Abstract
Purpose
The purpose of this study was to evaluate the efficacy of exercise, either alone or in combination with other interventions, compared to a control, for the preservation of bone mineral density (BMD) in early breast cancer (BC) patients.
Methods
A systematic search was conducted to identify randomized or quasi-randomized trials which met inclusion criteria including prescribed exercise for ≥12 months. Ten publications from seven randomized controlled trials (RCTs), involving 1199 participants, were identified. Data on primary and secondary outcome measures related to BMD at the lumbar spine, total hip, femoral neck and greater trochanter were analysed. Meta-analyses were limited to subgroups by menopausal status as other data could not be pooled.
Results
Based on mean differences or mean percentage differences between groups at 1 year, exercise did not preserve BMD or bone mineral content at any site in post-menopausal women. In contrast, evidence from one RCT (n = 498) found that exercise reduced bone loss in pre-menopausal women at the femoral neck [% MD = 1.20 (95% CI 0.22–2.18); P = 0.02] but not at the lumbar spine.
Conclusions
Although this review indicated that exercise may result in a clinically important preservation of bone health among pre-menopausal but not post-menopausal women, further studies are needed to confirm whether or not exercise is important in preservation of bone health in women diagnosed with early BC.
Implications for cancer survivors
Exercise alone may not be sufficient to prevent bone loss in post-menopausal women at high risk of osteoporosis. Further evidence is required to determine if it provides any benefit to pharmacological therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Early diagnosis and improvements in treatments for breast cancer (BC) have led to steady increases in survival rates [1]. However, women treated for BC are at increased risk of osteoporosis and fractures compared to women who have not been treated for BC [2, 3] due to treatment-induced loss of bone mineral density (BMD). Treatments for BC typically include adjuvant chemotherapy and/or hormone therapy in addition to surgery and radiotherapy, all of which can contribute, to varying degrees, to an increased risk of osteopenia and osteoporosis. This can occur as a direct side-effect of the treatments such as aromatase inhibitors (AIs) [4,5,6] and/or, indirectly, by triggering early-onset menopause [6,7,8,9].
Adjuvant chemotherapy and hormone therapy are the most significant risk factors underlying bone loss in women treated for BC. Up to 70% of women treated with chemotherapy experience premature menopause [10, 11], increasing bone loss by 1–2% per year [7, 12]. Bone loss and consequent increase in the risk of osteoporosis and fractures are related to the reduction of oestrogen [13].
Hormone therapy, including AIs and selective oestrogen-receptor modulators (SERMs), is used to inhibit growth of oestrogen-positive tumours [14]. These two hormone therapy approaches suppress circulating oestrogen levels by different mechanisms [15, 16], contributing to loss of BMD to different extents. In particular, third-generation AIs such as anastrozole, letrozole and exemestane, now standard care therapy for post-menopausal women, are associated with bone loss [4]. As a consequence, AIs cause more than double the rate of bone loss in women treated for BC compared to age-matched healthy post-menopausal women [4].
Exercise, and in particular interventions that include moderate-intensity impact exercise, has been studied as a non-pharmacological strategy for maintaining BMD and bone turnover in women treated for early BC [17,18,19,20,21,22,23,24,25,26,27,28]. However, the effect of exercise on bone health in this population is unclear, with some studies indicating a reduction in bone loss at certain sites and in specific subgroups, while others provide no such evidence. The outcomes might be confounded by (i) menopausal status at the time of the intervention; (ii) treatment with adjuvant hormone therapies; (iii) time since chemotherapy; and (iv) whether bisphosphonates and/or supplements such as vitamin D and calcium were also provided. The aim of this systematic review was to undertake a detailed qualitative and quantitative analysis of relevant high-quality randomized controlled trials (RCTs) to determine whether exercise is effective in limiting the effect of BC treatment on BMD in pre- and/or post-menopausal women treated for stages I–III BC. This clarification of benefits is needed for the optimal management of bone loss in a growing population of early BC survivors.
Methods
Search methods and study criteria
Searches were conducted for relevant RCTs in electronic databases and clinical trial websites. Five electronic databases searched were as follows: MEDLINE; EMBASE; CINHAL; AMED; and the Cochrane Central Register of Controlled Trials (CCTR). Search strategies were adapted from Cochrane reviews on exercise for preventing and treating osteoporosis [29] and from yoga for women with BC [30]. Clinical trials registries searched were as follows: the National Cancer Institute (http://www.cancer.gov/about-cancer/treatment/clinical-trials/search); Clinical Trials.Gov (http://clinicaltrials.gov); and the Australian and New Zealand Clinical trials Registry (http://www.anzctr.org.au/). The search occurred in June 2015 and was checked at time of submission in July 2016 and again in April 2017 in which two new potentially eligible RCTs were identified; however, closer examination led to their exclusion. There were no restrictions on dates of publication or language.
