al. 2001).
7.4.4 Pamidronate
PAM is an amino-bisphosphonate, which has been administered either orally or intravenously. In one trial, patients with advanced disease and at least one lytic lesion were randomized to placebo or intravenous PAM (Berenson et al. 1998). Administration of PAM resulted in a significant reduction in skeletal-related events (SREs, 24%) versus placebo (41%, p < 0.001). Patients receiving PAM also experienced reduced bone pain and no deterioration in the quality of life (QoL) during the 2-year study. By contrast, administration of oral PAM failed to reduce SREs relative to placebo (Brincker et al. 1998). However, patients treated with oral PAM experienced fewer episodes of severe pain. The overall negative result of this study was attributed to the low absorption of orally administered BPs (Brincker et al. 1998). A recent study for patients with newly diagnosed MM demonstrated that PAM 30 mg monthly had comparable time with SREs and SRE-free survival time as compared with PAM 90 mg monthly. After a minimum of 3 years, patients receiving PAM 30 mg showed a trend toward lower risks of osteonecrosis of the jaw (ONJ) and nephrotoxicity compared with the higher dose. However, the study was not powered to show SRE differences between the two PAM dosages but only to show QoL differences (Gimsing et al. 2010).
7.4.5 Zoledronic Acid (ZOL)
In a non-inferiority randomized phase II trial published by Berenson et al. escalating doses of ZOL were tested in comparison with 90 mg of PAM; in 280 patients, 108 of them affected by MM (the other had metastatic breast cancer to the bone). Both ZOL (at doses of 2 and 4 mg) and PAM significantly reduced SREs in contrast to 0.4 mg ZOL (Berenson et al. 2001). This phase II trial failed to show any superiority of ZOL compared with PAM in terms of SREs, but it was not powered to show differences between the groups.
Bisphosphonates Head to Head
There are only two large randomized studies comparing two different BPs. A Phase III, randomized, double-blind, study was performed to compare the effects of zoledronic acid with pamidronate for patients with myeloma and lytic bone disease or with metastatic breast cancer to the bone (Rosen et al. 2001; Rosen et al. 2003). In the myeloma cohort, there was no difference between the two treatment arms regarding incidence and time to first SRE. However, N-terminal cross-linking telopeptide of collagen type I (NTX) levels, a sensitive marker of bone resorption, normalized more often in the zoledronic acid arm compared with pamidronate-treated patients. More recently, the Medical Research Council (MRC) of the UK compared zoledronic acid (4 mg intravenous every 3–4 weeks or at doses according to creatinine clearance [CrCl] rates) and oral clodronate (1600 mg orally daily) for patients with newly diagnosed, symptomatic MM, who were treated with anti-myeloma therapy (n = 1960 evaluable for efficacy). Zoledronic acid reduced the incidence of SREs both in myeloma patients with or without bone lesions as assessed using conventional radiography, compared with clodronate (Morgan et al. 2010; Morgan et al. 2012). After a median follow-up of 3.7 years, 35% of patients receiving clodronate had experienced SREs versus 27% of patients receiving zoledronic acid (p = 0.004). More importantly, zoledronic acid reduced mortality and extended median survival. Further, subset analysis showed this that treatment extended survival by 10 months over clodronate for patients with osteolytic disease at diagnosis, whereas myeloma patients without bone disease at diagnosis as assessed using conventional radiography had no survival advantage with zoledronic acid (Morgan et al. 2012). These results confirm preclinical studies suggesting indirect and direct anti-myeloma effects of zoledronic acid (Croucher et al. 2003). Possible mechanisms for the anti-myeloma effects of zoledronic acid include direct cytotoxic effect on the tumor cells, the reduction of IL-6 secretion by bone marrow stromal cells, the expansion of gamma/delta T cells with possible anti-MM activity, anti-angiogenic effects, and inhibitory effects in the adhesion molecules. In specific subsets of patients, other BPs have also been associated with improved survival: patients receiving second-line anti-myeloma chemotherapy and treated with pamidronate experienced a borderline improvement in OS over placebo (Berenson et al. 1998), whereas clodronate had an OS advantage in patients without vertebral fractures at presentation relative to placebo (McCloskey et al. 2001). Nevertheless, a Cochrane Database meta-analysis showed that zoledronic acid was the only BP associated with superior OS compared with placebo (hazard ratio, 0.61; 95% CI, 0.28–0.98), but not compared with other BPs (Mhaskar et al. 2012).
Patients with Asymptomatic Myeloma (AMM)
Intravenous PAM (60–90 mg monthly for 12 months) in patients with AMM reduced bone involvement at progression but did not decrease the risk and increase the time to progression (D’Arena et al. 2011). Similarly, intravenous ZOL (4 mg monthly for 12 months) reduced the SRE risk at progression but did not influence the risk of progression of AMM patients (Musto et al. 2008).
Several studies have reported the value of MRI (presence of >1 focal lesion and presence of diffuse pattern of marrow infiltration) in detecting patients with AMM at high risk for progression (Moulopoulos et al. 1995; Hillengass et al. 2010). Since there is no data supporting PFS advantage with bisphosphonates in AMM, bisphosphonates should not be recommended except for a clinical trial of high-risk patients.
Patients with MGUS
MGUS patients are at high risk for developing osteoporosis and pathological fractures (Bida et al. 2009; Kristinsson et al. 2010). Three doses of ZOL (4 mg intravenously every 6 months) increased bone mineral density (BMD) by 15% in the lumbar spine and by 6% in the femoral neck in MGUS patients with osteopenia or osteoporosis (Berenson et al. 2008). Oral alendronate (70 mg/weekly) also increased BMD of the lumbar spine and total femur by 6.1% and 1.5%, respectively, in 50 MGUS patients with vertebral fractures and/or osteoporosis (Pepe et al. 2008).
Patients with Solitary Plasmacytoma (SPB)
Patients with solitary plasmacytoma and no evidence of MM do not require therapy with bisphosphonates. However, these patients should have a whole-body MRI since in a study of 17 patients diagnosed with a solitary plasmacytoma, all showed additional focal lesions or a diffuse infiltration on MRI, leading to a classification as stage I MM (76%), stage II MM (12%), or stage III MM (12%) using the Durie-Salmon PLUS system (Fechtner et al. 2010).
Route of Administration
Strict adherence to dosing recommendations is required for bisphosphonate therapy to effectively reduce and delay SREs in patients with MM. Each patient prescribed bisphosphonate therapy should be instructed about the crucial importance of adherence to the dosing regimen. Although a few randomized, placebo-controlled clinical studies suggest that long-term compliance with oral bisphosphonates such as CLO is satisfactory in MM patients (McCloskey et al. 1998; Lahtinen et al. 1992), compliance with oral bisphosphonate therapy is generally suboptimal (Cramer et al. 2007). Further, the MRC-IX data strongly support the use of intravenous ZOL over CLO in all outcomes measured, including reduction of SREs and improvement in OS (Morgan et al. 2010; Morgan et al. 2012; Morgan et al. 2011). According to the latest IMWG guidelines, intravenous administration of BPs is the preferred choice (grade A). However, oral administration remains an option for patients who cannot receive regular hospital care or in-home nursing visits (grade D) (Terpos et al. 2013a).
Treatment Duration
Intravenous bisphosphonates should be administered at 3- to 4-week intervals to all patients with active MM (grade A). ZOL improves OS and reduces SREs over CLO in patients who received treatment for more than 2 years; thus, it should be given until disease progression in patients not in complete remission (CR) or a very good partial remission (VGPR) and further continued at relapse (grade B). There is no similar evidence for PAM. PAM may be continued in patients with active disease at the physician’s discretion (grade D), and PAM therapy should be resumed after disease relapse (grade D). For patients in CR/VGPR, the optimal treatment duration of BPs is not clear; according to the IMWG BPs should be given for at least 12 months and up to 24 months and then at the physician’s discretion (grade D, panel consensus).
According to the latest IMWG guidelines and due to higher reported rates of ONJ with extended duration of therapy, ZOL or PAM should be discontinued after 1–2 years in patients who have achieved CR or VGPR (grade D, panel consensus) (Terpos et al. 2013a).
7.4.6 Adverse Events
Even though bisphosphonate therapy is well tolerated in patients with MM, clinicians should be alert for symptoms and signs suggesting adverse events (AEs), and patients and healthcare professionals should be instructed on how to prevent and recognize AEs. Potential AEs associated with bisphosphonate administration include hypocalcemia and hypophosphatemia, gastrointestinal events after oral administration, inflammatory reactions at the injection site, and acute-phase reactions after IV administration of amino-bisphosphonates. Renal impairment and ONJ represent infrequent but potentially serious AEs with bisphosphonate use.
Hypocalcemia
Hypocalcemia is usually relatively mild and asymptomatic with bisphosphonate use in most MM patients. The incidence of symptomatic hypocalcemia is much lower in MM patients compared to patients with solid tumors. Although severe hypocalcemia has been observed in some patients (Roux et al. 2003), these events are usually preventable via the administration of oral calcium and vitamin D3. Patients should routinely receive calcium (600 mg/day) and vitamin D3 (400 IU/day) supplementation since 60% of MM patients have vitamin D deficiency or insufficiency (Badros et al. 2008a; Laroche et al. 2010). In vitamin D-deficient patients, there is an increase in bone remodeling. This fact shows that MM patients should be calcium and vitamin D sufficient (Ross et al. 2011). Calcium supplementation should be used with caution in patients with renal insufficiency.
