Abstract
Three carbohydrate-modified polysiloxane bola surfactants (ATPS-GA) were synthesized using a two-step method. Their chemical structures were characterized by infrared spectroscopy (FT-IR) and proton nuclear magnetic resonance spectroscopy (1H NMR). Their surface properties and aggregation properties in aqueous solution were determined using surface tension measurements and transmission electron microscopy (TEM). Surface tension measurement results indicated that the three bola surfactants are under 25 mN m−1, and much lower than those of conventional hydrocarbon bola surfactants due to the siloxane moiety at the end of the hydrophobic chains. TEM analysis results indicated that the ATPS-GA can self-assemble into spherical micelles with a wide range of average diameters from 100 nm to above 600 nm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bola surfactants consisting of two or more hydrophilic groups connected by a hydrophobic spacer have attracted great attention because of their fundamental importance and their potential applicability [1, 2]. Most of these bola surfactants have been studied for their aggregate behavior in water as a function of their structure [3, 4]. In an aqueous environment, these bola surfactants often self-assemble into unilamellar vesicles, which are stable over a long period of time and could be used as membrane models [5].
The hydrophilic groups of bola surfactants can be nonionic, anionic, cationic or zwitterionic. Recently, bola surfactants involving carbohydrate as the hydrophilic group have attracted increasing attention because they provide the required hydrosolubility to the aggregates and reduce toxicity [6–10]. The hydrophobic chains of bola surfactants can be hydrocarbon or heteroatom in nature. However, most of research publications have focused on the hydrocarbon-tailed bola surfactants. Only a few reported studies of heteroatom-tailed bola surfactants are available [9].
Siloxane-tailed surfactants, as heteroatom-tailed surfactants, have been widely used in many industrial fields such as foam stabilizers, emulsifying agents, detergents and antifoaming agents due to their thermal stability, lower surface tension, and excellent spreading and wetting properties [11]. Most commonly, the hydrophilic groups in siloxane-tailed surfactants consist of polyethers [12]. Because the polyethers are often produced from nonrenewable petrochemical ethylene oxide and propylene oxide, the polyether siloxane-tailed surfactants are not environmental-friendly surfactants [13, 14]. Recently, siloxane surfactants with carbohydrate as hydrophilic group have been synthesized and studied [15–29]. Compared with hydrophilic polyether groups, carbohydrates possess important properties such as biodegradability, biocompatibility, good hydrophilicity and better environmental.
It is therefore of particularly advantageous to combine carbohydrate-modified bola surfactants with carbohydrate-modified siloxane surfactants to develop novel carbohydrate-modified polysiloxane bola surfactants. Compared with conventional bola surfactants, changing the hydrocarbon-tailed group to a siloxane-tailed group and the use of a carbohydrate hydrophilic group may lead to significant variations to the surface properties and aggregate behavior.
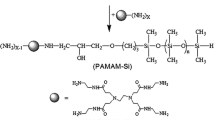
In this paper, carbohydrate-modified polysiloxane bola surfactants of different molecular weight were synthesized by two-step reactions as shown in Fig. 1. Their chemical structures were confirmed by FT-IR and 1H NMR. Surface activity and aggregation properties of the three bola surfactants were investigated to compare with conventional hydrocarbon-tailed bola surfactants.
Experimental Procedures
Materials
Octamethylcyclotetrasiloxane (D4), tetramethylammonium hydroxide (TMAH), gluconolactone (GA) were purchased from Energy Chemical Co. Ltd. 1,3-bis(aminopropyl)tetramethyldisiloxane (AT) was purchased from Alfa Aesar (Tianjin) Chemical Co. Ltd. Absolute methanol was obtained from Nanjing Chemical Reagent Co. Ltd. All regents were used as received without any further purification.
Synthesis of Amine-Terminated Polysiloxanes (ATPS)
The reaction was carried out in a 250-mL four-neck round-bottomed flask, equipped with a magnetic stirrer, a thermometer, and a reflux condenser. The mixture of D4 (14.83 g, 50.0 mmol), AT (12.43 g, 50.0 mmol) and TMAH (0.68 g, 7.5 mmol) were added based on the ingredients and stirred for 3 h at 90 °C under a nitrogen atmosphere. The resulting TMAH was then deactivated by heating to a temperature of 140 °C for 1 h. To remove unreacted starting materials and the low weight oligomer, the liquid product was subjected to fractional distillation. The amine-terminated polysiloxanes (ATPS-1) was obtained as a colorless liquid with a yield of 94.8 % (25.85 g). The amine value of ATPS-1, which was determined as reported earlier [30], was 3.89 mmol g−1. The molecular weights of ATPS-1, which was determined by end-group titration also as reported earlier [31], was 517 g mol−1.