Studies were identified if they were randomized or quasi-randomized trials (i.e. assignment of participants to study groups was not truly random, such as alternating allocation). Trials were excluded if they included cancers other than BC unless separate data were available. Studies presenting data for bisphosphonates and supplement interventions only were excluded.
Trials with women of any menopausal status, 18 years and older, who underwent surgical treatment for early BC (i.e. stages I–IIIA) were included. Trials including women with metastatic or stage IV BC were excluded.
Trials were included in which an osteogenic exercise program was given to one group and not to the other and the prescribed exercise was for at least 12 months [31], either with or without bisphosphonates, hormone therapy and supplements. Only trials in which interventions occurred after surgical intervention for early BC were considered. However, exercise interventions could occur during or following adjuvant therapy and in a home, hospital or gym setting.
Primary outcomes
The primary outcome was the post-intervention difference in mean BMD or bone mineral content (BMC) between exercise and control groups for the total hip, greater trochanter, femoral neck, proximal femur or lumbar spine (L1–4).
Secondary outcome
The secondary outcome measure was reported adverse events such as fractures and sprains over the course of the study and intervention safety in general.
Data collection and analysis
Both authors (SK and CF) screened all publications, including conference abstracts, identified by our searches, firstly on title and then on the abstract. A study was excluded if it did not meet the eligibility criteria. The reference lists of all full publications extracted were hand-checked to identify all previous studies. The full-text of each publication was then reviewed against the inclusion and exclusion criteria. Any discrepancies in retaining publications between the two authors were resolved by discussion.
Quality assessment
Both authors extracted and recorded data independently into forms modified to include all relevant information from the articles. The information recorded included study methods, participant selection criteria, group numbers and baseline demographics, type of exercise intervention and comparators (usual care, bisphosphonates and vitamin D and calcium supplements) and outcomes as raw data, effect sizes, 95% confidence intervals (CI) and statistical testing results. Continuous data were recorded as unadjusted means or percentage MD and standard deviations (SD), while categorical data was recorded as events per total in group. Where studies provided only percentage MDs and 95% CIs, we calculated SDsFootnote 1 [32].
Disagreements were resolved by discussion. We contacted study authors when there was missing relevant data reported or for further clarifications.
Assessment of risk of bias in included studies
Methodological quality was assessed for each included trial using established criteria for the appraisal of RCTs [33]. Both authors assessed each paper independently for the following ten quality items, worth 1 point each: (i) evidence of randomization and allocation concealment, (ii) absence of statistically significant differences between groups at baseline, (iii) specification of eligibility criteria, (iv) blinding of outcome assessor, (v) compliance with exercise regimen reported, (vi) supervision of exercise intervention, (vii) reporting of reasons for dropouts, (viii) reporting of data for primary and secondary outcomes, (ix) intention-to-treat analysis of results and (x) reporting of adverse events. Any disagreements between authors were resolved by discussion.
Statistical methods
We pooled studies using the random effects meta-analysis model. To compute point estimates of treatment effects for each trial, we plotted (i) post-intervention means and standard deviations from five trials [17, 18, 20, 23, 34] and (ii) percentage MD and standard deviations from two trials [19, 21]. Thus, the point estimates derived for BMD from the former five publications [17, 18, 20, 23, 34] did not take baseline values into account while baseline values were accounted for in the latter two trials [19, 21]. In trials in which only post-intervention data were used, we checked for statistically significant baseline differences in BMD where available and performed sensitivity analyses if differences were identified. As the data were continuous, MDs or mean percentage change in BMD were calculated, along with the 95% CIs between treatment and control groups. Where data for BMC was included [34], standardized MDs (SMD) and 95% CIs were computed. Authors were contacted regarding study data if graphic representation of mean difference or SMD appeared to be markedly distinct from other trials.
Heterogeneity among comparable trials was examined within subgroups visually and tested using the I-squared statistic (I 2) and chi-squared test of goodness of fit. If heterogeneity was identified, potential causes of divergences would be explored. If a factor in the study design was thought to potentially skew outcomes, a sensitivity analysis will be undertaken where the specific trial(s) would be removed and a new estimate of effect would be reported if it differed significantly. Conversely, a subgroup analysis would be undertaken, in which data from individual trials would be regrouped by a new factor with new estimates calculated for each subgroup.
We planned a priori subgroup analysis to examine the effect of exercise on outcome measures in relation to women’s menopausal status, use of vitamin D and calcium supplement, use of hormones and intensity and compliance of exercise supervision. We decided a priori that sensitivity analyses would be conducted (i) to evaluate the effect of variations in methodological quality if they existed or (ii) to estimate the effect of any trial(s) which differed importantly in study design from others pooled in the same comparison. Publication bias was not undertaken as the small number of trials that met inclusion criteria (<10) would not likely lead to a meaningful analysis.