Renal Impairment
Bisphosphonate infusions are associated with both dose- and infusion rate-dependent effects on renal function. The potential for renal damage is dependent on the concentration of bisphosphonate in the bloodstream, and the highest risk is observed after administration of high dosages or rapid infusion. Both ZOL and PAM have been associated with acute renal damage or increases in serum creatinine (Rosen et al. 2001; Berenson et al. 1996). Patients should be closely monitored for compromised renal function by measuring CrCl before administration of each IV bisphosphonate infusion. Current guideline recommendations (Terpos et al. 2013a) states that the dosages of zoledronic acid and clodronate, when administered intravenously, should be reduced for patients who have preexisting renal impairment (CrCl 30–60 mL/min), but there are no clinical studies demonstrating the efficacy of this approach. For patients with CrCl between 30 and 60 mL/min, zoledronic acid dose should be adjusted. Zoledronic acid has not been studied for patients presented with severe renal impairment (CrCl <30 mL/min), and it is not recommended for patients with severe renal impairment (CrCl <30 mL/min). We suggest that pamidronate may be given at a dose of 90 mg infused over 4–6 h for myeloma patients with osteolytic disease and renal insufficiency. Furthermore, serum creatinine and CrCl should be measured before each infusion of pamidronate or zoledronic acid, while BPs should not be administered in short infusion times (<2 h for pamidronate and less than 15 min for zoledronic acid). Bisphosphonate therapy can be resumed, after withholding zoledronic acid or pamidronate for patients who develop renal deterioration during therapy, when serum creatinine returns to within 10% of the baseline (Terpos et al. 2013a).
Osteonecrosis of the Jaw
It is an uncommon complication of intravenous bisphosphonates. It is potentially serious and its main characteristic is the presence of exposed bone in the mouth. Incidence may vary from 2% to 10% (Bamias et al. 2005; Dimopoulos et al. 2006). Longer exposure increases the cumulative incidence of ONJ. One of the main risk factors for the development of ONJ is the invasive dental procedures (Bamias et al. 2005). Other risk factors include poor oral hygiene, age, and duration of myeloma. Zoledronic acid was associated with a higher incidence of ONJ in retrospective evaluations (Zervas et al. 2006). In approximately one half of patients, ONJ lesions will heal (Badros et al. 2008b), but approximately one half of patients who restart bisphosphonate therapy after having stopped it will develop recurrence of ONJ. According to recent IMWG guidelines (Coleman et al. 2005), preventive strategies should be adopted to avoid ONJ. A dental examination is necessary before beginning of bisphosphonate’s course. Patients should also be alerted regarding dental hygiene (grade C, panel consensus). All existing dental condition should be treated before initiation of bisphosphonate therapy (grade C, panel consensus). After bisphosphonate treatment initiation, unnecessary invasive dental procedures should be avoided, and dental health status should be monitored on annual basis (grade C). Patients’ dental health status should be monitored by a physician and a dentist (grade D, panel consensus). Dental problems should be managed conservatively if possible (grade C). If invasive dental procedures are necessary, there should be temporary suspension of bisphosphonate treatment (grade D). The panel consensus suggests the interruption of bisphosphonates before and after dental procedures for a total of 180 days (90 days before and 90 days after procedures such as tooth extraction, dental implants, and surgery to the jaw). Bisphosphonates do not need to be discontinued for routine dental procedures including root canal. Initial treatment of ONJ should include discontinuation of bisphosphonates until healing occurs (grade C). The physician should consider the advantages and disadvantages of continued treatment with bisphosphonates, especially in the relapsed/refractory MM setting (grade D). Preventive measures during bisphosphonate treatment have the potential to reduce the incidence of ONJ about 75% (Dimopoulos et al. 2009b). Prophylactic antibiotic treatment may prevent ONJ occurrence after dental procedures (Montefusco et al. 2008). Management of patients depends on ONJ stage. Stage I (asymptomatic exposed bone, no soft tissue infection) can be managed conservatively with oral antimicrobial rinses. Stage II (exposed bone and associated pain/swelling and/or soft tissue infection) requires culture-directed long-term and maintenance antimicrobial therapy, analgesic management, and, occasionally, minor bony debridement. Stage III disease (pathological fracture and exposed bone or soft tissue infection not manageable with antibiotics) requires surgical resection in order to reduce the volume of necrotic bone in addition to the measures described in stage II (Migliorati et al. 2005). When ONJ occurs, initial therapy should include discontinuation of bisphosphonates until healing occurs (Terpos et al. 2009). The administration of medical ozone (O3) as an oil suspension directly to the ONJ lesions that are below ≤2.5 cm may be another possible therapeutic strategy for those patients who fail to respond to conservative treatment. In such patients, there are reports suggesting that ONJ lesions resolved with complete reconstitution of oral and jaw tissue, with three to ten applications (Ripamonti et al. 2011; Agrillo et al. 2012). In addition, treatment with hyperbaric oxygen has been reported to be helpful.
7.4.7 Future Treatment Options
7.4.7.1 RANKL/RANK Pathway Regulators: Targeting the Osteoclast
RANKL Antagonists
Preclinical models of MM demonstrated that RANKL inhibition can prevent bone destruction from MM. RANKL inhibition with recombinant RANK-Fc protein not only reduced MM-induced osteolysis but also caused a marked decline in tumor burden (Yaccoby et al. 2002; Croucher et al. 2001). Similar results were obtained using recombinant OPG for the treatment of MM-bearing animals (Vanderkerken et al. 2003). These data gave the rationale for using RANKL inhibition in the clinical setting.
Denosumab
A fully human monoclonal antibody has showed high affinity and specificity in binding RANKL and inhibits RANKL-RANK interaction, mimicking the endogenous effects of OPG. In knock-in mice with chimeric (murine/human) RANKL expression, denosumab showed inhibition of bone resorption (Kostenuik et al. 2009).
In a phase I trial, 54 patients with breast cancer (n = 29) or MM (n = 25) with radiologically confirmed bone lesions received a single dose of either denosumab or pamidronate. Denosumab decreased bone resorption within 24 h of administration, as reflected by levels of urinary and serum NTX. That was similar in magnitude but more sustained than with intravenous pamidronate (Body et al. 2006). These results were confirmed in another phase I trial, in which denosumab was given at multiple doses (Yonemori et al. 2008).
In a phase II trial, the ability of denosumab (120 mg given monthly as a subcutaneous injection) to affect bone resorption markers and monoclonal protein levels in MM patients who relapsed after response to prior therapy and in patients with response to most recent therapy and who had stable disease for at least 3 months was evaluated. No patients experienced complete or partial response (≥ 50% reduction in M protein), but seven patients had maximum reduction of ≥25% in serum M protein. Bone resorption markers were reduced by more than 50% with denosumab (Vij et al. 2007).
In another phase II trial, Fizazi et al. evaluated the effect of denosumab in patients with bone metastases and elevated urinary NTX levels despite ongoing intravenous bisphosphonate therapy. Patients were stratified by tumor type (total 111 patients: 9 patients with multiple myeloma, 50 patients with prostate cancer, 46 patients with breast cancer, and 6 patients with another solid tumor) and screening NTX levels and randomly assigned to receive subcutaneous denosumab 180 mg every 4 or every 12 weeks or continue intravenous bisphosphonates every 4 weeks. Denosumab normalized urinary NTX levels more frequently than the continuation of intravenous bisphosphonate (64% vs. 37% respectively, p = 0.01), while fewer patients receiving denosumab experienced on-study SREs than those receiving intravenous bisphosphonate (8% vs. 17%) (Fizazi et al. 2009). This study showed that denosumab inhibits bone resorption and prevents SREs even in patients who are refractory to bisphosphonate therapy.
A meta-analysis of major phase III studies comparing denosumab versus zoledronic acid including mainly patients with solid tumors showed that denosumab was superior in terms of delaying the time to first on-study SRE by 8 months and reducing the risk of the first SRE by 17%. No difference between the two drugs was reported regarding disease progression and overall survival. Hypocalcaemia was more common in denosumab arm, while ONJ was similar with the two drugs (Lipton et al. 2012).
Denosumab appears to have little toxicity, mainly asthenia, and multiple phase III trials of denosumab in patients with bone metastasis are ongoing. However, it is crucial to mention that RANKL is involved in dendritic cell survival and that the anti-RANKL strategy may have an effect on the immune system and a possible increase in infection rate, especially in cancer patients who have already had severe immunodeficiency. For MM patients, while denosumab was comparable to zoledronic acid with respect to the occurrence of SREs, inferior survival occurred in denosumab compared to zoledronic acid-treated patients, but this was a subset analysis from a large phase III trial that involved mostly solid tumor patients with metastatic bone disease (Henry et al. 2011). Interpretation is limited based on the small numbers of MM patients who were enrolled on the trial and imbalance in baseline disease characteristics.
7.4.7.2 Activin-A Inhibitors
Sotatercept (ACE-011) is a fusion protein of the extracellular domain of the high-affinity activin receptor IIA (ActRIIA) and human immunoglobulin G (IgG) Fc domain with potent inhibitory effect on activin, enhancing the deposition of new bone tissue and preventing bone loss. In the preclinical setting, RAP-011, a murine counterpart of sotatercept, prevented the formation of osteolytic lesions in a murine MM model by stimulating bone formation through osteoblasts, while having no effect on osteoclast activity (Chantry et al. 2010).
In a phase I study, in healthy postmenopausal volunteers, single-dose sotatercept was associated with increased serum levels of the bone formation marker bone-specific alkaline phosphatase (bALP) and decreased bone resorption markers CTX and tartrate-resistant acid phosphatase isoform 5b (TRACP-5b), reflecting a decrease in bone resorption and an increase in bone formation (Ruckle et al. 2009). No safety concerns were noted in this study.
