Abstract
Purpose
Certain cancer treatments are associated with bone loss and increased fracture risk. Weight-bearing impact exercise, resistance training or the combination, are recommended to preserve or improve bone mineral density (BMD) inhealthy older adults, but their efficacy in cancer survivors is less well understood. The aim of this systematic review with meta-analysis of randomised control trials (RCT) was to review the evidence regarding the role of exercise to counteract cancer treatment-induced bone loss.
Methods
Four databases were searched systematically with 12 RCTs of at least 6-month duration investigating the effects of exercise on BMD compared to a control group in adult cancer survivors identified.
Results
Meta-analysis was completed using available data from six studies enrolling 814 participants, with lumbar spine, femoral neck and/or total hip BMD as the primary outcome measures. Overall, there was no significant benefit of exercise compared to controls on BMD at the lumbar spine (0.0071 g/cm , 95% CI −0.0002 to 0.0145, p = 0.057), femoral neck (0.0044 g/cm , 95% CI −0.0005 to 0.0093, p = 0.077), or total hip (0.0024 g/cm , 95% CI −0.0038 to 0.0086, p = 0.443). Subgroup analysis revealed a positive effect on lumbar spine BMD in three studies implementing a combined resistance and impact exercise intervention (0.015 g/cm , 95% CI 0.003 to 0.028, p = 0.019).
Conclusions
From the evidence available, exercise may not be sufficient to improve bone health in cancer survivors, but given the heterogeneity in the participant characteristics and several exercise programs which may not have been designed to specifically optimise bone health, these findings should be interpreted with caution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy and endocrine therapy for breast cancer (selective oestrogen receptor modulators (SERM) or aromatase inhibitors (AI)), as well as androgen deprivation therapy (ADT) for prostate cancer, have all been shown to improve survival outcomes in cancer patients, but a common side effect for all these treatments is that they have been associated with accelerated bone loss and an increased risk of fracture [1, 2]. Pharmacotherapy has been shown to be effective in managing the treatment-related bone loss in cancer patients [1,2,3]; however, it does not appear to provide any additional benefits to the multiple other adverse effects of cancer treatment. Exercise has been shown to improve a range of cancer-related adverse effects including physical function, sexual function, body composition, and fatigue in men treated with ADT for prostate cancer [4, 5] as well as psychological symptoms, physical function, body composition, fatigue, and quality of life in women treated for breast cancer [6, 7]. Currently, however, there are no specific exercise prescription guidelines to combat treatment-related adverse effects for different types of cancer, stages or therapies, and even less is known about the efficacy of exercise to prevent bone loss in cancer patients.
In healthy older adults, current clinical practice guidelines recommend high-intensity progressive resistance training, moderate to high-impact or diverse multidirectional weight-bearing activities or the combination of these modalities, to maintain or improve BMD at the hip and/or lumbar spine [8,9,10,11]. However, there is less definitive research replicating these results in cancer populations. A 2010 qualitative review that included eight studies, three of which were controlled exercise trials with a usual care control group, reported that there were too few studies and they were too varied to warrant conclusions regarding the skeletal benefits of exercise during or after cancer treatment [12]. Given that there have been several recent intervention trials in this area, the aim of this systematic review with meta-analysis was to provide a comprehensive update on the efficacy of RCT’s investigating the effect of exercise on BMD in adult cancer survivors compared to a control group.
Materials and methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (PRISMA) [13].
Search strategy
An electronic search of the PubMed, MEDLINE, EMBASE and Scopus databases was performed to identify peer-reviewed articles published before January 2017. The search terms used for all databases were [exercise OR training OR physical activity] AND cancer AND [bone density OR bone mineral density OR BMD] (Supplementary Table 1). Reference lists of all included studies were examined for other potentially relevant articles.
Selection strategy
Titles and abstracts of all search results were screened for relevance by two independent reviewers (JDV and SFF). Full-text versions of all potentially relevant articles were then assessed for eligibility by the same reviewers according to predetermined inclusion and exclusion criteria (Table 1). A control comparison group was one that was not expected to influence BMD (non-intervention, usual care or exercise placebo) and only differed from the intervention group by the exercise intervention. Multiple publications from the same study were included once to avoid duplication of data reviewed. Disagreements between the reviewers were resolved by consensus discussion.
Quality assessment
The methodological quality of included studies was assessed by two independent reviewers (JDV and SFF) using the Cochrane collaboration risk of bias tool [14]. This tool involves assigning a judgement of low, high or unclear risk of bias for specific domains including sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of systematic bias. Disagreement between the reviewers was resolved by consensus discussion and reanalysis of the article.
