Abstract
The synthesis of quaternized glucosamide-based trisiloxane surfactant (QGS) of the general formula Me3SiOSiMeR1OSiMe3 (R1 = (CH2)3N+(CH3)2(CH2)2R2, R2 = glucosamide group) was described, and the surface activity properties of the surfactant were studied. The N-[2-(dimethylamino)ethyl]-d-gluconamide was synthesized by amidation of the ethylenediamine with d-gluconolactone. The 3-(3-chloropropyl)-1,1,1,3,5,5,5-heptamethyltrisiloxane was prepared by the acid-catalyzed reaction of a silane monomer. The QGS was prepared by quaternization of the precursor halogenated hydrocarbon with a tertiary amine. They were structurally characterized by IR, 1H NMR and MS. And it reduced the surface tension of water to approximately 21 mN m−1 at concentration levels of 10−3 mol L−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years silicone surfactants have attracted considerable interest because of their many technical applications. The trisiloxane surfactants are a kind of silicone surfactant. The exceptional surface properties of the trisiloxanes, some having “super-wetting” ability, are attributed to their unique molecular architecture. They have aroused the great interests of many scientific researchers [1–9].
In contrast, alkyl glucosamide surfactants have been known for many years. In recent years, their use has been gradually increasing because of their valuable properties, such as good dermatological compatibility, excellent biodegradability, and the absence of toxic effects.
The QGS are synthesized and they possess fine properties [10–18]. However, the trisiloxane with the quaternary glucosamide as the hydrophilic head-group has been rarely reported in the literature.
In this paper, we report the preparation of a QGS and the study of the surface activity of this compound by measuring the equilibrium surface tension of the dilute aqueous solutions.
Experimental Procedures
Preparation of QGS
-
Preparation of N-[2-(dimethylamino)ethyl]-d-gluconamide. First, 8.8 g (0.1 mol) of N,N-dimethylethylenediamine, 17.8 g (0.1 mol) of d-gluconolactone and 200 mL methanol were introduced into a 500-mL round-bottomed flask equipped with a refluxing condenser and a stirrer. The mixture was heated to reflux temperature in methanol. After 7 h, the solvent was removed under reduced pressure and the crude solid product obtained was recrystallized from ethanol to give the compound in a yield of 90.8 %. The structure of the compound was confirmed by IR and 1H NMR.
-
Preparation of 3-(3-chloropropyl)-1,1,1,3,5,5,5-heptamethyltrisiloxane. First, 81.1 g (0.5 mol) of hexamethyldisiloxane, 18.3 g (0.1 mol) of 3-chloropropylmethyldimethoxysilane and 14.7 g (0.15 mol) of concentrated sulfuric acid were placed in a 500-mL three-neck flask equipped with a refluxing condenser and a magnetic stirrer. The mixture was heated with stirring for 2 h at 60 °C. After the reaction had finished, the mixture was cooled to room temperature and neutralized with sodium carbonate to neutral. The solid material was filtered off. The excess hexamethyldisiloxane was removed under reduced pressure. The liquid product was purified by fractional distillation (b.p. 130 °C/76 mmHg, purity >99 %). The structure of the compound was confirmed by IR and 1H NMR.
-
Preparation of the QGS. First, 5.3 g (0.02 mol) of N-[2-(dimethylamino)ethyl]-d-gluconamide, 17.9 g (0.06 mol) of 3-(3-chloropropyl)-1,1,1,3,5,5,5-heptamethyltrisiloxane, 0.1 g (0.0006 mol) of potassium iodide and 150 mL benzyl alcohol were introduced into a 500-mL reactor vessel. The mixture was heated with stirring for 48 h at 90 °C. The excess 3-(3-chloropropyl)-1,1,1,3,5,5,5-heptamethyltrisiloxane was distilled off in vacuum and the brown viscous product obtained was extracted with ethyl acetate and water (ethyl acetate/water = 30/1 volume). The water layer was separated by silica gel column chromatography (ethyl acetate/methanol/water = 5/1/1 volume) to give the compound as a yellowish paste. The structure of the compound was confirmed by IR, 1H NMR and MS.