In a multicenter phase II trial, patients with osteolytic bone lesions due to MM were randomized to receive either four 28-day cycles of sotatercept or placebo as subcutaneous injection with concomitant anticancer therapy consisting of oral melphalan, prednisolone, and thalidomide (MPT). Sotatercept treatment demonstrated clinically significant increases in biomarkers of bone formation, decreases in bone pain, and anti-tumor activity as well as increase in hemoglobin levels (Chantry et al. 2010), but further research is needed to support these findings. Moreover, increased activin-A secretion was induced by lenalidomide and was canceled by the addition of an activin-A-neutralizing antibody. This effectively restored osteoblast function and subsequently inhibited myeloma-related osteolysis without abrogating the cytotoxic effects of lenalidomide on malignant cells (Scullen et al. 2013) and thus supporting the combination of lenalidomide with an anti-activin-A molecule.
7.4.7.3 Future Agents Targeting the Osteoclast
The pathophysiology of myeloma bone disease is complex. Interactions between myeloma cells, stromal cells, osteoclasts, and osteoblasts create vicious cycles that lead to the development of osteolytic disease and support the myeloma cell growth and survival. The better understanding of this biology has revealed several other pathways that enhance osteoclastogenesis, including the PI3K/AKT/mTOR pathway, the extracellular signal-regulated kinase 1/2 pathway, the nuclear export protein CRM1/XPO1 signaling, the MAPK pathways, the parathyroid hormone-related protein, chemokines and their receptors such as the C-C chemokine receptor types I and II (CCR1 and -2), the C-C motif ligand 3 (CCL-3, previously known as macrophage inflammatory protein 1a) pathways, and others (Christoulas et al. 2009; Oranger et al. 2013; Cao et al. 2013; Breitkreutz et al. 2007; Tai et al. 2013; Cafforio et al. 2013; Moreaux et al. 2011; Choi et al. 2001; Roussou et al. 2009). This knowledge has led to the development of novel drugs that may be used in the near future for the management of lytic bone disease in myeloma patients. AKT pathway is upregulated in marrow monocytes from MM patients, leading to a sustained high expression of RANK in osteoclast precursors. AKT inhibition blocks this upregulation of RANK expression and the subsequent osteoclast formation. In the clinical setting, the novel AKT inhibitor LY294002 blocked the formation of myeloma masses in the bone marrow cavity and dramatically reduced osteoclast formation and osteolytic lesions in SCID mice, suggesting a potential role in the management of MM patients with bone disease in the future (Cao et al. 2013). AZD6244 is a mitogen-activated or extracellular signal-regulated protein kinase (MEK) inhibitor. It has been reported in preclinical models that AZD6244 blocked osteoclast formation in a dose-dependent manner and inhibited bone resorption targeting a later stage of osteoclast differentiation (Breitkreutz et al. 2007). Novel, oral, irreversible, selective nuclear export inhibitors (SINEs) that target CRM1 have shown strong anti-myeloma activity, and they inhibit the MM-induced osteolysis. SINEs have direct anti-osteoclastic function through the blockade of RANKL-induced NF-kB and NFATc1, with almost no impact on osteoblasts, supporting their clinical development for myeloma-related bone disease (Tai et al. 2013). MLN3897 is a novel antagonist of the chemokine receptor CCR1 that demonstrated reduction of osteoclast formation and function by inhibiting the AKT signaling and the CCL-3 pathway in preclinical models (Vallet et al. 2007).
7.4.7.4 Wnt Pathway Regulators: Helping the Osteoblast
DKK-1 Antagonists
DKK-1 plays an important role in the dysfunction of osteoblasts observed in MM. The production of this soluble Wnt inhibitor by MM cells inhibits osteoblast activity, and its serum level reflects the extension of focal bone lesions in MM (Durie 2006; Brincker et al. 1998). Serum DKK-1 is increased not only in symptomatic MM patients at diagnosis but also in relapsed MM, correlating with advanced disease features and the presence of lytic lesions, while serum DKK-1 levels of asymptomatic patients at diagnosis and plateau do not differ from control values (Politou et al. 2006; Terpos et al. 2010a).
BHQ880, an IgG antibody, the first in class, fully human anti-Dkk-1 neutralizing antibody, seems to promote bone formation, and thus it has been shown to inhibit tumor-induced osteolytic disease in preclinical studies (Lipton et al. 2012). Inhibiting Dkk-1 with BHQ880 in the 5T2MM murine model of myeloma reduced the development of osteolytic bone lesions and in vivo growth of MM cells (Steinman et al. 2005). A phase I/II study of BHQ880 in combination with zoledronic acid in relapsed or refractory myeloma patients is ongoing as well as phase II studies in patients with high-risk smoldering MM or untreated MM and renal insufficiency. Results are highly anticipated.
Sclerostin Antagonists
Sclerostin is another Wnt inhibitor, specifically expressed by osteocytes, which inhibits osteoblast-driven bone formation and induces mature osteoblast apoptosis (Moester et al. 2010). Sclerostin deficiency leads to the development of rare bone sclerosing disorders, including sclerosteosis and van Buchem disease. On the other hand, elevated sclerostin is implicated in the mechanisms of bone loss in metabolic bone diseases, such as postmenopausal osteoporosis and thalassemia-associated osteoporosis (Polyzos et al. 2012; Voskaridou et al. 2012). Elevated circulating sclerostin levels correlate with advanced disease features and abnormal bone remodeling in symptomatic myeloma (Terpos et al. 2012b). In particular, MM patients who presented with fractures at diagnosis had very high levels of circulating sclerostin compared with all others (p < 0.01), while sclerostin serum levels correlated negatively with bALP (r = −0.541, p < 0.0001) and positively with CTX (r = 0.524, p < 0.0001) (Terpos et al. 2012b). Romosozumab (AMG 785, CDP7851), an investigational humanized monoclonal antibody that inhibits the activity of sclerostin, has been used in phase II clinical studies in postmenopausal women with low bone mineral density (BMD), demonstrating significant increases in lumbar spine BMD after 12 months (Lewiecki 2011). Studies in MM are planned to start soon.
7.5 Anti-Myeloma Agents
7.5.1 Bortezomib
Bortezomib is the first proteasome inhibitor with established activity against myeloma, with subsequent effects on osteoclasts that leads to reduced bone resorption (von Metzler et al. 2007; Boissy et al. 2008). For patients with relapsed/refractory MM, bortezomib reduces circulating RANKL, osteoclast function, and bone resorption, as assessed by TRACP-5b and CTX serum levels, respectively (Terpos et al. 2006). Furthermore, bortezomib increases osteoblast activity and bone formation both in vitro and for patients with relapsed/refractory MM (Giuliani et al. 2007; Zangari et al. 2005). More specifically, bortezomib increased bone formation markers such as bALP; this increase was observed both among responders and non-responders to bortezomib suggesting a direct effect of bortezomib on osteoblastic activity (Heider et al. 2006). Another proteasome inhibitor, carfilzomib, has been reported to increase bALP in patients with relapsed/refractory MM that responded to therapy (Zangari et al. 2011). Bortezomib in combination with zoledronic acid increased BMD in a subset of MM patients at first relapse even in the presence of dexamethasone (Terpos et al. 2010b). However, when bortezomib was given in combination with other anti-myeloma drugs, such as melphalan and thalidomide (VMDT regimen), no increase in bALP and osteocalcin was observed suggesting that in such combinations, bortezomib seems to lose its beneficial effect on osteoblasts (Terpos et al. 2008). Even in post-autologous stem cell transplantation patients with low myeloma burden, bortezomib in combination with thalidomide and dexamethasone as consolidation therapy failed to produce a significant bone anabolic effect (Terpos et al. 2013b). Nevertheless, in this specific cohort of patients who did not receive BPs during consolidation, bone resorption was reduced, and there were no SREs in responding patients. In a subanalysis of a phase III study in newly diagnosed patients (VISTA trial), bortezomib in combination with melphalan and prednisone (VMP) reduced substantially DKK-1 in responding patients, while the MP regimen increased DKK-1 even in responders (Delforge et al. 2011). In the same study, there was evident bone formation effect in conventional radiography in a subset of VMP patients but not in MP patients (Delforge et al. 2011).
These findings suggest that proteasome inhibition and especially bortezomib, in addition to its antineoplastic effects on tumor cells, may directly stimulate osteoblast differentiation and function and lead to increased bone formation and increased BMD, at least in responders. However, it is unclear if bortezomib alone is sufficient to reverse bone disease in MM patients and heal lytic lesions as evidence of the effect of bortezomib on clinical end points specific to the bone, such as SREs is limited, possibly as a result of relatively short follow-up periods. Prospective trials that specifically investigate end points related to bone formation are needed.
7.5.2 Immunomodulatory Agents
Immunomodulatory agents (IMiDs), such as thalidomide, lenalidomide, and pomalidomide, are highly active agents in the treatment of both newly diagnosed and relapsed/refractory MM. These agents also alter interactions between bone marrow microenvironment and malignant plasma cells and modify abnormal bone metabolism in MM (Christoulas et al. 2009).
Thalidomide
Thalidomide almost completely blocks RANKL-induced osteoclast formation in vitro. In relapsed/refractory MM patients, intermediate dose of thalidomide (200 mg/d) in combination with dexamethasone produced a significant reduction of serum markers of bone resorption [C-telopeptide of collagen type I (CTX) and tartrate-resistant acid phosphatase isoform-5b (TRACP-5b)] and also of sRANKL/OPG ratio (Terpos et al. 2005).