Data extraction and management
Two reviewers (JDV and SFF) independently extracted the following information from each eligible study: first authors surname, publication year, country, number of participants allocated to each group, participant cancer type, stage and treatment details, mean age and menopausal status, study duration, type of control group, BMD results, analysis type as well as exercise type, setting, prescription and whether it was targeted to improve BMD. Additionally, outcome data was extracted for DXA assessed BMD measurements of the lumbar spine, femoral neck and/or total hip. These sites are clinically relevant as they are common osteoporotic fracture sites and were assessed in most included studies. Authors were contacted to obtain absolute net differences for the change in BMD between groups with 95% confidence intervals, if not reported in the original publication. If a study did not report, or authors could not provide appropriate data, it was excluded from the meta-analysis but included in the systematic review. Where possible, data from an intention-to-treat (ITT) analysis was extracted.
Statistical analysis
Absolute net differences for the change in BMD between the intervention and control group were used to combine study effect estimates in the meta-analysis. If a study reported bilateral BMD results, the left side was arbitrarily used to avoid duplicating data. Outcomes for each BMD site were analysed using a random-effects meta-analysis. A random-effects model was chosen as the effect of the interventions on BMD in the included studies may vary due to variability in the samples and interventions used [14]. Statistical heterogeneity was assessed using the I 2 statistic. Values of 25, 50, and 75% were considered to indicate low, moderate and high heterogeneity, respectively [15]. To investigate sources of heterogeneity, a list of predefined variables that may influence the effect of exercise on BMD were chosen for subgroup analysis. These were cancer type, menopausal status for women, type of analysis (ITT or per protocol), exercise modality and whether the exercise program was targeted at improving bone health (yes or no). Sensitivity analysis was also completed by excluding studies with high or unclear risk of bias for each of the detection bias and attrition bias domains of the Cochrane collaboration risk of bias tool. Funnel plots to assess the risk of publication bias are only recommended when there are at least 10 studies [14], and so would only be used if sufficient studies were available. All analyses were conducted using STATA software version 14.2 (Stata Corp, College Station, TX, USA). A P value < 0.05 was considered statistically significant.
Results
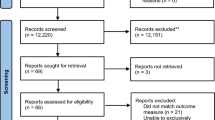
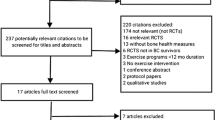
A total of 1002 unique studies were identified through the four electronic databases. Full-text articles were reviewed from 79 potentially relevant publications, of which 67 did not meet the inclusion criteria (Fig. 1). Consequently, 12 studies were included in the systematic review [16,17,18,19,20,21,22,23,24,25,26,27]. There was 100% agreement between reviewers on which studies to include. No additional relevant articles were identified from reference lists of included articles. Included studies were published between 2007 and 2017. Study characteristics are summarised in Table 2.
Overall, there was a low risk of bias in the included studies (Fig. 2). Only studies that reported appropriate randomisation and allocation concealment methods were considered to have low risk of selection bias. All studies were considered to have a high risk of performance bias as it is difficult to blind an exercise intervention. Only studies that reported blinding of DXA technicians were considered to have a low risk of detection bias. Attrition bias was assessed by considering attrition, retention and the analysis used in each study. Studies with similar attrition, retention and reasons for missing data between groups, as well as those using ITT analysis with appropriate imputation methods were considered to have a low risk of attrition bias. Only studies that reported outcomes as specified in a protocol paper were considered to have a low risk of reporting bias. The design, limitations and any other aspects of the study that may suggest bias were considered when assessing other sources of bias. A study was considered to have unclear risk of bias if there was insufficient information provided to classify the risk of bias for each domain.
Study characteristics
Nine studies were in women with breast cancer [16,17,18,19,20,21, 23, 24, 27], two in men with prostate cancer [22, 26] and one in female cancer patients (83% breast cancer) [25]. Prostate cancer studies (n = 2) included men currently treated with ADT [22, 26] for either any duration [22] or for at least 6 months [26], with some men previously treated with radiation therapy [22, 26] or chemotherapy [22]. Female cancer patients had commonly completed non-hormonal treatment (surgery, chemotherapy and/or radiotherapy) within the previous 5 years [17,18,19,20,21, 23,24,25]. One study included participants with a previous history of breast cancer, but did not define post-treatment duration [23] and another included participants commencing breast cancer treatment with chemotherapy with or without radiation therapy [16]. All female cancer studies included participants who were also treated with endocrine therapy with both SERM’s and AI’s. All breast cancer patients in one study were treated with AI’s for at least 6 months prior to enrolment [27].
Mean age of participants was 67 and 70 years in the two prostate cancer studies [22, 26] and between 46 and 62 years [16,17,18,19,20,21, 23,24,25, 27] in the female cancer studies. Sample sizes were 51 and 57 in the two prostate cancer studies [22, 26] and between 43 and 573 in female cancer studies [16,17,18,19,20,21, 23,24,25, 27]. All studies had a similar number of participants randomised to the intervention and control groups. Six studies completed ITT analysis only [16, 17, 20, 23, 25,26,27], while five reported completing both ITT and per-protocol analyses [18, 19, 21, 22, 24]. Of those, three studies only reported data from per-protocol analysis, only including participants who completed the intervention [19, 21, 22].