Characterization of the QGS and Surface Activity Measurements
The proton nuclear magnetic resonance (1H NMR) spectra were acquired on a 300 MHz Bruker DRX–300 NMR spectrometer. Samples were prepared in 5-mm-OD tubes with deuterated solvents D2O or CDCl3. Chemical shifts were referenced to tetramethylsilane. The infrared (IR) spectra were recorded on a Nicolet FT-IR Spectrometer iS10 (KBr). The mass spectra were scanned on a AB SCIEX API3200 instrument.
Aqueous solution equilibrium surface tension values were obtained by the Wilhelmy plate method using a Dataphysics tensiometer, model DCAT21. The CMC value was taken at the intersection of the linear portions of the plots of the surface tension against the logarithm of the surfactant concentration. Surfactant solutions were prepared with distilled, de-ionized water. Sample temperatures were stable at 25 ± 0.2 °C. Prior to measurements on the surfactant solutions, the surface tension of the distilled, de-ionized water was measured. These water values were in the range of 72.3 ± 0.3 mN m−1. Samples were aged for 15 min prior to their surface tension measurement.
Results and Discussion
Preparation and Spectroscopic Characterization of the QGS
The general formula and the preparation process of the QGS are shown in Scheme 1. The N-[2-(dimethylamino)ethyl]-d-gluconamide was prepared by amidation of the ethylenediamine with d-gluconic acid δ-lactone. The d-gluconic acid δ-lactone was ring-opened by nucleophilic attack of the primary amine. The amidation of the primary amine proceeded at the presence of methanol at reflux temperature. The reaction conditions were moderate. The 3-(3-chloropropyl)-1,1,1,3,5,5,5-heptamethyltrisiloxane was prepared by the acid-catalyzed reaction of a silane monomer. The excess hexamethyldisiloxane was added, which allowed the reaction to produce more trisiloxane and distilled off after reaction. The quaternized glucosamide-based trisiloxane was prepared by using the precursor halogenated hydrocarbon and tertiary amine as the starting material.
These compounds were structurally characterized by their IR, 1H-NMR and MS spectra. The details of these spectral characterizations were as follows. In all cases, the spectra acquired were consistent with the assigned structures of the compounds.
N-[2-(dimethylamino)ethyl]-d-gluconamide
IR (KBr, υ, cm−1): 3,250–3,450 (–OH in sugar), 2,830–2,950 (C–H), 1,650–1,660 (C=O), 1,540–1,550 (N–H), 1,030–1,090 (C–O); 1H NMR (D2O, 300 MHz, δ ppm): 2.10 (s, 6H, N–(CH3)2), 2.34–2.47 (t, 2H, N–CH2–), 3.20–3.30 (t, 2H, N–C–CH2–), 3.42–3.72 (m, 4H, –(CH)4– in sugar), 3.93, 4.15 (d, 2H, –CH2– in sugar).
3-(3-Chloropropyl)-1,1,1,3,5,5,5-heptamethyltrisiloxane
IR (KBr, υ, cm−1): 2,880–2,960 (C–H), 1,050–1,100 (Si–O–Si), 1,260, 840, 760 (Si(CH3)3); 1H NMR (CDCl3, 300 MHz, δ ppm): 0.04 (s, 3H, Si–CH3), 0.10 (s, 18H, Si(CH3)3·2), 0.55–0.61 (t, 2H, Si–CH2–), 1.74–1.85 (m, 2H, Si–C–CH2–), 3.49–3.54 (t, 2H, Si–C–C–CH2–).
QGS
IR(KBr, υ, cm−1): 3,250–3,450 (–OH in sugar), 2,890–2,960 (C–H), 1,650–1,660 (C=O), 1,540–1,550 (N–H), 1,259, 842, 755 (Si–CH3), 1,030–1,140 (C–O, Si–O); 1H NMR (CDCl3, 300 MHz, δ ppm): 0.06 (s, 3H, Si–CH3), 0.10 (s, 18H, Si(CH3)3·2), 0.42–0.58 (t, 2H, Si–CH2–), 1.65–1.75 (m, 2H, Si–C–CH2–), 3.26 (s, 6H, N–(CH3)2), 3.32–3.45 (t, 2H, N–C–CH2–), 3.52–3.97 (m, 8H, Si–C–C–CH2–N–CH2–, –(CH)4– in sugar, overlap), 4.20, 4.40 (d, 2H, –CH2– in sugar), 4.85–6.00 (d, 5H, –OH in sugar), 8.25–8.45 (s, H, –NH–); MS: m/z = 529 ([M + 1]+ of cation radical).