Cochrane Review Manager software (RevMan 5.3, version 5.3.5, 30 October 2014) was used for meta-analyses of the data.
Results
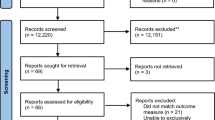
Potential studies identified for this review and reasons for their inclusion or exclusion are outlined in a flow diagram (Fig. 1). We identified 298 potentially relevant records through our search methods; three additional publications were identified by other means. After removal of duplicate and triplicate records, 236 records were screened by their titles and abstracts against the inclusion and exclusion criteria. A further 220 records were excluded, most commonly due to design, i.e. not a RCT or pseudo-RCT, leaving 17 records for full text screening. Of these, seven RCTs met eligibility criteria, and resulted in ten published journal articles.
Descriptive data synthesis
Table 1 presents the quality of the included publications, and Table 2 presents the main study characteristics of the included publications. Studies were published between 2009 and 2016, with six trials conducted in the USA [17, 18, 20,21,22,23] and one in Finland [19]. One study comprised four publications, including publications based on (i) analysis of data at the end of 1 year of training [20]; (ii) re-analysis of the intervention and control data by age subgroups [35]; (iii) analysis of 1 year follow-up from the original cohort [36]; and iv) an erratum [37]. One study comprised two publications in which the data from all sites involved in the study were analysed to determine the effect of exercise on BMD and total BMC in most participants [19]; and a second publication described the effect of exercise on femoral neck BMC among a subset of 86 participants from the multicentre trial [34]. The number of participants in the included trials ranged between 48 [18] and 498 [19], with a total of 1282 participants analysed across all trials, excluding a follow-up study [36], a subgroup analysis [35] and a substudy [34]. Mean age of the participants in the included trials ranged from 46 years [18] to 62 years [20].
Women diagnosed with BC were included in a trial if they had undergone surgery and were commencing chemotherapy [22] or had completed chemotherapy within the last 4 months [19], more than 6 months ago [21], more than 6 months ago but less than 5 years [18], within the last year [20], or within the last 3 years [23]. One trial did not specify inclusion criteria related to chemotherapy [17], although most women in both groups had undergone chemotherapy. All studies specified women were eligible if they presented with stages 0–III BC but not stage IV. In one trial, risk of lymphedema or diagnosis of stable lymphedema and resection of >1 lymph node(s) comprised the inclusion criteria [17, 38]. Diagnoses of BC were histologically confirmed in one trial [19, 34]. Menopausal status was confirmed by estradiol and follicle stimulating hormone (FSH) measures in two trials [18, 20] and determined by self-report in the remaining trials [18, 20, 35, 36]. One trial included only women within 1 month of commencing chemotherapy and those planning to have chemotherapy treatment [22]. Another trial included women who were treated with adjuvant chemotherapy or radiotherapy within 4 months or those who started adjuvant endocrine therapy within 4 months [19].
Women were excluded from trials if they took part in exercise deemed to be osteogenic. This included participants who were competitive athletes [19] or who participated in any weight training during the previous year [17], any resistance and/or impact training, comprising ≥2 sessions per week of at least 30 min duration during the past month [20], >60 min of resistance training per week [18], regular resistive exercise ≥3 times per week [23] and currently participating in strength training [21]. All but one study whose primary outcome measure was not bone health [17] excluded either current or recent bisphosphonate use. All trials but one [19] did not exclude women on an AI and/or tamoxifen although the proportion of women using either of these adjuvant therapies varied. The proportion of women on AIs varied from 1% [17] to 56% [19], in which the latter comprised only post-menopausal women while the proportion of women on a SERM varied from 15% [20] to 85% [19], in which the latter comprised only pre-menopausal women. One trial excluded women who had used tamoxifen for more than 6 months [19]. One trial did not exclude women with osteoporosis [17], while another trial excluded women who had either osteopenia or osteoporosis [21]. The remaining four trials excluded women with osteoporosis [18,19,20, 39] with one trial also excluding those with severe osteopenia (T-score ≥−2) [22]. Of the three trials which did not exclude women with osteopenia, two administered bisphosphonates to one study arm [21, 22]. Other general exclusion criteria were the inability to exercise, other major physical or mental health problems and conditions or use of drugs which interfered with bone metabolism.
Post-menopausal women only were included in three trials [20, 21, 23]; only women who were prematurely menopausal were included in one trial [18]; and both pre- and post-menopausal women were included in three trials [19, 20, 22]. In the studies which combined women with pre- and post-menopause, results were analysed and reported separately for pre- and post-menopausal participants in two publications [17, 19] and together in two publications [22, 34].