Inclusion criteria commonly excluded participants completing regular resistance and/or impact exercise (deemed to be osteogenic) [18, 19, 21,22,23,24,25]. Other studies excluded competitive athletes [20], participants with high aerobic fitness (VO2 maximum > 35 ml/kg/min) [26] or high overall weekly exercise duration (> 250 min per week) [16]. Two studies only included inactive participants (< 90 min physical activity per week) [17, 27]. Six studies included participants who were not osteoporotic [19,20,21,22, 25, 26], two studies included osteopenic or osteoporotic participants [18, 24], while others did not have baseline BMD criteria [16, 17, 23, 27]. Other inclusion criteria included participants with at least mild arthralgia [27], or at risk of developing or having breast cancer-related lymphedema [23].
Intervention characteristics
Intervention duration was most commonly 12 months (n = 8) [17, 19,20,21,22,23, 25, 27], except for two 6-month studies [16, 24], one 8-month study [26] and one 24-month study [18]. Interventions were completely supervised (n = 1) [26] or unsupervised (n = 3) [16, 18, 24] or most commonly a combination (n = 8) [17, 19,20,21,22,23, 25, 27]. Five studies used resistance training either alone (n = 2) [18, 23] or combined with impact training (n = 3) [19, 21, 22], two studies used aerobic training either alone (n = 1) [17] or combined with impact training (n = 1) [20], while three studies used a combination of resistance and aerobic training [24, 25, 27]. Additionally, one study used both a resistance and aerobic training group in a three-arm RCT [16], while another implemented a soccer training intervention [26]. Exercise training was completed at home (n = 2) [16, 24], in a health and fitness centre (n = 2) [23, 25], a combination of both (n = 7) [17,18,19,20,21,22, 27] or on an outdoor soccer field (n = 1) [26]. Aerobic training typically involved walking or any other aerobic activity of choice, resistance training typically involved free weights, weight machines or resistance bands, while weight-bearing impact exercises were completed using either body weight or weight belts. Resistance training incorporated upper and lower body exercises [16, 18, 19, 21,22,23,24, 27] or only lower body exercises [25]. Impact exercises included two-footed jumping [19, 21, 22] or alternating weeks of step aerobics and circuit training [20]. Aerobic exercise was prescribed two to five times per week [16, 17, 20, 24, 25], except for one study [27] prescribing a total weekly duration. Resistance training sessions were generally prescribed two to three times per week [18, 23, 24, 27], including studies incorporating impact exercises [19, 21, 22] and aerobic exercises [25] within the same session. Exceptions were Schwartz et al. [16] who prescribed four sessions and Saarto et al. [20] who prescribed one impact session per week. Aerobic training intensity ranged from 11 to 16 on the Rating of Perceived Exertion (RPE) scale [20, 24, 25], 50–85% maximum heart rate [17, 25, 27] or simply moderate intensity (not defined) [16]. Resistance and impact training intensity was 70% 1RM [25], 0–15% body weight [19, 21, 22], 14–16 RPE [20], undefined low-moderate intensity [23, 24] or not specifically reported [16, 18, 19, 21, 22, 27]. Uth et al. [26] did not report the prescribed intensity of soccer training. Session duration was 15–60 min for aerobic training in most studies [16, 20, 24, 25] and 30–90 min for resistance training sessions [18, 23] including those combined with impact training [19, 21, 22] and 30–60 min for soccer training sessions [26]. Four studies did not report resistance training duration [16, 24, 25, 27].
Eight studies were specifically aimed at improving bone outcomes [16, 18,19,20,21,22, 24, 25], with five of these studies reporting that the exercises prescribed were targeted at specific skeletal sites [18, 19, 21, 22] or were focused on increasing the load placed on bone to induce adaptation [19,20,21,22]. Other interventions were primarily aimed at improving lean mass [26], muscle strength [23], arthralgia [27] or body composition in general [17].
Eight studies used a usual care control group [16,17,18, 20, 23, 24, 26, 27], three a flexibility training program as an exercise placebo [19, 21, 22] and one a home exercise information program [25]. Additionally, three studies provided calcium and vitamin D supplementation to both the control and intervention groups [18, 24, 25], one of which also provided bisphosphonates [18].