Equilibrium Surface Tension Measurements
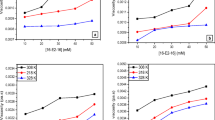
The equilibrium surface tension of dilute aqueous solutions of the QGS was measured. For comparison, the glucosamide-based trisiloxane (no quaternization, the structure is shown in Scheme 2, abbreviated as GS in what follows) was also listed [13, 14]. The minimum surface tension (γ CAC) values were acquired by analyzing the plateau region of the plots. The critical aggregation concentrations (CAC) of the surfactants were acquired by analyzing the intersection point of the plateau region and the steeply downward sloping portion of the plots. The surface excess concentration (Γmax) and the surface area per molecule ( \(a_{\text{m}}^{\text{s}}\)) were acquired by analyzing the application of the Gibbs equation to the steeply downward sloping section of the plots. A summary of the data iss compiled in Table 1 and in Fig. 1.
Inspection of the data in the Table 1 shows that the GS and QGS significantly reduce the surface tension of the solution at low concentration, indicating that these molecules adsorb strongly at the air/water surface and they are highly effective aqueous surfactants, reducing the surface tension of water to approximate 21 mN m−1.
Examination of Fig. 1 shows that the two plots have significant changes in slope as a minimum surface tension is reached. For most surfactants, this behavior implies that aggregation is occurring in the bulk solution.
Micelle formation by siloxane surfactants in aqueous solution has not been extensively investigated. Most of the studies to date report critical micelle concentrations (CMC) values from surface tension versus log surfactant concentration plots. Although the structure of the micelles formed was investigated in the last few years, no conclusion was drawn on the tendency of these surfactants to form well-defined micelles [1–9].
Assuming that the changes in slopes of the surface tension versus log concentration curves in Fig. 1 represent the onset of surfactant aggregation, possibly into micelles, the CAC values for the surfactants are reported in Table 1.
Our interpretation of the plots is that aggregation is occurring in solution, not necessarily micellization, and that the CAC values can be determined by extrapolation. This type of extrapolation is universally applied to this type of plot to estimate critical aggregation concentration, and we use it uncritically here. Some caution is needed in that the presence of a break point in the surface tension versus log concentration plot is only circumstantial evidence of aggregate formation.
The CAC values are determined from extrapolation to the break points in the surface tension versus log surfactant concentration curves (Fig. 1). The CAC values vary with the structure of the hydrophilic group. The CAC value increase after the glucosamide-based trisiloxane is quaternized.
The saturation adsorption values, Γmax, at the air/water interface and the minimum area per surfactant molecule, \(a_{\text{m}}^{\text{s}}\), at the air/water interface were obtained from the slope of the surface tension versus log concentration plots (Fig. 1) by using the approximate form of the Gibbs adsorption isotherm equations (Eqs. 1, 2).
where R = 8.3144 J mol−1 K−1, N A = Avogadro’s number, Γmax is in mol cm−2, and \(a_{\text{m}}^{\text{s}}\)is in 102 nm2 molecule−1. In our solution, we can set n = 1. π (=γ0 − γ) is the surface pressure in the region of surface saturation and C π is the molar concentration of surfactant in the aqueous phase at a surface pressure π (in mN m−1).
The polar region of the silicone surfactant molecule determines the required surface area and the siloxane group is responsible for the distance between the molecules in the adsorption layer, so the measured or estimated required surface area per molecule depends on both the size of the siloxanyl group and the nature of the polar part of the surfactant molecule.
It is to be expected, and confirmed by the \(a_{\text{m}}^{\text{s}}\) values in Table 1, that the surface area per molecule ( \(a_{\text{m}}^{\text{s}}\)) (Eq. 3) of the QGS at the interface increase after the glucosamide-based trisiloxane is quaternized.
The free energy of aggregation \({{\Delta }}G_{\text{agg}}^{ \circ }\) (Eq. 3) and adsorption ( \({{\Delta }}G_{\text{ads}}^{ \circ }\)) (Eq. 4) of the GS and QGS are negative value, indicating that the processes are thermodynamically favored.