Timing of the interventions with regard to diagnosis, chemotherapy and radiotherapy varied between studies. Participants could be newly diagnosed but within 4 months of receiving chemotherapy [19], newly diagnosed but within 1 month of receiving chemotherapy [22], diagnosed from between one to 15 years before study entry [17], post-chemotherapy ≥6 months to <5 years prior to study enrolment [18], at least 6 months post-chemotherapy and/or radiotherapy [21] or at least 1 year post-chemotherapy or radiotherapy (25).
In all but one trial [17], the primary outcomes were related to bone health with exercise prescribed specifically to target BMD (see Table 2). Six trials administered exercise programs consisting of either progressive resistance training (PRT) alone [17, 21] or in combination with impact loading exercises [18, 20, 23]. One exercise program comprised step aerobics and circuit training [19] whereas another prescribed daily walking to a total of approximately 10,000 steps, equivalent to 5 miles [22]. PRT was prescribed using weight machines (upper and lower body) and/or free weights (replaced by resistance bands at home) performed at moderate to high forces [17,18,19,20,21, 23]. Additionally, some programs included weighted vests/belts and body weight resistance exercises such as wall sits, squats, lateral lunges and push ups [18,19,20, 23]. Participants were typically instructed to complete 1–3 sets of 8–12 repetitions. Weighted vests [18, 20] or loaded belts (30) were used with jumps as part of impact exercises. Step aerobics comprised jumps and leaps in different directions using benches which increased in height in three phases (10, 20 and 30 cm). Home-based aerobic exercise included walking [19, 22, 23], Nordic walking [19] or aerobic training [19, 23]. Circuit training comprised steps and hops that progressed in difficulty to high impact jumps with a magnitude of up to four times the body weight [19].
The length of the prescribed exercise programs was 12 months in six trials [17,18,19,20, 22, 23] and 24 months in one trial [21]. The duration of each exercise session was 30 min including 5–10 min warm-up stretching exercises [23], between 30 and 45 min [21], 45 and 60 min [18, 20], and 60 and 90 min including warm-ups [17]; duration for one trial was for 60 min [19]. The frequency of exercise sessions was twice weekly in two trials [17, 21], thrice weekly in three trials [18, 20, 23], three to four times per week in one trial [19] and daily in one trial [22]. Exercise sessions were supervised once per week in one trial [19], twice per week in two trials [18, 20], twice-weekly for the first 3 months only of a 12 month intervention [17], thrice-weekly for the first 6 months of a 12 month intervention [23], and once every 2 weeks for the first 9 months and once every 2 months in the remaining 15 months [21]. In one trial, participants were not supervised [22]. Mean rates of adherence to prescribed exercises in the RCTs ranged from 57% [20] to 77% [23].
Supplementation with vitamin D and calcium was provided to the intervention and control groups in three studies [21,22,23]. Dosages were 600 mg of calcium + 200 IU vitamin D twice daily [22], 1200 mg calcium + 400 IU vitamin D [21] or a multivitamin that contained 400 IU vitamin D + calcium supplementation to a total of 1200 mg calcium based on dietary intake questionnaire [23]. An additional 50,000 IU of vitamin D was given to participants in whom the vitamin D levels were <20 ng/ml [23]. Vitamin D and calcium intake were monitored by pill counts in two of the trials in which supplementation was provided [21, 22] and measured at baseline and post-intervention [21]. Vitamin D and calcium supplementation were noted at baseline and post-intervention in one trial [19], while calcium intake was measured at baseline and post-intervention in three trials using the Block Food Frequency Questionnaire (BFFQ) questionnaire [17, 18, 20] and a 4-day diet recall in one trial [23].
The control group received a stretching intervention in two trials [18, 20], encouragement to maintain regular levels of physical activity in one trial [19], home-based health promotion guidelines for moderate intentisy exercise in one trial [23], a 1-h education lecture on exercise for lymphedema based on the National Lymphedema Network material in one trial [17], bisphosphonate (oral risedronate, 35 mg/week) in addition to exercise in one trial [21] and bisphosphonates only (IV zoledronic acid, 5 × 4 mg every 3 months) and standard exercise counselling in one trial [22]. Adherence to interventions was determined in all trials by patient diaries [19] and/or questionnaires including the Community Healthy Activity Model Program for Seniors (CHAMPS) [18, 20], the International Physical Activity Questionnaire (IPAQ) [17, 23], the Paffenbarger Physical Activity Questionnaire (PPAQ) [22] and the 7-Day Physical Activity Record-Adapted (7PAR-A) [21].
Primary outcome measures were BMD at the lumbar spine (L1–4), femoral neck, greater trochanter and total hip in four trials [17, 18, 20, 23], BMD at the lumbar spine (L1–4), femoral neck and total hip in two trials [21, 22] and BMD at the lumbar spine (L1–4) and femoral neck in one trial [19]. Total BMC [19] and femoral neck BMC [34] were also measured in different subsets of participants from one trial [19].
Safety data was recorded as adverse events [17,18,19,20,21] or fractures [22] in all but one trial [23], including a follow-up [36].