Mean retention of participants was 87% (range 66 to 100%) in intervention groups and 86% (range 57 to 95%) in control groups [17,18,19,20,21,22,23,24,25,26,27]. One study reported 93% retention overall for the study [16]. Adherence to supervised exercise sessions ranged from 61 to 84% [18,19,20,21,22, 25], while adherence to unsupervised sessions was 23–43% in resistance and impact training interventions [19, 21, 22] and 100% in a study prescribing unsupervised aerobic exercise [20]. One study reported adherence for supervised but not unsupervised sessions [25], while another did not report exercise adherence at all [16]. One study reported 82% adherence in the first 6 months (first 3 months were supervised) compared to 58% in the remaining 6 months (unsupervised) [23]. Uth et al. [26] reported 77% adherence in the first 12 weeks of soccer training and 46% in the remaining 20 weeks. Kim et al. [24] reported 70% adherence for aerobic exercise sessions but 49% for resistance training sessions. Thomas et al. [27] reported that participants completed slightly above the prescribed weekly exercise duration while Irwin et al. [17] reported that 73% of participants completed at least 80% of the prescribed aerobic training goal.
No study-related adverse events or injuries were reported in five studies [18, 19, 22, 24, 25], while six studies did not report on adverse events [16, 17, 20, 21, 23, 27]. The soccer training intervention [26] reported two fractured fibulas and three other muscular or tendon injuries. Three of these men resumed training once recovered.
BMD outcomes
All studies used DXA to assess BMD. One study [26] reported results for both left and right hips, while all other studies reported a single result without specifying the side measured. Overall, 50% of included studies did not report any significant between group differences at any site (Table 2) [18, 21, 23,24,25, 27]. Significant benefits of exercise on lumbar spine BMD were reported in two studies [16, 19]. Winters-Stone et al. [19] reported a significant benefit in the resistance and impact exercise compared to control group (0.41 vs −2.27%) while Schwartz et al. [16] reported a significant net difference (7.1%) between the aerobic training and control group, but not between their resistance training and control groups. One study reported a net benefit for L4 BMD in the resistance and impact exercise compared to control group (−0.8 vs −3.4%), but no effect for L1-L4 BMD [22]. Saarto et al. [20] reported a significant benefit to femoral neck BMD in the aerobic-impact exercise relative to control group among premenopausal participants (−0.2 vs −1.4%). Uth et al. [26] reported significant benefits of soccer training compared to controls for both left and right total hip (0.9 vs −0.75%) and femoral shaft (0.7 vs −1.05%), while differences in left and right femoral neck BMD approached significance (p = 0.07). One study reported improved total body BMD following aerobic exercise training compared to usual care [17] while another did not [27]. In five of the studies which reported a beneficial effect of exercise on BMD, this was due to a maintenance in the exercise group and a decline (loss) in the control group [16, 17, 19, 20, 22].
Meta-analysis
Data for the meta-analysis were available for six studies [19,20,21,22, 25, 26]. Two studies measured total body BMD [17, 27], which was not measured in any other study and so were not included in the meta-analysis. Authors of four studies were not able to provide required data additional to what was published, and thus these results were not included [16, 18, 23, 24]. Of the remaining six studies, all measured lumbar spine and femoral neck BMD [19,20,21,22, 25, 26] and five measured total hip BMD [19, 21, 22, 25, 26]. One study [20] analysed pre- and postmenopausal women separately so these subgroups were included separately in the meta-analysis. In one study measuring both hips [26], data from the left hip only was included in the meta-analysis. Analysis was repeated with the right hip data and the results remained unchanged. Another study [25] including any female cancer patient was included with the breast cancer studies due to the high proportion of breast cancer patients (83%) in the final sample. The results remained unchanged if the analysis was completed with this study included as a separate group.
Primary analysis
There was no overall significant effect of exercise compared to controls on lumbar spine BMD (0.0071 g/cm2, 95% CI −0.0002 to 0.0145, p = 0.057), femoral neck BMD (0.0044 g/cm2, 95% CI −0.0005 to 0.0093, p = 0.077) or total hip BMD (0.0024 g/cm2, 95% CI −0.0038 to 0.0086, p = 0.443) (Fig. 3a–c). There was borderline significant moderate heterogeneity overall for the effect of exercise on lumbar spine BMD (I 2 = 52.1%, p = 0.051) and low non-significant heterogeneity for femoral neck (I 2 = 19.7%, p = 0.279) and total hip BMD (I 2 = 26.7%, p = 0.244).