The ATPS-2 and ATPS-3 were obtained using the same procedure as for ATPS-1. ATPS-2 was colorless liquid with a yield of 98.6 %. The amine value and molecular weight of ATPS-2 were 3.35 mmol g−1 and 596 g mol−1, respectively. ATPS-3 was a colorless liquid with a yield of 96.8 %. The amine value and molecular weight of ATPS-3 were 2.98 mmol g−1 and 691 g mol−1, respectively.
Synthesis of Carbohydrate-Modified Bola Polysiloxane Surfactants (ATPS-GA)
The ATPS-1 (25.71 g, 0.1 mol NH2) and gluconolactone (17.81 g, 0.1 mol) were put into a three-neck round-bottom flask equipped with a magnetic stirrer and refluxing condenser. The mixture was stirred and heated to reflux temperature for 12 h using methanol as solvent. After removal of the solvent, a solid residue was obtained and dried under reduced pressure to a constant mass. The carbohydrate modified bola polysiloxane surfactants (ATPS-GA-1) were obtained as a white powder with a yield of 94.6 % (39.5 g).
The ATPS-GA-2 and ATPS-GA-3 were synthesized using the same procedure as for ATPS-GA-1. ATPS-GA-2 was a white powder with a yield of 95.3 %. ATPS-GA-3 was a white powder with a yield of 93.8 %.
Characterization
Results of Fourier transform infrared spectroscopy (FT-IR) of ATPS and ATPS-GA were recorded using a Nicolet 380 (Thermo, USA) Spectrometer. The ATPS were smeared directly onto a KBr plate, whereas the ATPS-GA were mixed with KBr and pressed onto a plate for measurement.
Proton nuclear magnetic resonance spectroscopy (1H NMR) of ATPS and ATPS-GA were performed on a Bruker Avance DPX-300 Hz spectrometer. CDCl3 and D2O were used as solvent, respectively.
Measurement of Surface Tension
The surface tensions of aqueous solutions of the bolaform surfactants were measured on a KRUSS K12 Processor Tensiometer by the Wilhelmy plate method at 25 ± 0.1 °C.
Because aqueous solutions of the surfactants were prepared in doubly distilled water, before measurement, the surface tension of water was confirmed to be in the range of 72.0 ± 0.3 mN m−1.
Transmission Electron Microscopy
The aggregation behaviors of the bola surfactants in solutions were studied by a negative-staining method using JEM-1011 transmission electron microscopy (TEM) at 100 kV. One drop of the aggregates solution was laid on a 400-mesh copper grid coated with a thin film of carbon. The grids were negatively stained using 1.5 wt% phosphor-tungstic acid. The excess liquid was also tapped with filter paper.
Results and Discussion
Synthesis and Characterization of ATPS-GA Surfactants
Results from the synthesis indicated that the amine values of the three ATPS copolymers gradually decrease with increasing of the feed of D4, and the molecular weights increase with increasing of the feed of D4.
The structures of ATPS and ATPS-GA were determined by FT-IR and 1H NMR. The FT-IR spectra of ATPS and ATPS-GA are shown in Fig. 2. As illustrated in Fig. 2a, the peaks at 1259.2 and 800.2 cm−1 were attributed to Si–C group, the peaks at 1022.1, 1089.6 cm−1 were assigned to the Si–O–Si group, and the peaks at 3353.0 and 1579.2 cm−1 were ascribed to the NH2 group. As shown in Fig. 2c, the successful chemical incorporation of GA with ATPS was proved through the obtained of amide bond peak at 1643.8 and 1552.6 cm−1, and the disappearance of ester peak of gluconolactone at 1725.9 cm−1. Figure 3 shows the 1H NMR spectrum of ATPS and ATPS-GA. As illustrated in Fig. 3a, the peak at 0.07 ppm was assigned to –Si–CH 3, the peak at 0.47 ppm was attributed to –Si–CH 2, the peak at 1.42 ppm was ascribed to –Si–CH2–CH 2, and the peak at 2.63 ppm was attributed to –N–CH 2. Compared with the 1H NMR spectrum of the ATPS copolymer in Fig. 3a, the spectrum of ATPS-GA in Fig. 3b indicated that the new peaks in the range from 3.50 to 4.60 ppm were assigned to protons of the incorporated GA, and the peak for –N–CH 2 shifted to 3.30 ppm. Based on the FT-IR and 1H NMR analyses, indicating that ATPS-GA surfactants were successful synthesized using two-step reactions.