Quantitative data synthesis
Primary outcomes
Pre- and post-menopausal women were analysed as subgroups in the meta-analysis; however, no other a priori subgroup analysis was performed due to the unavailability of separate data of other factors of interest.
Exercise did not lead to a significant increase in either post-intervention mean BMD or percentage change in mean BMD from baseline at the lumbar spine (Fig. 2), femoral neck (Fig. 3), total hip or greater trochanter (Fig. 4) in post-menopausal women. For example, the MD at the lumbar spine between exercise only and control (placebo (FLEX), no exercise, or bisphosphonates + exercise) groups post-intervention for 478 women derived from four trials [17, 18, 20, 23] was 0.01 g/cm2 BMD (95% CI −0.02 to 0.03; P = 0.69) in favour of exercise. Similarly, for all regions of interest except the femoral neck, exercise did not lead to significant increase in BMD or percent change in BMD in pre-menopausal women (Figs. 2, 3 and 4). Exercise significantly increased BMD at the femoral neck (Fig. 3) in pre-menopausal women (% MD 1.2 (95% CI 0.22–2.18); P = 0.02), indicating that for this region of interest, pre- and post-menopausal women differed significantly from each other in their response to exercise (P = 0.04, I 2 = 77%). In all analyses, there was no heterogeneity between trials within subgroups.
Sensitivity analyses
Only one trial did not exclude women currently on bisphosphonates or who had taken them for at least 6 months [17]. In this trial, the primary outcome was not related to bone health. Removal of this trial from the meta-analysis did not result in any substantive changes in estimates or significant P levels (Table 3).
Secondary outcomes
No adverse events or injuries were reported in four of the trials [17, 18, 20, 21], including a 1-year follow-up study [36]. In contrast, two trials did not mention recording safety events [19, 23] although Nikander et al.’s report [34] on a subset of participants from Saarto et al.’s trial [19] reported four moderate overuse injuries which resolved. One trial [22] reported a fracture rate of 10.3% (3/29) in the physical activity group and a rate of 3% (1/33) in the bisphosphonate group; both groups had one pre-existing fracture at baseline.
Discussion
Among post-menopausal women diagnosed with stages I–III BC, neither mean BMD/BMC nor mean percentage difference in BMD at the lumbar spine, total hip, femoral neck or greater trochanter was significantly different after 1 year of exercise compared to a group who did not exercise. These findings are based on meta-analyses from seven RCTs [17,18,19,20,21,22,23]. In addition, exercise provided no additional benefit to bisphosphonate use among post-menopausal women when compared to a control group prescribed bisphosphonate only for preserving BMD [21]. In contrast, among pre-menopausal women who exercised, BMD at the femoral neck was significantly higher compared to the control group while no effect was observed at the lumbar spine. Although the finding comes from only one study [19], the effect was large and statistically significant. The net effect of exercise in this subgroup was to preserve BMD at the femoral neck, whereas the control group lost BMD. This was also the only trial which included pre-menopausal women in which the exercise intervention was specifically designed to improve bone health. The other trial that included pre-menopausal women was designed to assess safety of weight training among women at risk of lymphedema [17].
A couple of factors may contribute to the overall lack of responsiveness of exercise in post-menopausal women compared to pre-menopausal women. First, in post-menopausal women, oestrogen’s role in osteoclast-mediated bone resorption is suppressed, independent of cancer treatment [13, 40, 41]. Second, endocrine therapy may influence the responsiveness of bone to exercise differently for pre- and post-menopausal women. AIs, which are prescribed to post-menopausal women, lead to bone loss [4, 42], while SERMs, prescribed mainly to pre-menopausal women, preserve BMD and reduce bone turnover markers and fractures [43]. In the current review, we were unable to perform a meta-analysis for the effect of endocrine therapy on bone health outcomes by intervention group. However, of the trials included in this review, two reported that endocrine therapy did not modify the effect of exercise on bone outcomes [18, 20]; one reported that women on AI therapy who exercised had significant bone loss compared to controls (data analysis for BC patients only provided by trial authors) [23]; and another tentatively reported that type of endocrine therapy was an independent predictor of BMD outcomes regardless of intervention group [19]. Although it has not been established that endocrine therapy can modulate the effect of exercise on BMD, the fact that AIs lead to increased bone loss and its disproportionate use among post-menopausal women suggests that it could potentially underlie the effect of menopausal status on exercise outcomes. (pre-menopausal use 1.6% vs post-menopausal use 84.7% in the trial which showed a significant benefit from exercise among pre-menopausal women only [19]).