Sensitivity and subgroup analysis
Intervention effects on lumbar spine and femoral neck BMD became significant when the analysis excluded one study with unclear risk of detection bias (Table 3), while results for total hip BMD did not differ from the overall analysis. Analysis of studies with low risk of attrition bias provided the same conclusions as the overall analysis at all skeletal sites. Heterogeneity did not diverge from the overall analysis at any site when only studies with low risk of detection bias were included, but was lower at the lumbar spine (I 2 = 9.3%, p = 0.346) and higher at the femoral neck (I 2 = 52.0%, p = 0.100) and total hip (I 2 = 73.1%, p = 0.054) when analysis only included studies at low risk of attrition bias. There were no significant effects when breast and prostate cancer studies were analysed separately. Subgroup analysis involving three trials [19, 21, 22] showed a significant benefit of exercise on lumbar spine BMD in studies implementing a resistance and impact exercise program (0.015 g/cm2, 95% CI 0.003 to 0.028, p = 0.019). The same results were observed by analysis type (ITT versus per protocol) since the same three studies as above used a per-protocol analysis. The only other significant subgroup effects were observed in subgroups containing results from a single study (Table 3).
Discussion
The main finding from this systematic review and meta-analysis of exercise RCTs in adults with cancer was that there were no significant effects on lumbar spine, femoral neck or total hip BMD. However, given that there was a trend for a beneficial effect of exercise on lumbar spine (p = 0.057) and femoral neck (p = 0.077) BMD, the lack of clear evidence to support the role of exercise to maintain or improve BMD in cancer patients was likely influenced by the limited and varied studies available, particularly the few studies (n = 6) with appropriate data to include in the meta-analysis. It is also worth highlighting that these trends became significant when one study with unclear risk of detection bias was excluded from the analysis. Additionally, subgroup analysis of three intervention trials implementing a resistance and impact exercise program revealed that there was a significant benefit on lumbar spine BMD. This is consistent with the findings from meta-analyses of exercise interventions in healthy older adults, where multi-component programs including progressive resistance training with weight-bearing activities have been shown to be most effective for improving hip and/or lumbar spine BMD [8,9,10,11]. Given that there are few well-designed, long-term RCTs examining the efficacy of such targeted exercise programs on skeletal health in adults with cancer, there is a clear need for further research in this area.
Previous meta-analyses of RCTs in healthy older adults have consistently reported small but significant net benefits (1–3%) of multi-component exercise programs on BMD at the hip and/or lumbar spine [8,9,10,11]. The equivocal results observed regarding the effects of exercise on BMD in cancer patients in this systematic review and meta-analysis is likely related to a number of factors associated with the limited RCTs available. Sensitivity analysis by detection bias excluded a single study and resulted in a significant positive overall effect of exercise on lumbar spine and femoral neck BMD. This was likely because the excluded study by Knobf et al. [25] reported a small but non-significant negative effect of exercise on BMD at these skeletal sites, while almost all other studies in the meta-analysis reported small but predominantly non-significant positive effects of exercise at these sites. Despite high retention of participants (mean 87%) in the exercise trials included, several of the studies described difficulties with recruitment [22] and limitations with regard to the final sample size [19, 21, 22, 24], potentially limiting the statistical power to detect any exercise-related benefits. Low exercise adherence in several studies, differences in the age, menopausal status, habitual physical activity levels and initial BMD of participants, the type and timing of cancer treatment, the use of calcium and/or vitamin D supplements or bisphosphonate therapy in some trials are all other factors that may have contributed to the mixed findings. For instance, previous research indicates that premenopausal women are more responsive to the osteogenic effects of exercise than postmenopausal women [28, 29]. In part support of this notion, two studies included in this review reported a greater skeletal response in pre- compared to postmenopausal women when analysed separately [16, 20]. Additionally, one study [21] reported a greater osteogenic response to exercise in women at least 1 year postmenopause compared to women within the first year of menopause, suggesting the oestrogen status at different stages of menopause may also influence skeletal adaptation to exercise beyond simply pre- compared to postmenopause. Of note, most studies only included postmenopausal women [17,18,19, 21, 24, 27] limiting subgroup analysis by menopausal status.
A greater skeletal response to exercise is also expected with lower initial BMD [30, 31]. All six studies included in the meta-analysis only included non-osteoporotic participants. In habitually active participants, higher intensity or higher magnitude loading patterns are likely required to achieve a sufficient overload to stimulate further bone adaptations [32]. While most studies excluded participants completing regular resistance or impact training at baseline [18, 19, 21,22,23,24,25], others used more generous physical activity inclusion criteria [16, 20, 26], potentially contributing to the varying results. Regarding the timing of cancer treatment, the rate of bone loss has been shown to be higher in women undergoing current endocrine treatment or chemotherapy compared to those not receiving current treatment [1, 2]. While three included studies reported no differences in the skeletal response to exercise between types of breast cancer treatment [17, 20, 23], they were limited by relatively small sample sizes in the subgroups.