Equilibrium Surface Tension and Critical Micelle Concentration
To evaluate the surface activity of the bola surfactants, the aqueous solution equilibrium surface tension was determined. As illustrated in Fig. 4, the surface tension was plotted against the aqueous solution concentration of ATPS-GA-1, ATPS-GA-2 and ATPS-GA-3. It is clearly seen that the surface tension of bola surfactants gradually decrease with the increasing in logarithmic scale concentration and then level off. The critical micelle concentrations (CMC) of these three bola surfactants are shown as sharp bends in the curves. The CMC and the surface tension at the CMC (γCMC) results of these three bola surfactants are summarized in Table 1. The CMC of ATPS-GA-1, ATPS-GA-2, and ATPS-GA-3 are 9.63, 3.35 and 4.52 10−5 mol L−1, respectively. The γCMC of ATPS-GA-1, ATPS-GA-2 and ATPS-GA-3 are 23.2, 24.5 and 23.6 mN m−1, respectively. It can be seen that these three bola surfactants significantly reduced the surface tension at low concentration, indicating that these molecules adsorbed strongly at the air–water interface. γCMC values are 23–25 mN m−1, significantly lower than those conventional hydrocarbon-based bola surfactants [1, 2]. This result may be attributed to the siloxane methyl groups in the hydrophobic part lying flat at the air–water interface [32–34].
Aggregation Properties
The aggregation behaviors of conventional hydrocarbon bola surfactants in aqueous solutions have been widely reported [10, 35, 36]. Hill et al. investigated the aggregation behavior of ABA and comb-type siloxane surfactants using optical microscopy, cryo-TEM and X-ray scattering [37, 38]. However, studies on the aggregation behaviors of the carbohydrate-modified polysiloxane bola surfactants in aqueous solution are rare. As reported in Table 1, the critical micelle concentration of the bola surfactants is very low due to the extreme hydrophobicity of polysiloxanes, indicating that the bola surfactants are easy to self-assemble into aggregates in water. In order to evaluate the size and shape of aggregates formed in the bola surfactant solutions, the TEM measurements was conducted by negative-staining methods. Figure 5 shows the TEM images of the ATPS-GA-1 in aqueous solution. As shown in the TEM images, spherical micelles with a wide range of average diameters from 100 nm to above 600 nm were observed and found to be bigger than conventional polymeric micelles usually with a diameter of less than 100 nm. The majority of smaller micelles are less than 200 nm in diameter, whereas large ones can reach more than 600 nm in diameter. Based on the literature reports, the formation of larger complex micelles is attributed to he further aggregation of simple micelles, which is induced by hydrogen bonding or van der Waals interactions among the hydrophilic shell [39, 40].
Conclusions
Three novel carbohydrate-modified polysiloxane bola surfactants were successfully synthesized using two-step reactions and characterized by FT-IR and 1H NMR spectrometry. The surface activity of the three surfactants, ATPS-GA-1, ATPS-GA-2 and ATPS-GA-3, in water was determined by surface tension measurements and found to have excellent efficiency in surface tension reduction due to the incorporation of a polysiloxane moiety. TEM analysis of ATPS-GA aqueous solutions revealed that ATPS-GA can self-assemble into micelles with a wide range in diameter.