Other factors may contribute to the overall lack of responsiveness of exercise on bone health in post-menopausal women compared to pre-menopausal women in both the general population and in those diagnosed with stages I–III BC. Age may modify the effect of exercise, even among post-menopausal women. Age-related bone loss occurs through increases in oxidative stress and decreases in growth factors [44]. As treatments used for cancer accelerate the ageing process [45], skeletal responsiveness in the older post-menopausal women may be blunted compared to younger women [35]. Furthermore, the rate of bone loss is highest during peri- and recently menopausal women [46]. Another explanation may be related to the intensity at which older women exercise compared their younger counterparts. While the same programs were used for both older and younger women, the effort which older women exert, and thus level of activation of muscles, may not be equivalent to that exerted by the younger women [19].
Other factors that may contribute to the overall lack of responsiveness of exercise in women treated for BC, regardless of menopausal status, include timing of chemotherapy, the exercise program, including type of intervention, intensity at which it was delivered and adherence to the prescribed exercise regimen. Timing of the exercise intervention with regards to chemotherapy treatment may affect BMD outcomes as rapid loss of BMD during chemotherapy could mask the effect of the concurrent exercise intervention.
Several issues related to the exercise program may have contributed to the general lack of effect of exercise on bone factors. Systematic reviews of trials that aim to improve BMD in post-menopausal women reveal that that exercise interventions which have efficacy for maintenance and/or improvement of bone density include impact exercises such as occurs with jogging, jumping and hopping combined with resistance training [29, 47]. One trial used only a walking program in which women were asked to achieve 10,000 steps per day [22]. However, a previous meta-analysis for dynamic low-force weight bearing exercises that included walking found a significant effect in favour of exercise on percentage change in BMD at the spine but not at the hip [29]. In contrast and in another meta-analyses, the opposite was found in that walking had no significant effect on BMD at the spine but did have positive effects at the hip [48]. Two trials used only resistance training but not impact exercise [17, 21]. Adherence to the prescribed exercise program ranged from 57% [20] to 77% [23] while retention rates ranged from 63% [20] to 90% [21]. Where reasons for dropouts were reported, lack of time to exercise was commonly cited for declining to participate in the trial or discontinuing it [18,19,20, 22]. Even the addition of resources to motivate participants did not result in high compliance: in a home-based, daily walking intervention without supervision with high levels of patient-reported adherence (90%), pedometer readings found that only 67% of prescribed exercise was performed [49].
The low compliance and/or adherence to prescribed activity in exercise trials raises the issue of whether exercise to preserve or increase BMD is the best strategy. Furthermore, the challenge in recruitment and/or low ratio (<50%) of randomized participants from the available pool [17, 18, 20, 21, 23] is particularly relevant to practicality given that effective pharmaceutical alternatives (bisphosphonates) exist and are currently recommended for post-menopausal women taking AI who have osteoporosis [50, 51]. Consequently, current use of bisphosphonates may be an obstacle in recruiting women diagnosed with BC from being eligible for exercise trials as was reported in a separate publication about one trial [21, 52]. Moreover, exercise added no benefit to risedronate and supplementation in one trial [21] while daily walking failed to prevent bone loss compared to women on zoledronic acid [22].
Limitations
Several limitations are noted in our review. First, only seven trials were identified that examined the effect of exercise programs lasting one year or more on BMD/BMC outcomes in women diagnosed with early BC. However, these RCTs were of relatively high quality (Table 1). The single RCT from which improvements in BMD following exercise in pre-menopausal women was identified excluded 18 participants from each group (6–7% per exercise and control group, respectively) after randomization but before the intervention took place [19] which may have compromised randomisation. Second, participants’ baseline characteristics differed among the included trials in terms of menopausal and bone health status, endocrine therapy and time from diagnosis of BC and/or chemotherapy. Third, the exercise interventions varied in terms of type, length and frequency, as well as participants’ compliance and adherence. Fourth, a range of comparators were used, including usual care, placebo exercise, bisphosphonates and use of vitamin D and calcium supplementation. Despite this clinical heterogeneity, I 2 statistics showed no methodological heterogeneity among the trials grouped in our meta-analysis.
The diversity of studies could have led to relevant subgroup analyses; however there were too few comparable trials without separate data for all planned subgroup analyses. Consequently, subgroup analysis was only conducted on menopausal status. There was also diversity in reporting units of outcome and lack of variability data necessary to consolidate results. It is preferable for meta-analyses to be undertaken on the mean percentage change as it accounts for any baseline differences. However, as most trials reported pre- and post-intervention data with SDs rather than change scores (absolute or percentage change), we were restricted to undertaking the meta-analysis on post-intervention measures [17, 18, 20, 23, 34]. Use of post-intervention data may have introduced bias as baseline values were not considered; however, there were no significant differences in inter-group mean baselines BMD.