It is well established that not all types of exercise are equally osteogenic. Walking and other low/non-impact activities such as cycling have been shown to have little or no effects on BMD in healthy middle aged and older adults [33,34,35]. Several studies included in this review involved aerobic exercise (walking or another aerobic activity of choice) alone or with resistance training and reported little benefit on BMD at any site [24, 25, 27]. However, in women with breast cancer, Schwartz et al. [16] reported improved lumbar spine BMD following aerobic exercise (predominantly walking) compared to usual care, but there was no benefit of resistance training, which is likely due to an insufficient training load due to using resistance bands. Consistent with these findings, the lack of a marked effect of resistance training alone on BMD in many of the other studies included in this review is likely related to the dose or intensity prescribed, which appeared to be moderate (70% 1-RM or 8–15 repetitions maximum) in studies using free weights or weight machines [18, 19, 21,22,23, 25, 27] and low in studies using resistance bands [16, 24]. Resistance training volume may have also been insufficient to maximise skeletal adaptation, with just 1 or 2 sets per exercise prescribed in several studies [16, 18, 19, 22, 24, 25]. Current exercise guidelines for preventing osteoporosis recommended 2–3 sets of 8–10 repetitions at moderate to high intensity (75–85% 1-RM) [30]. Specificity and progression of an exercise program and training (overload principle) are also critical elements to stimulate bone adaptation [35]. Most included studies planned to progress resistance exercises predominantly by increasing the weight [18, 19, 21,22,23, 27] or the level of resistance of the band used [16, 24]; however, the only two studies [19, 22] that reported the actual level of progression achieved both reported falling short of the targeted progression.
Exercise guidelines for weight-bearing impact exercise with regard to skeletal health highlight that moderate to high-magnitude dynamic loading, applied rapidly and interspersed with rest periods and with loads that are novel and varied in direction, provides the greatest stimulus to elicit bone adaptation [35, 36]. Impact exercises in three studies consisted of the same two-footed jumping for the entire 12-month intervention [19, 21, 22], and thus, participants were not subjected to novel or diverse loading patterns so potentially adapted to the prescribed exercise, blunting the osteogenic response. Additionally, the achieved progression of these exercises was either lower than planned [22] or not reported [19, 21]. Men in the soccer training intervention [26] completed frequent accelerations and decelerations that likely subjected the bones to large muscle and ground reaction forces, which the authors suggested contributed to the positive skeletal adaptations. However, the safety of this mode of training is questionable given the five serious musculoskeletal injuries reported during this intervention. Alternating weeks of step aerobics and circuit training in the study by Saarto et al. [20] involved multidirectional impact loading patterns progressing in intensity; however, these impact training sessions were completed just once per week. When evaluating the exercise prescribed in the included studies to current evidence-based exercise recommendations for preventing and managing osteoporosis in older adults [32, 35], only three studies implemented combined resistance and impact exercise training [19, 21, 22] and subgroup analysis revealed a significant positive effect of such programs on lumbar spine BMD. The magnitude of skeletal adaptation following exercise is known to be dependent on a number of factors, including the specificity of loading, progressive overload and the loading characteristics (magnitude, rate, number and frequency of loads) [37]. Each of these three interventions specifically prescribed exercises aimed to load the spine or the muscles attached to or near the spine, which likely contributed to the positive effect on lumbar spine BMD. While these results must be interpreted with caution, they are consistent with the skeletal benefits reported following multi-component exercise interventions that are targeted to load specific skeletal sites in healthy older adults [8,9,10,11].
Two included studies in this review only reported total body BMD [17, 27], without measuring clinically relevant sites such as the hip and spine. Although the focus of these studies was on body composition (muscle and fat) changes, in which case total body DXA scans are appropriate, total body BMD is not used clinically for the diagnosis of osteoporosis. Future research should focus on measuring clinically relevant skeletal sites that are prone to osteoporotic fracture, in addition to total body DXA scans to provide a more complete and informative assessment of body composition.
A strength of this systematic review and meta-analysis is that it was conducted in accordance with the PRISMA guidelines [13]. Additionally, only studies with an intervention 6 months or longer were included as exercise programs shorter than this are unlikely to achieve true meaningful changes in BMD given that the typical bone remodelling cycle lasts 6 to 9 months. However, there are some limitations that should be considered when interpreting the results. Firstly, a small number of studies were identified overall and even fewer included or provided appropriate data to include in the meta-analysis. Additionally, substantial heterogeneity existed between studies in terms of the participant characteristics, exercise interventions prescribed and control groups used. Finally, with insufficient studies available to complete a formal assessment, publication bias cannot be discounted.
In summary, this review provides an important update on the effects of exercise training on cancer treatment-induced bone loss. While the results are largely equivocal and do not provide conclusive evidence for clinicians and other health care professionals to recommend exercise training to selected cancer patients to prevent bone loss, the results must be interpreted in the context of the individual study limitations discussed. Well-designed RCT’s implementing appropriately prescribed exercise programs are still required to better understand the role of exercise to combat cancer treatment-induced bone loss. Future studies should be conducted for at least 12 months and use a combination of targeted and progressive resistance training and impact exercises to maximise bone adaptation. However, future studies also need to evaluate the safety and tolerability of prescribing high-intensity resistance or high-impact weight-bearing activities in such clinical populations, with detailed recording of any potential adverse effects. If these studies are safe and well tolerated, future studies could consider increasing the dose of impact training or increasing the rate of progression to elicit greater bone adaptation. These studies could also implement longer term follow-up to assess important outcomes such as fractures, exercise maintenance and quality of life following an exercise intervention.