References
Fuhrhop JH, Wang TY (2004) Bolaamphiphiles. Chem Rev 104:2901–2938
Mullerfahrnow A, Saenger W, Fritsch D, Schneider P, Fuhrhop JH (1993) Molecular and crystal structure of the bola-amphiphile N-[8-(D-gluconamido)octyl]-D-gluconamide. Carbohydr Res 242:11–20
Benvegnu T, Brard M, Plusquellec D (2004) Archaebacteria bipolar lipid analogues: structure, synthesis and lyotropic properties. Curr Opin Colloid Interface Sci 8:469–479
Franceschi S, Andreu V, de Viguerie N, Riviere M, Lattes A, Moisand A (1998) Synthesis and aggregation behavior of two-headed surfactants containing the urocanic acid moiety. New J Chem 22:225–231
Fuhrhop JH, David HH, Mathieu J, Liman U, Winter HJ, Bockema E (1986) Bolaamphiphiles and monolayer lipid membranes made from 1,6,19,24-tetraoxa-3,21-cyclohexatriacontadiene-2,5,20,23-tetrone. J Am Chem Soc 108:1785–1791
Bertho JN, Coue A, Ewing DF, Goodby JW, Letellier P, Mackenzie G, Plusquellec D (1997) Novel sugar bola-amphiphiles with a pseudo macrocyclic structure. Carbohydr Res 300:341–346
Prata C, Mora N, Polidori A, Lacombe JM, Pucci B (1999) Synthesis and molecular aggregation of new sugar bola-amphiphiles. Carbohydr Res 321:12–23
Schuur B, Wagenaar A, Heeres A, Heeres EHJ (2004) A synthetic strategy for novel nonsymmetrical bola amphiphiles based on carbohydrates. Carbohydr Res 339:1147–1153
Denoyelle S, Polidori A, Brunelle M, Vuillaume PY, Laurent S, Elazhary Y, Pucci B (2006) Synthesis and preliminary biological studies of hemifluorinated bifunctional bolaamphiphiles designed for gene delivery. New J Chem 30:629–646
Deleu M, Damez C, Gatard S, Nott K, Paquot M, Bouquillon S (2011) Synthesis and physico-chemical characterization of bola amphiphiles derived from alkenyl D-xylosides. New J Chem 35:2258–2266
Hill RM (1999) Silicone surfactants. Marcel Dekker, New York, pp 1–48
Wang GY, Du ZP, Zhang W, Cao QY (2009) Synthesis and surface properties of trisiloxane-modified oligo(ethylene oxide). Tenside Surfactant Det 46:214–217
Black RE, Hurley FJ, Havery DC (2001) Occurrence of 1,4-dioxane in cosmetic raw materials and finished cosmetic products. J AOAC Int 84:666–670
Fu CB, Lai M, Tsai HY, Chang CM (2005) Impurity analysis of 1,4-dioxane in nonionic surfactants and cosmetics using headspace solid-phase microextraction coupled with gas chromatography and gas chromatography-mass spectrometry. J Chromatogr A 1071:141–145
Berson S, Viet DS, Driguez H, Fleury E, Hamaide T (2008) Synthesis of new cellobiose-based glycopolysiloxanes and their use as polymer stabilizers in miniemulsion polymerisation. Macromol Chem Phys 209:1814–1825
Wang GY, Qu WS, Du ZP, Cao QY, Li QX (2011) Adsorption and aggregation behavior of tetrasiloxane-tailed surfactants containing oligo(ethylene oxide) methyl ether and a sugar moiety. J Ind Eng Chem 115:3811
Du ZP, Wang L, Wang GY, Wang SJ (2011) Synthesis, surface and aggregation properties of glucosamide-grafted amphiphilic glycopolysiloxanes. Colloid Surface A 381:55
Wang L, Du ZP, Wang GY, Wang SJ, Cao Y (2011) Synthesis and properties of lactobionamide-based polysiloxane surfactant. Tenside Surfactants Deterg 48:281
Wang GY, Qu WS, Du ZP, Wang WX, Li QX (2013) Adsorption and aggregation behaviors of tetrasiloxane-tailed gemini surfactants with (EO)m spacers. J Phys Chem B 117:3154
Wang GY, Zhang DL, Du ZP, Li P (2013) Spontaneous vesicle formation from trisiloxane-tailed gemini surfactant. J Phys Chem B 20:1247
Zeng XJ, Lu ZP, Liu Y (2013) Synthesis and solution properties of novel sugar-based polysiloxane surfactants. J Surfactants Deterg 16:131–137