Our findings from the meta-analysis that exercise did not affect BMD in post-menopausal women may have been compromised by less than ideal adherence and/or compliance with the prescribed exercise regimen in most trials [20, 23]. Issues with measures of physical activity included self-reported exercise adherence and compliance [22] and no [22] or partial supervision of exercise for only a period of the intervention duration [17, 23] or a fraction of exercise sessions [18,19,20,21]. The validity of effect estimates derived from the trials and the efficacy of the intervention may have been underestimated due to low adherence and/or compliance of participants in most trials.
Another limitation which may impact on the responsiveness of bone to exercise is related to the subgroup of women who were on endocrine therapy. It is unknown to what extent women were compliant with their medication. Compliance to AIs is an important factor which was never verified in any of the trials. It is likely that some women on AIs discontinued their therapy due to side-effects such as arthralgia, a common side-effect leading to discontinuation in 20–30% of cases [53]. This could confound the effect of exercise on bone and reduce the difference in effect estimates between exercise and control groups.
Conclusions
In post-menopausal women with stages I–III BC, exercise did not improve or prevent bone loss or add any benefit to bisphosphonates. In pre-menopausal women diagnosed with stages I–III BC, exercise favourably impacted on bone outcomes at the femoral neck but not the spine compared to those who did not exercise. However, these need to be viewed cautiously due to the small numbers of studies available to investigate this topic and the methodological issues evident in the published trials, particularly in relation to the exercise programs. Future high-quality studies are required to address limitations identified in the current studies.
Notes
SD = (upper 95% CI − lower 95% CI) × √N/(1.96 × 2) [32].
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Chen Z, Maricic M, Bassford TL, Pettinger M, Ritenbaugh C, Lopez AM, et al. Fracture risk among breast cancer survivors: results from the Women’s Health Initiative Observational Study. Arch Intern Med. 2005;165:552–8.
Kanis JA, McCloskey EV, Powles T, Paterson AH, Ashley S, Spector T. A high incidence of vertebral fracture in women with breast cancer. Br J Cancer. 1999;79:1179–81.
Hadji P. Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol. 2009;69:73–82.
Santen RJ. Clinical review: effect of endocrine therapies on bone in breast cancer patients. J Clin Endocrinol Metab. 2011;96:308–19.
Hadji P, Asmar L, van Nes JG, Menschik T, Hasenburg A, Kuck J, et al. The effect of exemestane and tamoxifen on bone health within the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial: a meta-analysis of the US, German, Netherlands, and Belgium sub-studies. J Cancer Res Clin Oncol. 2011;137:1015–25.
Vehmanen L, Saarto T, Elomaa I, Makela P, Valimaki M, Blomqvist C. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment. Eur J Cancer. 2001;37:2373–8.
Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19:3306–11.
Tonezzer T, Pereira CM, Filho UP, Marx A. Hormone therapy/adjuvant chemotherapy induced deleterious effects on the bone mass of breast cancer patients and the intervention of physiotherapy: a literature review. Eur J Gynaecol Oncol. 2010;31:262–7.
Bruning PF, Pit MJ, de Jong-Bakker M, van den Ende A, Hart A, van Enk A. Bone mineral density after adjuvant chemotherapy for premenopausal breast cancer. Br J Cancer. 1990;61:308–10.
Goodwin PJ, Ennis M, Pritchard KI, McCready D, Koo J, Sidlofsky S, et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol. 1999;17:120–9.
Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, et al. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol. 2008;26:5465–76.
Riggs BL. The mechanisms of estrogen regulation of bone resorption. J Clin Invest. 2000;106:1203–4.
Freedman OC, Fletcher GG, Gandhi S, Mates M, Dent SF, Trudeau ME, et al. Adjuvant endocrine therapy for early breast cancer: a systematic review of the evidence for the 2014 Cancer Care Ontario systemic therapy guideline. Curr Oncol. 2015;22:S95–S113.
Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717.
Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–42.
Winters-Stone KM, Laudermilk M, Woo K, Brown JC, Schmitz KH. Influence of weight training on skeletal health of breast cancer survivors with or at risk for breast cancer-related lymphedema. J Cancer Surviv. 2014;8:260–8.
Winters-Stone KM, Dobek J, Nail LM, Bennett JA, Leo MC, Torgrimson-Ojerio B, et al. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporos Int. 2013;24:1637–46.
Saarto T, Sievanen H, Kellokumpu-Lehtinen P, Nikander R, Vehmanen L, Huovinen R, et al. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int. 2012;23:1601–12.
Winters-Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. [Erratum appears in Breast Cancer Res Treat. 2011 Jun;127(2):457]. Breast Cancer Res Treat. 2011;127:447–56.
Waltman NL, Twiss JJ, Ott CD, Gross GJ, Lindsey AM, Moore TE, et al. The effect of weight training on bone mineral density and bone turnover in postmenopausal breast cancer survivors with bone loss: a 24-month randomized controlled trial. Osteoporos Int. 2010;21:1361–9.