Studies using more advanced imaging technology (pQCT, high resolution CT) to investigate bone structure, strength and distribution outcomes are also needed to gain a greater understanding of the potential benefits of exercise on whole bone strength. Further research investigating the combination of exercise training with other common management practices such as nutritional approaches or anti-resorptive medication would also be of benefit to inform best practice guidelines to manage cancer treatment-induced bone loss. Research to investigate the effects of exercise on skeletal health with different cancer treatment types and at different stages of cancer treatment when the rate of bone loss may differ may also allow more effective tailoring of exercise training to cancer patients. More well-designed trials showing benefits of exercise for not only bone health, but other cancer treatment-related adverse effects, would ideally lead to more integrated oncology and exercise physiology practices to optimise the care of cancer survivors. Ideally, strategies such as exercise training, to manage the adverse effects of cancer treatment would be implemented concurrently with the initiation of cancer treatment to ameliorate the impact of these adverse effects on cancer patients.
References
Reid DM, Doughty J, Eastell R, Heys SD, Howell A, McCloskey EV, Powles T, Selby P, Coleman RE (2008) Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev 34:S3–S18
Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, Pritchard KI (2008) Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol 26(33):5465–5476
Grossmann M, Hamilton EJ, Gilfillan C, Bolton D, Joon DL, Zajac JD (2011) Bone and metabolic health in patients with non-metastatic prostate cancer who are receiving androgen deprivation therapy. Med J Aust 194(6):301–306
Gardner JR, Livingston PM, Fraser SF (2014) Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol Off J Am Soc Clin Oncol 32(4):335–346. https://doi.org/10.1200/JCO.2013.49.5523
Østergren PB, Kistorp C, Bennedbæk FN, Faber J, Sønksen J, Fode M (2016) The use of exercise interventions to overcome adverse effects of androgen deprivation therapy. Nat Rev Urol 13(6):353–364
Loprinzi PD, Cardinal BJ (2012) Effects of physical activity on common side effects of breast cancer treatment. Breast Cancer 19(1):4–10
Casla S, Hojman P, Márquez-Rodas I, López-Tarruella S, Jerez Y, Barakat R, Martín M (2015) Running away from side effects: physical exercise as a complementary intervention for breast cancer patients. Clin Transl Oncol 17(3):180–196
Kelley GA, Kelley KS, Kohrt WM (2012) Effects of ground and joint reaction force exercise on lumbar spine and femoral neck bone mineral density in postmenopausal women: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 13(1):177
Marques E, Mota J, Carvalho J (2012) Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age (Dordr) 34(6):1493–1515. https://doi.org/10.1007/s11357-011-9311-8
Kelley GA, Kelley KS, Kohrt WM (2013) Exercise and bone mineral density in premenopausal women: a meta-analysis of randomized controlled trials. Int J Endocrinol 2013:741639
Zhao R, Zhao M, Xu Z (2015) The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: a meta-analysis. Osteoporos Int 26(5):1605–1618. https://doi.org/10.1007/s00198-015-3034-0
Winters-Stone KM, Schwartz A, Nail LM (2010) A review of exercise interventions to improve bone health in adult cancer survivors. J Cancer Surviv 4(3):187–201. https://doi.org/10.1007/s11764-010-0122-1
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Higgins JPT, Green S (editors) The Cochrane Collaboration (2011) Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. Available from www.cochrane-handbook.org.