Iwakiri N, Nishikawa T, Kaneko Y, Kadokawa JI (2009) Synthesis of amphiphilic polysiloxanes and their properties for formation of nano-aggregates. Colloid Polym Sci 287:577–582
Racles C, Hamaide T (2005) Synthesis and characterization of water soluble saccharide functionalized polysiloxanes and their use as polymer surfactants for the stabilization of polycaprolactone nanoparticles. Macromol Chem Phys 206:1757–1768
Racles C, Hamaide T, Ioanid A (2006) Siloxane surfactants in polymer nanoparticles formulation. Appl Organome Chem 20:235–245
Zhou CH, Guan RF, Feng SY (2004) The preparation of a new polysiloxane copolymer with glucosylthioureylene groups on the side chains. Eur Polym J 40:165–170
Wagner R, Richter L, Wersig R, Schmaucks G, Weiland B, Weissmuller J, Reiners J (1996) Silicon-modified carbohydrate surfactants I: synthesis of siloxanyl moieties containing straight-chained glycosides and amides. Appl Organome Chem 10:421–435
Wagner R, Richter L, Weiland B, Reiners J, Weissmuller J (1996) Silicon-modified carbohydrate surfactants II: siloxanyl moieties containing branched structures. Appl Organome Chem 10:437–450
Wagner R, Richter L, Wersig R, Schmaucks G, Weiland B, Weissmuller J, Reiners J (1997) Silicon-modified carbohydrate surfactants III: cationic and anionic compounds. Appl Organome Chem 11:523–538
Wagner R, Richter L, Weissmuller J, Reiners J, Klein KD, Schaefer D, Stadtmuller S (1997) Silicon-modified carbohydrate surfactants: IV. The impact of substructures on the wetting behaviour of siloxanyl-modified carbohydrate surfactants on low-energy surfaces. Appl Organome Chem 11:617–632
Wu YZ, Feng SY (2001) Viscosity-molecular weight relationship for aminopropyl-terminated poly(dimethylsiloxane). J Appl Poly 80:975–978
Basu S (1950) Molecular weight of nylon by end-group titration. J Poly Sci 5:975–978
Chung D-W, Lim JC (2009) Study on the effect of structure of polydimethylsiloxane grafted with polyethyleneoxide on surface activities. Colloid Surface A 336:35–40
Wang AF, Jiang LP, Mao GZ, Liu YH (2001) Direct force measurement of comb silicone surfactants in alcoholic media by atomic force microscopy. J Colloid Interf Sci 242:337–345
Svitova T, Hoffmann H, Hill RM (1996) Trisiloxane surfactants: surface/Interfacial tension. Langmuir 12:1712–1721
Li YM, Zhang HX, Bao M, Chen QG (2011) Aggregation behavior of surfactants with different molecular structures in aqueous solution: DPD simulation study. J Disper Sci Technol 33:1437–1443
Zhang DM, Liu FL, Hao X, Guo YQ, Chen YS (2012) Large vesicles of ethylenediaminediacetic in ethanol due to hydrogen bonding. Colloid Surface A 415:167–173
Hill RM (1993) Lyotropic liquid crystal phase behavior of polymeric siloxane surfactants. Langmuir 9:2789–2798
Hill RM (1994) Comparison of the liquid crystal phase behavior of four trisiloxane superwetter surfactants. Langmuir 10:1724–1734
Zhang HC, An W, Liu ZN, Hao AY, Hao JC, Shen J, Zhao XH, Sun HY, Sun LZ (2010) Redox-responsive vesicles prepared from supramolecular cyclodextrin amphiphiles. Carbohydr Res 345:87–96
Liu M, Fu ZS, Wang Q, Xu JT, Fan ZQ (2008) Study of amphiphilic poly(1-dodecene-co-para-methylstyrene)-graft-poly(ethylene glycol). Part II: preparation and micellization behavior of the amphiphilic copolymers. Eur Polym J 44:4122–4128
Acknowledgments
The authors gratefully acknowledge the Suzhou SUNBO Chemical Building Materials Co., Ltd for financial support.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zeng, X., Wang, H., Chen, Y. et al. Synthesis and Solution Properties of Carbohydrate-Modified Polysiloxane Bola Surfactants. J Surfact Deterg 18, 1089–1094 (2015). https://doi.org/10.1007/s11743-015-1730-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1730-6