Lenalidomide
Lenalidomide also inhibited osteoclast formation, by targeting PU.1, a critical transcription factor for the development of osteoclasts, and downregulating cathepsin K. The downregulation of PU.1 in hematopoietic progenitor cells resulted in a complete shift of lineage development toward granulocytes. Lenalidomide also reduced the serum levels of sRANKL/OPG ratio in MM patients (Breitkreutz et al. 2008).
Pomalidomide
Pomalidomide, like thalidomide, blocks RANKL-induced osteoclastogenesis in vitro, even at concentrations of 1 μM, which is similar or even lower than that achieved in vivo after the therapeutic administration of this agent. Pomalidomide downregulates transcription factor PU.1, affecting the lineage commitment of osteoclast precursors toward granulocytes instead of mature osteoclasts (Anderson et al. 2006).
7.5.3 Other Novel Agents
Panobinostat is a histone deacetylase inhibitor, which has shown significant preclinical anti-myeloma activity and is currently in phase III trials for relapsed MM. Recently, a potent synergistic antiproliferative effect of panobinostat with zoledronic acid was described in three myeloma cell lines and may result in clinical trials in myeloma patients (Bruzzese et al. 2013).
Bruton’s tyrosine kinase (BTK) has been reported to play an important role in myeloma cell homing to the bone and the subsequent myeloma-induced bone disease (Bam et al. 2013). Several BTK inhibitors have been developed including ibrutinib, which was recently approved for the treatment of mantle cell lymphoma. This new category of drugs has entered into clinical trials in myeloma patients and may be used in the future in patients with bone disease.
Other novel anti-myeloma agents have also shown effects on bone disease in preclinical models. Antibodies against B-cell-activating factor (anti-BAFF) have produced direct anti-myeloma effects and reductions in tartrate-resistant acid phosphatase-positive osteoclasts and in lytic lesions in anti-BAFF-treated animals (Neri et al. 2007). Similarly, SCIO-469, a selective p38a MAPK inhibitor, inhibited MM growth and prevented bone disease in the 5T2MM and 5T33MM animal models (Vanderkerken et al. 2007).
7.6 Kyphoplasty and Vertebroplasty
Several studies have demonstrated that balloon kyphoplasty (BKP) and vertebroplasty are well-tolerated and effective procedures that provide pain relief and improve functional outcomes in patients with painful neoplastic spinal fractures. A single randomized study of 134 patients with bone metastases due to solid tumors and MM demonstrated that treatment of VCFs with BKP was associated with clinically meaningful improvements in physical functioning, back pain, QoL, and ability to perform daily activities relative to nonsurgical management. These benefits persisted throughout the 12-month study (Berenson et al. 2011). A meta-analysis of seven nonrandomized studies of patients with MM or osteolytic metastasis revealed that BKP was associated with reduced pain and improved functional outcomes, benefits that were maintained up to 2 years post-procedure (N = 306). BKP also improved early vertebral height loss and spinal deformity, but these effects were not long term (Bouza et al. 2009). Similarly, a retrospective review of 67 patients with MM-related vertebral compression fractures (VCFs) demonstrated that vertebroplasty provided clinically meaningful improvements in physical functioning, pain, and mobility throughout 12 months of follow-up (McDonald et al. 2008). Several small nonrandomized studies of BKP or BKP and vertebroplasty generated comparable results (Huber et al. 2009; Zou et al. 2010; Dalbayrak et al. 2010). However, the role of vertebroplasty for myeloma patients remains debatable in the absence of prospective data (Zou et al. 2010), as two randomized trials failed to show any benefit of vertebroplasty in patients with osteoporotic fractures versus conservative therapy (Buchbinder et al. 2009; Kallmes et al. 2009). Furthermore, a meta-analysis of 59 studies (56 case series) showed that BKP appears to be more effective than vertebroplasty in relieving pain secondary to cancer-related VCFs and is associated with lower rates of cement leakage (Bhargava et al. 2009).
7.7 Radiation Therapy
Several studies, the majority of which were retrospective and included relatively small patient cohorts, demonstrated that radiotherapy provided pain relief, decreased analgesic use, promoted recalcification, reduced neurologic symptoms, and improved motor function and QoL in patients with MM (Rades et al. 2006; Hirsch et al. 2011; Balducci et al. 2011). In addition, the total administered dose should be limited and the field of therapy restricted, especially when the aim of treatment is pain relief rather than treatment or prevention of pathologic fractures. A single 8–10 Gy fraction is generally recommended. Indeed, single fractions are increasingly preferred to fractionated treatment. No difference in rapidity of onset or duration of pain relief was observed between a single 8 Gy fraction and a fractionated 2-week course of 30 Gy in a randomized study of 288 patients with widespread bony metastases, including 23 patients with MM (Price et al. 1986).
MM accounts for 11% of the most prevalent cancer diagnoses causing spinal cord compression (SCC) (Mak et al. 2011). In the largest retrospective series to date, radiotherapy alone improved motor function in 75% of patients with MM and SCC. One-year local control was 100%, and 1-year survival was 94% (Rades et al. 2007).
7.8 Surgery
Surgery is usually directed toward preventing or repairing axial fractures, unstable spinal fractures, and SCC in myeloma patients. Decompression laminectomy is rarely required in MM patients, but radioresistant MM or retropulsed bone fragments may require surgical intervention (Wedin 2001). In a relatively large study, 75 MM patients were treated surgically (83 interventions) for skeletal complications of the disease. Most of the lesions were in the axial skeleton or the proximal extremities apart from one distal lesion of the fibula, and most surgery was performed in the spine (35 patients). Surgical treatment in these patients was mostly limited to a palliative approach and was well tolerated (Utzschneider et al. 2011).
References
Abe M, Hiura K, Wilde J et al (2004) Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood 104(8):2484–2491
Agrillo A, Filiaci F, Ramieri V et al (2012) Bisphosphonate-related osteonecrosis of the jaw (BRONJ): 5 year experience in the treatment of 131 cases with ozone therapy. Eur Rev Med Pharmacol Sci 16(12):1741–1747
Alongi P, Zanoni L, Incerti E et al (2015) 18F-FDG PET/CT for early post-radiotherapy assessment in solitary bone plasmacytomas. Clin Nucl Med 40:e399–e404
Anderson G, Gries M, Kurihara N et al (2006) Thalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1. Blood 107(8):3098–3105
Aparicio A, Gardner A, Tu Y et al (1998) In vitro cytoreductive effects on multiple myeloma cells induced by bisphosphonates. Leukemia 12:220
Attariwala R, Picker W (2013) Whole body MRI: improved lesion detection and characterization with diffusion weighted techniques. J Magn Reson Imaging 38:253–268
Badros A, Goloubeva O, Terpos E et al (2008a) Prevalence and significance of vitamin D deficiency in multiple myeloma patients. Br J Haematol 142:492–494
Badros A, Terpos E, Katodritou E et al (2008b) Natural history of osteonecrosis of the jaw in patients with multiple myeloma. J Clin Oncol 26(36):5904–5909
Balducci M, Chiesa S, Manfrida S et al (2011) Impact of radiotherapy on pain relief and recalcification in plasma cell neoplasms: long-term experience. Strahlenther Onkol 187:114–119
Bam R, Ling W, Khan S et al (2013) Role of Bruton’s tyrosine kinase in myeloma cell migration and induction of bone disease. Am J Hematol 88(6):463–471
Bamias A, Kastritis E, Bamia C et al (2005) Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 23(34):8580–8587
Bannas P, Hentschel HB, Bley TA et al (2012) Diagnostic performance of whole-body MRI for the detection of persistent or relapsing disease in multiple myeloma after stem cell transplantation. Eur Radiol 22:2007–2012
Bartel TB, Haessler J, Brown TL et al (2009) F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood 114:2068–2076
Bataille R, Chappard D, Marcelli C et al (1991) Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. J Clin Invest 88(1):62–66
Bauerle T, Hillengass J, Fechtner K et al (2009) Multiple myeloma and monoclonal gammopathy of undetermined significance: importance of whole-body versus spinal MR imaging. Radiology 252:477–485
Baur A, Stabler A, Bruning R et al (1998) Diffusion-weighted MR imaging of bone marrow: differentiation of benign versus pathologic compression fractures. Radiology 207:349–356
Baur-Melnyk A, Buhmann S, Durr HR et al (2005) Role of MRI for the diagnosis and prognosis of multiple myeloma. Eur J Radiol 55:56–63
Baur-Melnyk A, Buhmann S, Becker C et al (2008) Whole-body MRI versus whole-body MDCT for staging of multiple myeloma. AJR Am J Roentgenol 190:1097–1104
Belch AR, Bergsagel DE, Wilson K et al (1991) Effect of daily etidronate on the osteolysis of multiple myeloma. J Clin Oncol 9(8):1397–1402
Berenson J, Pflugmacher R, Jarzem P et al (2011) Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 12:225–3596
Berenson JR, Lichtenstein A, Porter L et al (1996) Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med 334(8):488–493