Higgin J, Thompson S, Deeks J, Altman D (2003) Measuring inconsistency in meta-analysis. BMJ 327:557–560
Schwartz AL, Winters-Stone K, Gallucci B (2007) Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum 34(3):627–633. https://doi.org/10.1188/07.onf.627-633
Irwin ML, Alvarez-Reeves M, Cadmus L, Mierzejewski E, Mayne ST, Yu H, Chung GG, Jones B, Knobf MT, DiPietro L (2009) Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity (Silver Spring, Md) 17(8):1534–1541. https://doi.org/10.1038/oby.2009.18
Waltman NL, Twiss JJ, Ott CD, Gross GJ, Lindsey AM, Moore TE, Berg K, Kupzyk K (2010) The effect of weight training on bone mineral density and bone turnover in postmenopausal breast cancer survivors with bone loss: a 24-month randomized controlled trial. Osteoporos Int 21(8):1361–1369. https://doi.org/10.1007/s00198-009-1083-y
Winters-Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, Schwartz A (2011) Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat 127(2):447–456. https://doi.org/10.1007/s10549-011-1444-z
Saarto T, Sievänen H, Kellokumpu-Lehtinen P, Nikander R, Vehmanen L, Huovinen R, Kautiainen H, Järvenpää S, Penttinen HM, Utriainen M, Jääskeläinen AS, Elme A, Ruohola J, Palva T, Vertio H, Rautalahti M, Fogelholm M, Luoto R, Blomqvist C (2012) Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int 23(5):1601–1612
Winters-Stone KM, Dobek J, Nail LM, Bennett JA, Leo MC, Torgrimson-Ojerio B, Luoh SW, Schwartz A (2013) Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporos Int 24(5):1637–1646. https://doi.org/10.1007/s00198-012-2143-2
Winters-Stone KM, Dobek JC, Bennett JA, Maddalozzo GF, Ryan CW, Beer TM (2014) Skeletal response to resistance and impact training in prostate cancer survivors. Med Sci Sports Exerc 46(8):1482–1488. https://doi.org/10.1249/MSS.0000000000000265
Winters-Stone KM, Laudermilk M, Woo K, Brown JC, Schmitz KH (2014) Influence of weight training on skeletal health of breast cancer survivors with or at risk for breast cancer-related lymphedema. J Cancer Surviv 8(2):260–268. https://doi.org/10.1007/s11764-013-0337-z
Kim SH, Cho YU, Kim SJ, Hong S, Han MS, Choi E (2016) The effect on bone outcomes of adding exercise to supplements for osteopenic breast cancer survivors: a pilot randomized controlled trial. Cancer Nurs 39(2):144–152. https://doi.org/10.1097/ncc.0000000000000245
Knobf MT, Jeon S, Smith B, Harris L, Kerstetter J, Thompson AS, Insogna K (2016) Effect of a randomized controlled exercise trial on bone outcomes: influence of adjuvant endocrine therapy. Breast Cancer Res Treat 155(3):491–500. https://doi.org/10.1007/s10549-016-3693-3
Uth J, Hornstrup T, Christensen JF, Christensen KB, Jørgensen NR, Schmidt JF, Brasso K, Jakobsen MD, Sundstrup E, Andersen LL, Rørth M, Midtgaard J, Krustrup P, Helge EW (2016) Efficacy of recreational football on bone health, body composition, and physical functioning in men with prostate cancer undergoing androgen deprivation therapy: 32-week follow-up of the FC prostate randomised controlled trial. Osteoporos Int 27(4):1507–1518
Thomas GA, Cartmel B, Harrigan M, Fiellin M, Capozza S, Zhou Y, Ercolano E, Gross CP, Hershman D, Ligibel J, Schmitz K, Li F-Y, Sanft T, Irwin ML (2017) The effect of exercise on body composition and bone mineral density in breast cancer survivors taking aromatase inhibitors. Obesity 25(2):346–351. https://doi.org/10.1002/oby.21729
Bassey E, Rothwell M, Littlewood J, Pye D (1998) Pre-and postmenopausal women have different bone mineral density responses to the same high-impact exercise. J Bone Miner Res 13(12):1805–1813
Sugiyama T, Yamaguchi A, Kawai S (2002) Effects of skeletal loading on bone mass and compensation mechanism in bone: a new insight into the “mechanostat” theory. J Bone Miner Metab 20(4):196–200
Beck BR, Snow CM (2003) Bone health across the lifespan—exercising our options. Exerc Sport Sci Rev 31(3):117–122
Winters-Stone KM, Snow CM (2003) Musculoskeletal response to exercise is greatest in women with low initial values. Med Sci Sports Exerc 35(10):1691–1696
Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR (2004) ACSM position stand: physical activity and bone health. Med Sci Sports Exerc 36(11):1985–1996
Martyn-St James M, Carroll S (2008) Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone 43(3):521–531
Ma D, Wu L, He Z (2013) Effects of walking on the preservation of bone mineral density in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Menopause 20(11):1216–1226
Beck BR, Daly RM, Singh MAF, Taaffe DR (2017) Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. J Sci Med Sport 20(5):438–445. https://doi.org/10.1016/j.jsams.2016.10.001
Turner C (1998) Three rules for bone adaptation to mechanical stimuli. Bone 23(5):399–407
Winters-Stone KM, Snow CM (2006) Site-specific response of bone to exercise in premenopausal women. Bone 39(6):1203–1209
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
Supplementary Table 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Dalla Via, J., Daly, R.M. & Fraser, S.F. The effect of exercise on bone mineral density in adult cancer survivors: a systematic review and meta-analysis. Osteoporos Int 29, 287–303 (2018). https://doi.org/10.1007/s00198-017-4237-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-017-4237-3