Swenson KK, Nissen MJ, Anderson E, Shapiro A, Schousboe J, Leach J. Effects of exercise vs bisphosphonates on bone mineral density in breast cancer patients receiving chemotherapy. J Support Oncol. 2009;7:101–7.
Knobf MT, Jeon S, Smith B, Harris L, Kerstetter J, Thompson AS, et al. Effect of a randomized controlled exercise trial on bone outcomes: influence of adjuvant endocrine therapy. Breast Cancer Res Treat. 2016;155:491–500.
Peppone LJ, Mustian KM, Janelsins MC, Palesh OG, Rosier RN, Piazza KM, et al. Effects of a structured weight-bearing exercise program on bone metabolism among breast cancer survivors: a feasibility trial. Clin Breast Cancer. 2010;10:224–9.
Irwin ML, Alvarez-Reeves M, Cadmus L, Mierzejewski E, Mayne ST, Yu H, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity. 2009;17:1534–41.
Schwartz AL, Winters-Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum. 2007;34:627–33.
Sellmeyer D, Stewart S, Bloom J. A randomized controlled trial of exercise to prevent bone loss and adverse cardiovascular changes in premenopausal women with breast cancer. J Bone Miner Res. 2013;28(S1):FR0428.
Simonavice E, Liu PY, Ilich JZ, Kim JS, Arjmandi B, Panton LB. The effects of a 6-month resistance training and dried plum consumption intervention on strength, body composition, blood markers of bone turnover, and inflammation in breast cancer survivors. Appl Physiol Nutr Metab. 2014;39:730–9.
Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;7:CD000333.
Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for women diagnosed with breast cancer. Cochrane Database Syst Rev. 2013;(10):CD010802. doi:10.1002/14651858.CD010802.
Winters-Stone KM, Schwartz A, Nail LM. A review of exercise interventions to improve bone health in adult cancer survivors. J Cancer Surviv. 2010;4:187–201.
Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. Available from www.handbook.cochrane.org.
Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32.
Nikander R, Sievanen H, Ojala K, Kellokumpu-Lehtinen PL, Palva T, Blomqvist C, et al. Effect of exercise on bone structural traits, physical performance and body composition in breast cancer patients—a 12-month RCT. J Musculoskelet Neuronal Interact. 2012;12:127–35.
Winters-Stone KM, Leo MC, Schwartz A. Exercise effects on hip bone mineral density in older, post-menopausal breast cancer survivors are age dependent. Arch Osteoporos. 2012;7:301–6.
Dobek J, Winters-Stone KM, Bennett JA, Nail L. Musculoskeletal changes after 1 year of exercise in older breast cancer survivors. J Cancer Surviv. 2014;8:304–11.
Winters-Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, et al. Erratum to: Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat. 2011;127:457.
Twiss JJ, Waltman NL, Berg K, Ott CD, Gross GJ, Lindsey AM. An exercise intervention for breast cancer survivors with bone loss. J Nurs Scholarsh. 2009;41:20–7.
Knobf M, Winters-Stone K. Exercise and cancer. Annu Rev Nurs Res. 2013;31:327–65.
Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11:1043–51.
Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106:1229–37.
Perez EA, Weilbaecher K. Aromatase inhibitors and bone loss. Oncology (Williston Park). 2006;20:1029–39.
Maximov PY, Lee TM, Jordan VC. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol. 2013;8:135–55.
Kohrt WM. Aging and the osteogenic response to mechanical loading. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S137–42.
Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypotheses. 2006;67:212–5.
Arnold P, Bautmans I. The influence of strength training on muscle activation in elderly persons: a systematic review and meta-analysis. Exp Gerontol. 2014;58:58–68.
Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med. 2009;43:898–908.
Martyn-St James M, Carroll S. Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone. 2008;43:521–31.
Swenson KK, Nissen MJ, Henly SJ. Physical activity in women receiving chemotherapy for breast cancer: adherence to a walking intervention. Oncol Nurs Forum. 2010;37:321–30.
Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL, et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol. 2016;34:611–35.
Hadji P, Aapro MS, Body JJ, Bundred NJ, Brufsky A, Coleman RE, et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol. 2011;22:2546–55.
Ott CD, Twiss JJ, Waltman NL, Gross GJ, Lindsey AM. Challenges of recruitment of breast cancer survivors to a randomized clinical trial for osteoporosis prevention. Cancer Nurs. 2006;29:21–31.
Fernandez Ortega A, Jolis Lopez L, Vinas Villaro G, Villanueva Vazquez R, Garcia Arias A, Gonzalez Farre X, et al. Individualization of treatment strategies. Adv Ther. 2011;28(Suppl 6):19–38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fornusek, C.P., Kilbreath, S.L. Exercise for improving bone health in women treated for stages I–III breast cancer: a systematic review and meta-analyses. J Cancer Surviv 11, 525–541 (2017). https://doi.org/10.1007/s11764-017-0622-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-017-0622-3