Berenson JR, Lichtenstein A, Porter L et al (1998) Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol 16(2):593–602
Berenson JR, Rosen LS, Howell A et al (2001) Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 91(7):1191–1200
Berenson JR, Yellin O, Boccia RV et al (2008) Zoledronic acid markedly improves bone mineral density for patients with monoclonal gammopathy of undetermined significance and bone loss. Clin Cancer Res 14:6289–6295
Bhargava A, Trivedi D, Kalva L et al (2009) Management of cancer-related vertebral compression fracture: comparison of treatment options: a literature meta-analysis. J Clin Oncol (Meeting Abstracts) 27:e20529
Bida JP, Kyle RA, Therneau TM et al (2009) Disease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17,398 patients. Mayo Clin Proc 84:685–693
Body JJ, Facon T, Coleman RE et al (2006) A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res 12(4):1221–1228
Boissy P, Andersen TL, Lund T et al (2008) Pulse treatment with the proteasome inhibitor bortezomib inhibits osteoclast resorptive activity in clinically relevant conditions. Leuk Res 32(11):1661–1668
Boonekamp PM, van der Wee-Pals LJ, van Wijk-van Lennep MM et al (1986) Two modes of action of bisphosphonates on osteoclastic resorption of mineralized matrix. Bone Miner 1:27
Borggrefe J, Giravent S, Campbell G et al (2015) Association of osteolytic lesions, bone mineral loss and trabecular sclerosis with prevalent vertebral fractures in patients with multiple myeloma. Eur J Radiol 84:2269–2274
Bouza C, Lopez-Cuadrado T, Cediel P et al (2009) Balloon kyphoplasty in malignant spinal fractures: a systematic review and meta-analysis. BMC Palliat Care 8:12
Bredella MA, Steinbach L, Caputo G et al (2005) Value of FDG PET in the assessment of patients with multiple myeloma. AJR Am J Roentgenol 184:1199–1204
Breitkreutz I, Raab MS, Vallet S et al (2007) Targeting MEK1/2 blocks osteoclast differentiation, function and cytokine secretion in multiple myeloma. Br J Haematol 139(1):55–63
Breitkreutz I, Raab MS, Vallet S et al (2008) Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia 22(10):1925–1932
Breyer RJ 3rd, Mulligan ME, Smith SE et al (2006) Comparison of imaging with FDG PET/CT with other imaging modalities in myeloma. Skelet Radiol 35:632–640
Brincker H, Westin J, Abildgaard N et al (1998) Failure of oral pamidronate to reduce skeletal morbidity in multiple myeloma: a double-blind placebo-controlled trial. Danish-Swedish co-operative study group. Br J Haematol 101(2):280–286
Bruce NJ, McCloskey EV, Kanis JA et al (1999) Economic impact of using clodronate in the management of patients with multiple myeloma. Br J Haematol 104:358–364
Bruzzese F, Pucci B, Milone MR et al (2013) Panobinostat synergizes with zoledronic acid in prostate cancer and multiple myeloma models by increasing ROS and modulating mevalonate and p38-MAPK pathways. Cell Death Dis 4:e878
Buchbinder R, Osborne RH, Ebeling PR et al (2009) A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 361:557–568
Cafforio P, Savonarola A, Stucci S et al (2013) PTHrP produced by myeloma plasma cells regulates their survival and pro-osteoclast activity for bone disease progression. J Bone Miner Res 29(1):55–66
Cao H, Zhu K, Qiu L et al (2013) Critical role of AKT protein in myeloma-induced osteoclast formation and osteolysis. J Biol Chem 288(42):30399–30410
Carlson K, Aström G, Nyman R et al (1995) MR imaging of multiple myeloma in tumour mass measurement at diagnosis and during treatment. Acta Radiol 36:9–14
Cascini GL, Falcone C, Console D et al (2013) Whole-body MRI and PET/CT in multiple myeloma patients during staging and after treatment: personal experience in a longitudinal study. Radiol Med 118(6):930–948
Chantry AD, Heath D, Mulivor AW et al (2010) Inhibiting activin-a signaling stimulates bone formation and prevents cancer-induced bone destruction in vivo. J Bone Miner Res 25(12):2633–2646
Choi SJ, Oba Y, Gazitt Y et al (2001) Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. J Clin Invest 108(12):1833–1841
Christoulas D, Terpos E, Dimopoulos MA (2009) Pathogenesis and management of myeloma bone disease. Expert Rev Hematol 2:385–398
Cocks K, Cohen D, Wisloff F et al (2007) An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer 43:1670–1678
Coleman RE (2007) Skeletal complications of malignancy. Cancer 80:1588–1594
Coleman RE, Major P, Lipton A et al (2005) Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol 23(22):4925–4935
Colucci S, Brunetti G, Oranger A et al (2011) Myeloma cells suppress osteoblasts through sclerostin secretion. Blood Cancer J 1:e27
Cramer JA, Gold DT, Silverman SL et al (2007) A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int 18:1023–1031
Cretti F, Perugini G (2016) Patient dose evaluation for the whole-body low-dose multidetector CT (WBLDMDCT) skeleton study in multiple myeloma (MM). Radiol Med 121(2):93–105
Croucher PI, Apperley JF (1998) Bone disease in multiple myeloma. Br J Haematol 103:902–910
Croucher PI, Shipman CM, Lippitt J et al (2001) Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood 98(13):3534–3540
Croucher PI, De Hendrik R, Perry MJ et al (2003) Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res 18(3):482–492
D’Arena G, Gobbi PG, Broglia C et al (2011) Pamidronate versus observation in asymptomatic myeloma: final results with long-term follow-up of a randomized study. Leuk Lymphoma 52:771–775
Dalbayrak S, Onen M, Yilmaz M et al (2010) Clinical and radiographic results of balloon kyphoplasty for treatment of vertebral body metastases and multiple myelomas. J Clin Neurosci 17:219–224
Daragon A, Humez C, Michot C et al (1993) Treatment of multiple myeloma with etidronate: results of a multicentre double-blind study. Eur J Med 2(8):449–452
Delforge M, Terpos E, Richardson PG et al (2011) Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with Bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur J Haematol 86:372–384
Derlin T, Peldschus K, Münster S et al (2013) Comparative diagnostic performance of 18F-FDG PET/CT versus whole-body MRI for determination of remission status in multiple myeloma after stem cell transplantation. Eur Radiol 23:570–578
Dhodapkar MV, Singh J, Mehta J et al (1998) Anti-myeloma activity of pamidronate in vivo. Br J Haematol 103:530
Dhodapkar MV, Sexton R, Waheed S et al (2014) Clinical, genomic, and imaging predictors of myeloma progression from asymptomatic monoclonal gammopathies (SWOG S0120). Blood 123:78–85
Diel IJ, Solomayer EF, Costa SD et al (1998) Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med 339:357
Dimopoulos M, Terpos E, Comenzo RL et al (2009a) International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia 23:1545–1556
Dimopoulos MA, Kastritis E, Bamia C et al (2009b) Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol 20(1):117–120
Dimopoulos MA, Moulopoulos A, Smith T et al (1993) Risk of disease progression in asymptomatic multiple myeloma. Am J Med 94:57–61
Dimopoulos MA, Moulopoulos LA, Maniatis A et al (2000) Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood 96:2037–2044
Dimopoulos MA, Kastritis E, Anagnostopoulos A et al (2006) Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: evidence of increased risk after treatment with zoledronic acid. Haematologica 91(7):968–971
Dimopoulos MA, Hillengass J, Usmani S et al (2015) Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol 33(6):657–664. https://doi.org/10.1200/JCO.2014.57.9961
Durie BGM (2006) The role of anatomic and functional staging in myeloma: description of Durie/Salmon plus staging system. Eur J Cancer 42:1539–1543
Fechtner K, Hillengass J, Delorme S et al (2010) Staging monoclonal plasma cell disease: comparison of the Durie-Salmon and the Durie-Salmon PLUS staging systems. Radiology 257:195–204
Fizazi K, Lipton A, Mariette X et al (2009) Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol 27(10):1564–1571
Fonti R, Pace L, Cerchione C et al (2015) 18F-FDG PET/CT, 99mTc-MIBI, and MRI in the prediction of outcome of patients with multiple myeloma: a comparative study. Clin Nucl Med 40:303–308
Fouquet G, Guidez S, Herbaux C et al (2014) Impact of initial FDG-PET/CT and serum-free light chain on transformation of conventionally defined solitary plasmacytoma to multiple myeloma. Clin Cancer Res 20:3254–3260
Fraioli F, Punwani S (2014) Clinical and research applications of simultaneous positron emission tomography and MRI. Br J Radiol 87:20130464
Ghanem N, Lohrmann C, Engelhardt M et al (2006) Whole-body MRI in the detection of bone marrow infiltration in patients with plasma cell neoplasms in comparison to the radiological skeletal survey. Eur Radiol 16:1005–1014
Giles SL, Messiou C, Collins DJ et al (2014) Whole-body diffusion-weighted MR imaging for assessment of treatment response in myeloma. Radiology 271(3):785–794
Giles SL, deSouza NM, Collins DJ et al (2015) Assessing myeloma bone disease with whole-body diffusion-weighted imaging: comparison with x-ray skeletal survey by region and relationship with laboratory estimates of disease burden. Clin Radiol 70:614–621
Gimsing P, Carlson K, Turesson I et al (2010) Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): a double-blind, randomised controlled trial. Lancet Oncol 11(10):973–982
Giuliani N, Morandi F, Tagliaferri S et al (2007) The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood 110(1):334–338
Gleeson TG, Moriarty J, Shortt CP et al (2009) Accuracy of whole-body low-dose multidetector CT (WBLDCT) versus skeletal survey in the detection of myelomatous lesions, and correlation of disease distribution with whole-body MRI (WBMRI). Skelet Radiol 38:225–236