The CAC/C20 ratio is a measure of the tendency to form aggregates relative to the tendency to adsorb at the air/water interface. A higher CAC/C20 ratio indicates that the hydrophobic groups are less suitably oriented for accommodating themselves in the interior of the aggregates. The larger CAC/C20 values for the QGS compared to the GS indicate that the quaternized product has a greater preference to be adsorbed at the water/air interface relative to its preference to form aggregates than do the non-quaternized product.
References
Snow SA, Fenton WN, Owen MJ (1990) Synthesis and characterization of zwitterionic silicone sulfobetaine surfactants. Langmuir 6:385–391
Snow SA, Fenton WN, Owen MJ (1991) Zwitterionic organofunctional siloxanes as aqueous surfactants: synthesis and characterization of betaine functional siloxanes and their comparison to sulfobetaine functional siloxanes. Langmuir 7:868–871
Snow SA (1993) Synthesis, characterization, stability, aqueous surface activity, and aqueous solution aggregation of the novel, cationic siloxane surfactants (Me3SiO)2Si(Me)-(CH2) +3 NMe2(CH2)2ORX-(R=H,C(O)Me,C(O)NH(Ph);X=Cl,Br,I,NO3,MeOSO3). Langmuir 9:424–430
Zhu S, Miller WG (1994) Superspreading of water–silicone surfactant on hydrophobic surfaces. Colloids Surf A 90:63–78
Gentle TE, Snow SA (1995) Absorption of small silicone polyether surfactants at the air/water surface. Langmuir 11:2905–2910
Rosen MJ, Song L (1996) Superspreading, skein wetting, and dynamic surface tension. Langmuir 12:4945–4949
Svitova T, Hoffmann H, Hill RM (1996) Trisiloxane surfactants: surface/interfacial tension dynamics and spreading on hydrophobic surfaces. Langmuir 12:1712–1721
Peng Z, Lu C, Lai J (2009) Synthesis and properties of novel double-tail trisiloxane surfactants. J Surf Deterg 12:331–336
Peng Z, Huang S, Cao M (2011) Synthesis and properties of novel double-tail trisiloxane surfactants with high spreading ability. J Surf Deterg 14:521–528
Wagner R, Richter L, Wersig R, Schmaucks G, Weiland B, Weißmüller J, Reiners J (1996) Silicon-modified carbohydrate surfactants i: synthesis of siloxanyl moieties containing straight-chained glycosides and amides. Appl Organomet Chem 10:421–435
Wagner R, Richter L, Weiland B, Reiners J, Weißmüller J (1996) Silicon-modified carbohydrate surfactants II: siloxanyl moieties containing branched structures. Appl Organomet Chem 10:437–450
Wagner R, Richter L, Weiland B, Weißmüller J, Reiners J, Krämer W (1997) Silicon-modified carbohydrate surfactants iii: cationic and anionic compounds. Appl Organomet Chem 11:523–538
Han F, Zhang G (2003) New family of siloxane surfactants having glucosamide. Tenside Surf Deterg 40:332–337
Han F, Zhang G (2004) Synthesis and characterization of glucosamide-based trisiloxane gemini surfactants. J Surf Deterg 7:175–180
Han F, Zhang G (2004) New family of gemini surfactants with glucosamide-based trisiloxane. Colloids Surf A 237:79–85
Han F, Deng Y, Zhou Y, Xu B (2011) Carbohydrate-modified silicone surfactants. J Surf Deterg 15:123–129
Han F, Chen Y, Zhou Y, Xu B (2011) Synthesis and characterization of glycoside-based trisiloxane surfactant. J Surf Deterg 14:515–520
Han F, Wang P, Song J, Zhou Y, Xu B (2012) A surface rheological study of a glucosamide-based trisiloxane gemini surfactant at the air/water interface. J Dispers Sci Technol 33:1708–1714
Acknowledgments
The financial support of the Natural Science Foundation of China (No. 21376009, 21176004, 21203005), the National Key Technologies R&D Program of China for the 12th Five-Year Plan (No. 2013BAC01B04) and the Importation and Development of High-Caliber Talents Project of Beijing Municipal Institutions (No. CIT&TCD201304026) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Han, F., Liu, Yl., Gao, Yd. et al. Synthesis and Characterization of a Quaternized Glucosamide-Based Trisiloxane Surfactant. J Surfact Deterg 17, 733–737 (2014). https://doi.org/10.1007/s11743-014-1571-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-014-1571-8