Heider U, Kaiser M, Muller C et al (2006) Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol 77(3):233–238
Henry DH, Costa L, Goldwasser F et al (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29(9):1125–1132
Hillengass J, Landgren O (2013) Challenges and opportunities of novel imaging techniques in monoclonal plasma cell disorders: imaging “early myeloma”. Leuk Lymphoma 54:1355–1363
Hillengass J, Wasser K, Delorme S et al (2007) Lumbar bone marrow microcirculation measurements from dynamic contrast-enhanced magnetic resonance imaging is a predictor of event-free survival in progressive multiple myeloma. Clin Cancer Res 13:475–481
Hillengass J, Fechtner K, Weber MA et al (2010) Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol 28:1606–1610
Hillengass J, Bäuerle T, Bartl R et al (2011) Diffusion-weighted imaging for non-invasive and quantitative monitoring of bone marrow infiltration in patients with monoclonal plasma cell disease: a comparative study with histology. Br J Haematol 153:721–728
Hillengass J, Ayyaz S, Kilk K et al (2012) Changes in magnetic resonance imaging before and after autologous stem cell transplantation correlate with response and survival in multiple myeloma. Haematologica 97:1757–1760
Hillengass J, Weber MA, Kilk K et al (2014) Prognostic significance of whole-body MRI in patients with monoclonal gammopathy of undetermined significance. Leukemia 28:174–178
Hirsch AE, Jha RM, Yoo AJ et al (2011) The use of vertebral augmentation and external beam radiation therapy in the multimodal management of malignant vertebral compression fractures. Pain Physician 14:447–458
Horger M, Claussen CD, Bross-Bach U et al (2005) Whole-body low-dose multidetector row-CT in the diagnosis of multiple myeloma: an alternative to conventional radiography. Eur J Radiol 54:289–297
Horger M, Weisel K, Horger W et al (2011) Whole-body diffusion-weighted MRI with apparent diffusion coefficient mapping for early response monitoring inmultiple myeloma: preliminary results. AJR Am J Roentgenol 196:W790–W795
Huang SY, Chen BB, HY L et al (2012) Correlation among DCE-MRI measurements of bone marrow angiogenesis, microvessel density, and extramedullary disease in patients with multiple myeloma. Am J Hematol 87:837–839
Huber F, McArthur N, Tanner M et al (2009) Kyphoplasty for patients with multiple myeloma is a safe surgical procedure: results from a large patient cohort. Clin Lymphoma Myeloma 9:375–380
Ippolito D, Besostri V, Bonaffini PA et al (2013) Diagnostic value of whole-body low-dose computed tomography (WBLDCT) in bone lesions detection in patients with multiple myeloma. Eur J Radiol 82:2322–2327
Jemal A, Siegel R, Xu J et al (2010) Cancer statistics. CA Cancer J Clin 60:277–300
Kallmes DF, Comstock BA, Heagerty PJ et al (2009) A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 361:569–579
Kastritis E, Zervas K, Symeonidis A et al (2009) Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia 23:1152–1157
Kastritis E, Terpos E, Moulopoulos L et al (2013) Extensive bone marrow infiltration and abnormal free light chain ratio identifies patients with asymptomatic myeloma at high risk for progression to symptomatic disease. Leukemia 27:947–953
Khalafallah AA, Snarski A, Heng R et al (2013) Assessment of whole body MRI and sestamibi technetium-99m bone marrow scan in prediction of multiple myeloma disease progression and outcome: a prospective comparative study. BMJ Open 3:pii: e002025
Kostenuik P, Nguyen H, McCabe J et al (2009) Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases bone density in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res 24(2):182–195
Kristinsson SY, Tang M, Pfeiffer RM et al (2010) Monoclonal gammopathy of undetermined significance and risk of skeletal fractures: a population-based study. Blood 116:2651–2655
Kropil P, Fenk R, Fritz LB et al (2008) Comparison of whole- body 64-slice multidetector computed tomography and conventional radiography in staging of multiple myeloma. Eur Radiol 18:51–58
Kumar SK, Rajkumar SV, Dispenzieri A et al (2008) Improved survival in multiple myeloma and the impact of novel therapies. Blood 111:2516–2520
Kyle RA, Gertz MA, Witzig TE et al (2003) Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 78:21–33
Lafforgue P, Dahan E, Chagnaud C et al (1993) Early-stage avascular necrosis of the femoral head: MR imaging for prognosis in 31 cases with at least 2 years of follow-up. Radiology 187:199–204
Lahtinen R, Laakso M, Palva I et al (1992) Randomised, placebo-controlled multicentre trial of clodronate in multiple myeloma. Lancet 340(8832):1049–1052
Lapa C, Lückerath K, Malzahn U et al (2014) 18 FDG-PET/CT for prognostic stratification of patients with multiple myeloma relapse after stem cell transplantation. Oncotarget 5(17):7381–7391
Laroche M, Lemaire O, Attal M (2010) Vitamin D deficiency does not alter biochemical markers of bone metabolism before or after autograft in patients with multiple myeloma. Eur J Haematol 85:65–67
Lecouvet FE, Vande Berg BC, Michaux L et al (1998) Stage III multiple myeloma: clinical and prognostic value of spinal bone marrow MR imaging. Radiology 209:653–660
Lecouvet FE, Malghem J, Michaux L et al (1999) Skeletal survey in advanced multiple myeloma: radiographic versus MR imaging survey. Br J Haematol 106:35–39
Lemke A, Stieltjes B, Schad LR et al (2011) Toward an optimal distribution of b values for intravoxel incoherent motion imaging. Magn Reson Imaging 29:766–776
Lewiecki EM (2011) Sclerostin: a novel target for intervention in the treatment of osteoporosis. Discov Med 12(65):263–273
Libshitz HI, Malthouse SR, Cunningham D et al (1992) Multiple myeloma: appearance at MR imaging. Radiology 182:833–837
Liebross RH, Ha CS, Cox JD et al (1998) Solitary bone plasmacytoma: outcome and prognostic factors following radiotherapy. Int J Radiat Oncol Biol Phys 41:1063–1067
Lipton A, Fizazi K, Stopeck AT et al (2012) Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 48(16):3082–3092
Lonial S, Kaufman JL (2013) Non-secretory myeloma: a clinician’s guide. Oncology (Williston Park) 27:924–928
Ludwig H, Frühwald F, Tscholakoff D et al (1987) Magnetic resonance imaging of the spine in multiple myeloma. Lancet 2:364–366
Lütje S, de Rooy JW, Croockewit S et al (2009) Role of radiography, MRI and FDG-PET/CT in diagnosing, staging and therapeutical evaluation of patients with multiple myeloma. Ann Hematol 88:1161–1168
Mak KS, Lee LK, Mak RH et al (2011) Incidence and treatment patterns in hospitalizations for malignant spinal cord compression in the United States, 1998–2006. Int J Radiat Oncol Biol Phys 80:824–831
McCloskey EV, MacLennan IC, Drayson MT et al (1998) A randomized trial of the effect of clodronate on skeletal morbidity in multiple myeloma. MRC Working Party on Leukaemia in Adults. Br J Haematol 100:317–325
McCloskey EV, Dunn JA, Kanis JA et al (2001) Long-term follow-up of a prospective, double-blind, placebo-controlled randomized trial of clodronate in multiple myeloma. Br J Haematol 113(4):1035–1043
McDonald RJ, Trout AT, Gray LA et al (2008) Vertebroplasty in multiple myeloma: outcomes in a large patient series. AJNR Am J Neuroradiol 29:642–648
Menssen HD, Sakalova A, Fontana A et al (2002) Effects of long-term intravenous ibandronate therapy on skeletal-related events, survival, and bone resorption markers in patients with advanced multiple myeloma. J Clin Oncol 20(9):2353–2359
Merz M, Hielscher T, Wagner B et al (2014) Predictive value of longitudinal whole-body magnetic resonance imaging in patients with smoldering multiple myeloma. Leukemia 28(9):1902–1908
Messiou C, Collins DJ, Morgan VA et al (2011) Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur Radiol 21:1713–1718
Messiou C, Giles S, Collins DJ et al (2012) Assessing response of myeloma bone disease with diffusion-weighted MRI. Br J Radiol 85:e1198–e1203
Mhaskar R, Redzepovic J, Wheatley K et al (2012) Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev 5:CD003188
Migliorati CA, Casiglia J, Epstein J et al (2005) Managing the care of patients with bisphosphonate-associated osteonecrosis an American Academy of Oral Medicine position paper. J Am Dent Assoc 136(12):1658–1668
Moester MJ, Papapoulos SE, Löwik CW et al (2010) Sclerostin: current knowledge and future perspectives. Calcif Tissue Int 87(2):99–107
Montefusco V, Gay F, Spina F et al (2008) Antibiotic prophylaxis before dental procedures may reduce the incidence of osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates. Leuk Lymphoma 49(11):2156–2162
Moreau P, Attal M, Karlin L et al (2015) Prospective evaluation of MRI and PET-CT at diagnosis and before maintenance therapy in symptomatic patients with Multiple Myeloma included in the IFM/DFCI 2009 trial. Blood 126:395. (ASH abstract)
Moreaux J, Hose D, Kassambara A et al (2011) Osteoclast-gene expression profiling reveals osteoclast-derived CCR2 chemokines promoting myeloma cell migration. Blood 117(4):1280–1290
Morgan GJ, Davies FE, Gregory WM et al (2010) First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet 376(9757):1989–1999
Morgan GJ, Child JA, Gregory WM et al (2011) Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): secondary outcomes from a randomised controlled trial. Lancet Oncol 12:743–752
Morgan GJ, Davies FE, Gregory WM et al (2012) Effects of induction and maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: MRC Myeloma IX trial. Blood 119(23):5374–5383
Moulopoulos LA, Dimopoulos MA (1997) Magnetic resonance imaging of the bone marrow in hematologic malignancies. Blood 90:2127–2147
Moulopoulos LA, Varma DG, Dimopoulos MA et al (1992) Multiple myeloma: spinal MR imaging in patients with untreated newly diagnosed disease. Radiology 185:833–840
Moulopoulos LA, Dimopoulos MA, Weber D et al (1993) Magnetic resonance imaging in the staging of solitary plasmacytoma of bone. J Clin Oncol 11:1311–1315
Moulopoulos LA, Dimopoulos MA, Alexanian R et al (1994) Multiple myeloma: MR patterns of response to treatment. Radiology 193:441–446
Moulopoulos LA, Dimopoulos MA, Smith TL et al (1995) Prognostic significance of magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol 13:251–256
Moulopoulos LA, Gika D, Anagnostopoulos A et al (2005) Prognostic significance of magnetic resonance imaging of bone marrow in previously untreated patients with multiple myeloma. Ann Oncol 16:1824–1828
Moulopoulos LA, Dimopoulos MA, Christoulas D et al (2010) Diffuse MRI marrow pattern correlates with increased angiogenesis, advanced disease features and poor prognosis in newly diagnosed myeloma treated with novel agents. Leukemia 24:1206–1212
Moulopoulos LA, Dimopoulos MA, Kastritis E et al (2012) Diffuse pattern of bone marrow involvement on magnetic resonance imaging is associated with high risk cytogenetics and poor outcome in newly diagnosed, symptomatic patients with multiple myeloma: a single center experience on 228 patients. Am J Hematol 87:861–864
Muller MF, Edelman RR (1995) Echo planar imaging of the abdomen. Top Magn Reson Imaging 7:112–119
Mundy GR, Yoneda T (1998) Bisphosphonates as anticancer drugs. N Engl J Med 339:398
Musto P, Petrucci MT, Bringhen S et al (2008) A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer 113:1588–1595
Nanni C, Zamagni E, Versari A et al (2016) Image interpretation criteria for FDG PET/CT in multiple myeloma: a new proposal from an Italian expert panel. IMPeTUs (Italian Myeloma criteria for PET USe). Eur J Nucl Med Mol Imaging 43:414–421
Narquin S, Ingrand P, Azais I et al (2013) Comparison of whole-body diffusion MRI and conventional radiological assessment in the staging of myeloma. Diagn Interv Imaging 94:629–636
Neri P, Kumar S, Fulciniti MT et al (2007) Neutralizing B-cell activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin Cancer Res 13(19):5903–5909
Nonomura Y, Yasumoto M, Yoshimura R et al (2001) Relationship between bone marrow cellularity and apparent diffusion coefficient. J Mag Reson Imaging 13:757–760
Oranger A, Carbone C, Izzo M et al (2013) Cellular mechanisms of multiple myeloma bone disease. Clin Dev Immunol 2013:289458
Oshima T, Abe M, Asano J et al (2005) Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood 106:3160–3165
Paiva B, van Dongen JJ, Orfao A et al (2015) New criteria for response assessment: role of minimal residual disease in multiple myeloma. Blood 125:3059–3068
Parker SL, Davis KJ, Wingo PA et al (1998) Cancer statistics by race and ethnicity. CA Cancer J Clin 48:31–48
Patriarca F, Carobolante F, Zamagni E et al (2015) The role of positron emission tomography with 18F-fluorodeoxyglucose integrated with computed tomography in the evaluation of patients with multiple myeloma undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant 21:1068–1073
Pawlyn C, Fowkes L, Otero S et al (2015) Whole-body diffusion-weighted MRI: a new gold standard for assessing disease burden in patients with multiple myeloma? Leukemia 30(6):1446–1448. https://doi.org/10.1038/leu.2015.338
Pearse RN, Sordillo EM, Yaccoby S et al (2001) Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci U S A 98:11581–11586
Pepe J, Petrucci MT, Nofroni I et al (2006) Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br J Haematol 134:485–490
Pepe J, Petrucci MT, Mascia ML et al (2008) The effects of alendronate treatment in osteoporotic patients affected by monoclonal gammopathy of undetermined significance. Calcif Tissue Int 82:418–426
Pianko MJ, Terpos E, Roodman GD et al (2014) Whole-body low-dose computed tomography and advanced imaging techniques for multiple myeloma bone disease. Clin Cancer Res 20:5888–5897
Politou MC, Heath DJ, Rahemtulla A et al (2006) Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int J Cancer 119:1728–1731
Polyzos SA, Anastasilakis AD, Bratengeier C et al (2012) Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women—the six-month effect of risedronate and teriparatide. Osteoporos Int 23(3):1171–1176
Price P, Hoskin PJ, Easton D et al (1986) Prospective randomised trial of single and multifraction radiotherapy schedules in the treatment of painful bony metastases. Radiother Oncol 6:247–255
Princewill K, Kyere S, Awan O et al (2013) Multiple myeloma lesion detection with whole body CT versus radiographic skeletal survey. Cancer Investig 31:206–211
Rades D, Hoskin PJ, Stalpers LJ et al (2006) Short-course radiotherapy is not optimal for spinal cord compression due to myeloma. Int J Radiat Oncol Biol Phys 64:1452–1457
Rades D, Veninga T, Stalpers LJ et al (2007) Outcome after radiotherapy alone for metastatic spinal cord compression in patients with oligometastases. J Clin Oncol 25:50–56
Raje N, Roodman GD (2011) Advances in the biology and treatment of bone disease in multiple myeloma. Clin Cancer Res 17:1278–1286
Rajkumar SV, Dimopoulos MA, Palumbo A et al (2014) International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15:e538–e548
Regelink JC, Minnema MC, Terpos E et al (2013) Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: a systematic review. Br J Haematol 162:50–61
Ripamonti CI, Cislaghi E, Mariani L et al (2011) Efficacy and safety of medical ozone (O(3)) delivered in oil suspension applications for the treatment of osteonecrosis of the jaw in patients with bone metastases treated with bisphosphonates: preliminary results of a phase I-II study. Oral Oncol 47(3):185–190
Rogers MJ, Gordon S, Benford HL et al (2000) Cellular and molecular mechanisms of action of bisphosphonates. Cancer 88:2961
Roodman GD (2008) Novel targets for myeloma bone disease. Expert Opin Ther Targets 12:1377–1387
Rosen LS, Gordon D, Kaminski M et al (2001) Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 7(5):377–387
Rosen LS, Gordon D, Kaminski M et al (2003) Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 98(8):1735–1744
Ross AC, Manson JE, Abrams SA et al (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58
Roussou M, Tasidou A, Dimopoulos MA et al (2009) Increased expression of macrophage inflammatory protein-1alpha on trephine biopsies correlates with extensive bone disease, increased angiogenesis and advanced stage in newly diagnosed patients with multiple myeloma. Leukemia 23(11):2177–2181
Roux S, Bergot C, Fermand JP et al (2003) Evaluation of bone mineral density and fat-lean distribution in patients with multiple myeloma in sustained remission. J Bone Miner Res 18:231–236
Rowe DJ, Etre LA, Lovdahl MJ et al (1999) Relationship between bisphosphonate concentration and osteoclast activity and viability. In Vitro Cell Dev Biol Anim 35:383
Ruckle J, Jacobs M, Kramer W et al (2009) Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res 24(4):744–752
Sachpekidis C, Mosebach J, Freitag MT et al (2015a) Application of (18)F-FDG PET and diffusion weighted imaging (DWI) in multiple myeloma: comparison of functional imaging modalities. Am J Nucl Med Mol Imaging 5:479–492
Sachpekidis C, Hillengass J, Goldschmidt H et al (2015b) Comparison of (18)F-FDG PET/CT and PET/MRI in patients with multiple myeloma. Am J Nucl Med Mol Imaging 5:469–478
Sachpekidis C, Mai EK, Goldschmidt H et al (2015c) (18)F-FDG dynamic PET/CT in patients with multiple myeloma: patterns of tracer uptake and correlation with bone marrow plasma cell infiltration rate. Clin Nucl Med 40:e300–e307
Scullen T, Santo L, Vallet S et al (2013) Lenalidomide in combination with an activin A-neutralizing antibody: preclinical rationale for a novel anti-myeloma strategy. Leukemia 27(8):1715–1721
Shipman CM, Rogers MJ, Apperley JF et al (1997) Bisphosphonates induce apoptosis in human myeloma cell lines: a novel anti-tumour activity. Br J Haematol 98:665
Silbermann R, Roodman GD (2016) Current controversies in the management of myeloma bone disease. J Cell Physiol 231(11):2374–2379. https://doi.org/10.1002/jcp.25351
Siontis B, Kumar S, Dispenzieri A et al (2015) Positron emission tomography-computed tomography in the diagnostic evaluation of smoldering multiple myeloma: identification of patients needing therapy. Blood Cancer J 5:e364
Song MK, Chung JS, Lee JJ et al (2014) Magnetic resonance imaging pattern of bone marrow involvement as a new predictive parameter of disease progression in newly diagnosed patients with multiple myeloma eligible for autologous stem cell transplantation. Br J Haematol 165(6):777–785
Spinnato P, Bazzocchi A, Brioli A et al (2012) Contrast enhanced MRI and 18F-FDG PET-CT in the assessment of multiple myeloma: a comparison of results in different phases of the disease. Eur J Radiol 81:4013–4018
Steinman RM, Bonifaz L, Fujii S et al (2005) The innate functions of dendritic cells in peripheral lymphoid tissues. Adv Exp Med Biol 560:83–97
Sugatani T, Alvarez UM, Hruska KA (2003) Activin A stimulates IkappaB-alpha/NFkappaB and RANK expression for osteoclast differentiation, but not AKT survival pathway in osteoclast precursors. J Cell Biochem 90:59–67
Syed IS, Glockner JF, Feng D et al (2010) Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging 3:155–164
Tai YT, Landesman Y, Acharya C et al (2013) CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia 28(1):155–165
Tanaka Y, Abe M, Hiasa M et al (2007) Myeloma cell-osteoclast interaction enhances angiogenesis together with bone resorption: a role for vascular endothelial cell growth factor and osteopontin. Clin Cancer Res 13(3):816–823
Terpos E, Dimopoulos MA (2005) Myeloma bone disease: pathophysiology and management. Ann Oncol 16:1223–1231
Terpos E, Szydlo R, Apperley JF et al (2003) Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 102:1064–1069
Terpos E, Mihou D, Szydlo R et al (2005) The combination of intermediate doses of thalidomide with dexamethasone is an effective treatment for patients with refractory/relapsed multiple myeloma and normalizes abnormal bone remodeling, through the reduction of sRANKL/osteoprotegerin ratio. Leukemia 19(11):1969–1976
Terpos E, Heath DJ, Rahemtulla A et al (2006) Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-kappaB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol 135(5):688–692
Terpos E, Kastritis E, Roussou M et al (2008) The combination of bortezomib, melphalan, dexamethasone and intermittent thalidomide is an effective regimen for relapsed/refractory myeloma and is associated with improvement of abnormal bone metabolism and angiogenesis. Leukemia 22(12):2247–2256
Terpos E, Sezer O, Croucher PI et al (2009) The use of bisphosphonates in multiple myeloma recommendations of an expert panel on behalf of the European Myeloma Network. Ann Oncol 20:1303
Terpos E, Christoulas D, Papatheodorou A et al (2010a) Dickkopf-1 is elevated in newly-diagnosed, symptomatic patients and in relapsed patients with multiple myeloma; correlations with advanced disease features: a single-center experience in 284 patients. Presented at: 15th Congress of the European Hematology Association, Barcelona, Spain, 10–13 June 2010
Terpos E, Christoulas D, Kokkoris P et al (2010b) Increased bone mineral density in a subset of patients with relapsed multiple myeloma who received the combination of bortezomib, dexamethasone and zoledronic acid. Ann Oncol 21(7):1561–1562
Terpos E, Moulopoulos LA, Dimopoulos MA (2011) Advances in imaging and the management of myeloma bone disease. J Clin Oncol 29:1907–1915
Terpos E, Kastritis E, Christoulas D et al (2012a) Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy. Ann Oncol 23:2681–2686
Terpos E, Christoulas D, Katodritou E et al (2012b) Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int J Cancer 131:1466–1471
Terpos E, Morgan G, Dimopoulos MA et al (2013a) International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol 31(18):2347–2357
Terpos E, Christoulas D, Kastritis E et al (2013b) VTD consolidation, without bisphosphonates, reduces bone resorption and is associated with a very low incidence of skeletal-related events in myeloma patients post-ASCT. Leukemia 28(4):928–934
Terpos E, Berenson J, Raje N et al (2014) Management of bone disease in multiple myeloma. Expert Rev Hematol 7(1):113–125
Terpos E, Kleber M, Engelhardt M et al (2015a) European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica 100:1254–1266
Terpos E, Koutoulidis V, Fontara S et al (2015b) Diffusion-Weighted Imaging improves accuracy in the diagnosis of MRI patterns of marrow involvement in newly diagnosed myeloma: results of a prospective study in 99 patients. Blood 126:4178. (ASH abstract)
Tertti R, Alanen A, Remes K (1995) The value of magnetic resonance imaging in screening myeloma lesions of the lumbar spine. Br J Haematol 91:658–660
Tian E, Zhan F, Walker R et al (2003) The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 349:2483–2494
Tirumani SH, Sakellis C, Jacene H et al (2016) Role of FDG-PET/CT in extramedullary multiple myeloma: correlation of FDG-PET/CT findings with clinical outcome. Clin Nucl Med 41:e7–e13
Usmani SZ, Mitchell A, Waheed S et al (2013) Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy. Blood 121:1819–1823
Utzschneider S, Schmidt H, Weber P et al (2011) Surgical therapy of skeletal complications in multiple myeloma. Int Orthop 35:1209–1213
Vallet S, Raje N, Ishitsuka K et al (2007) MLN3897, a novel CCR1 inhibitor, impairs osteoclastogenesis and inhibits the interaction of multiple myeloma cells and osteoclasts. Blood 110(10):3744–3752
Van de Donk NW, Palumbo A, Johnsen HE et al (2014) The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica 99(6):984–996
Vande Berg BC, Michaux L, Lecouvet FE et al (1997) Nonmyelomatous monoclonal gammopathy: correlation of bone marrow MR images with laboratory findings and spontaneous clinical outcome. Radiology 202:247–251
Vanderkerken K, De Leenheer E, Shipman C et al (2003) Recombinant osteoprotegerin decreases tumor burden and increases survival in a murine model of multiple myeloma. Cancer Res 63(2):287–289
Vanderkerken K, Medicherla S, Coulton L et al (2007) Inhibition of p38alpha mitogen-activated protein kinase prevents the development of osteolytic bone disease, reduces tumor burden, and increases survival in murine models of multiple myeloma. Cancer Res 67(10):4572–4577
Varettoni M, Corso A, Pica G et al (2010) Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol 21:325–330
Vij R, Horvath N, Spencer A, Kitagawa K et al (2007) An open-label, Phase 2 trial of denosumab in the treatment of relapsed (R) or plateau-phase (PP) multiple myeloma (MM). Presented at: 49th ASH Annual Meeting and Exposition, 8–11 Dec 2007, Atlanta, GA, USA
von Metzler I, Krebbel H, Hecht M et al (2007) Bortezomib inhibits human osteoclastogenesis. Leukemia 21(9):2025–2034
Voskaridou E, Christoulas D, Plata E et al (2012) High circulating sclerostin is present in patients with thalassemia-associated osteoporosis and correlates with bone mineral density. Horm Metab Res 44(12):909–913
Waheed S, Mitchell A, Usmani S et al (2013) Standard and novel imaging methods for multiple myeloma: correlates with prognostic laboratory variables including gene expression profiling data. Haematologica 98:71–78
Walker R, Barlogie B, Haessler J et al (2007) Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. J Clin Oncol 25:1121–1128
Wang Y (2000) Description of parallel imaging in MRI using multiple coils. Magn Reson Med 44:495–499
Wedin R (2001) Surgical treatment for pathologic fracture. Acta Orthop Scand Suppl 72(2p):1–29
Weininger M, Lauterbach B, Knop S et al (2008) Whole-body MRI of multiple myeloma: comparison of different MRI sequences in assessment of different growth patterns. Eur J Radiol 69:339–345
Wolf MB, Murray F, Kilk K et al (2014) Sensitivity of whole-body CT and MRI versus projection radiography in the detection of osteolyses in patients with monoclonal plasma cell disease. Eur J Radiol 83:1222–1230
Xu X, Ma L, Zhang JS et al (2008) Feasibility of whole body diffusion weighted imaging in detecting bone metastasis on 3.0T MR scanner. Chin Med Sci J 23:151–157
Yaccoby S, Pearse RN, Johnson CL et al (2002) Myeloma interacts with the bone marrow microenvironment to induce osteoclastogenesis and is dependent on osteoclast activity. Br J Haematol 116(2):278–290
Yin JJ, Selander K, Chirgwin JM et al (1999) TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 103:197
Yonemori K, Fujiwara Y, Minami H et al (2008) Phase 1 trial of denosumab safety, pharmacokinetics, and pharmacodynamics in Japanese women with breast cancer-related bone metastases. Cancer Sci 99(6):1237–1242
Zamagni E, Nanni C, Patriarca F et al (2007) A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica 92:50–55
Zamagni E, Patriarca F, Nanni C et al (2011) Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood 118:5989–5995
Zamagni E, Nanni C, Gay F et al (2015a) 18F-FDG PET/CT focal, but not osteolytic, lesions predict the progression of smoldering myeloma to active disease. Leukemia 30(2):417–422
Zamagni E, Nanni C, Mancuso K et al (2015b) PET/CT improves the definition of complete response and allows to detect otherwise unidentifiable skeletal progression in multiple myeloma. Clin Cancer Res 21:4384–4390
Zangari M, Esseltine D, Lee CK et al (2005) Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol 131(1):71–73
Zangari M, Aujay M, Zhan F et al (2011) Alkaline phosphatase variation during carfilzomib treatment is associated with best response in multiple myeloma patients. Eur J Haematol 86(6):484–487
Zechmann CM, Traine L, Meissner T et al (2012) Parametric histogram analysis of dynamic contrast-enhanced MRI in multiple myeloma: a technique to evaluate angiogenic response to therapy? Acad Radiol 19:100–108
Zervas K, Verrou E, Teleioudis Z et al (2006) Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: a single-centre experience in 303 patients. Br J Haematol 134(6):620–623
Zou J, Mei X, Gan M et al (2010) Kyphoplasty for spinal fractures from multiple myeloma. J Surg Oncol 102:43–47
Zwick S, Brix G, Tofts PS et al (2010) Simulation-based comparison of two approaches frequently used for dynamic contrast-enhanced MRI. Eur Radiol 20:432–442
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Terpos, E., Kanellias, N., Raje, N. (2018). Bone Disease. In: Dimopoulos, M., Facon, T., Terpos, E. (eds) Multiple Myeloma and Other Plasma Cell Neoplasms. Hematologic Malignancies. Springer, Cham. https://doi.org/10.1007/978-3-319-25586-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-25586-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25584-2
Online ISBN: 978-3-319-25586-6
eBook Packages: MedicineMedicine (R0)