Abstract
Treatment of suspension cells of Ginkgo biloba with fungal endophytes resulted in accumulation of flavonoids, increased abscisic acid (ABA) production and activation of phenylalanine ammonia-lyase (PAL). Fluridone, an inhibitor of ABA biosynthesis, was effective in inhibiting fungal endophytes-induced ABA biosynthesis, increase of PAL activity and flavonoids accumulation. Moreover, exogenous application of ABA enhanced PAL activity and increased accumulation of flavonoids in G. biloba cells with or without fungal endophytes elicitor. These finding suggest a causal relationship between ABA release and both PAL activity and flavonoid accumulation under fungal endophytes treatment and that ABA is involved in fungal endophytes-induced flavonoids accumulation in this plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Production of secondary metabolites of distinct and complex structures in plant cell cultures has been extensively explored. This is due to the high commercial value of these compounds, scarcity of those plants synthesizing these compounds and, at times, low content levels of such compounds in plants. However, use of plant cell cultures for production of useful secondary metabolites remains limited due to low yields of targeted compounds.
Synthesis of secondary metabolites is part of the defense response of plants to environmental stresses and pathogen attacks. Strategies for improving the yield of secondary metabolites in plant cell suspension cultures should be based on this mechanism (Zhao et al. 2005). Molecules of biological and nonbiological origin that stimulate secondary metabolism are called elicitors (Dörnenburg and Knorr 1995). Elicitation is one of the most effective methods established to enhance the production of secondary metabolites in cell cultures. A wide variety of elicitors, such as fungal elicitors, benzoic acid, methyl jasmonate, and arachidonic acid, can induce the biosynthesis of secondary metabolites (Yukimune et al. 1996).
Fungal endophytes are common and highly diverse microorganisms that live within plant tissues, but usually remain asymptomatic. Endophytes traditionally have been considered plant mutualists, mainly by reducing herbivory via production of mycotoxins, such as alkaloids (Faeth and Fagan 2002). Endophytes may live in plant as a symbiont for all or part of their life cycle. The ecological relationships between fungal endophytes and their respective plant hosts are very complex. A fungal endophyte may survive in a plant as a symbiont by providing protective substances (e.g. antifungal, antibacterial) that may inhibit or kill tissue invading pathogens (Strobel et al. 1997), while the plant also provides nutrients for the fungus. Thus, endophytes may play an important role in their host’s growth and the formation of secondary metabolites related to plant defense. Li and Tao (2009) reported that endophytic fungi (Fusarium mairei) culture broth stimulated the accumulation of taxoids in suspension cultures of Taxus cuspidate. However, the signal transduction mechanism of secondary metabolite accumulation induced by endophytic fungi elicitor in plants is still unclear.
Abscisic acid (ABA) is an essential signal for plant resistance to pathogens and biosynthesis of secondary metabolites. Adie et al. (2007) showed that ABA is an essential signal for plant resistance to pathogens affecting jasmonic acid (JA) biosynthesis and the activation of defenses in Arabidopsis. Nagira et al. (2006) have reported that that changes in the amount of endogenous ABA may play an important role in the induction of anthocyanin synthesis and chlorophyll degradation in regenerated torenia shoots. Clinton et al. (1998) reported a strong correlation between the paclitaxel and ABA recovered from yew, and suggested that the increase in ABA concentration might lead to the increase paclitaxel production. Luo et al. (2001) showed that ABA stimulated the synthesis in paclitaxel in plant cell cultures which depended on the dosage of ABA as well as the growth stage of the cells. These observations suggested the existence of a ABA-mediated signaling pathway in defence response for pathogens and biosynthesis of induced secondary metabolites in plant cells.
Fungal endophytes can thus induce secondary metabolite accumulation in plants. ABA can also accelerate secondary metabolite biosynthesis in plants and is also involved in plant resistance to pathogens as an essential signal. However, our knowledge about fungal endophytes-mediated signaling transduction is limited. We proposed that ABA may be as an essential signal to medite secondary metabolites biosynthesis induced by fungal endophytes.
Ginkgo biloba is a common medicinal plant in China and the secondary metabolites of ginkgo, flavonoids and ginkgolides, are excellent drugs used in the treatment of peripheral arterial diseases, tinnitus, neurological disorders, sclerosis of cerebral arteries, and cerebral ageing (Cao et al. 2002). As a therapeutic drug, it has no side effects even after long periods of use, and phytopharmaceuticals made from G. biloba are readily accessible throughout the world. Cell cultures of G. biloba could provide an alternative way to produce flavonoids owing to the scarcity of G. biloba in the world and extremely low levels of the compound in the plant. Our previous study have showed that NO acts as an important signal in UV-B-induced activation of PAL and stimulation of flavonoid biosynthesis in G. biloba callus (Hao et al. 2009a). We have derived a fungal endophyte from the inner bark of the G. biloba and identified it as Sphaeropsis sp B301. The results of the inhibitory activity of its metabolic products against pathogenic microbial showed that its metabolic products have strong inhibitory effects on Staphylococcus aureus, Enterococcus faecalis, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Saccharomyces cerevisiae (unpublished data). There have been no reports of application of endophyte–Ginkgo plant associations based on natural ecosystems in flavonoid biosynthesis production. Based on this, we aim to investigate the effects of endophytic fungi on flavonoid biosynthesis in Ginkgo suspension cells in this work, and also to find out whether ABA might be the signal molecule involved in mediating fungal endophytes-induced flavonoid biosynthesis.
Materials and methods
Plant materials and cell culture conditions
Ginkgo leaves (Ginkgo biloba L. cv. Da jing zhui) were collected from Tancheng town, Shandong, China. Leaves were surface-sterilized by a brief immersion in 70% (v/v) ethanol followed by 10 min in a commercial bleach solution (5% active chlorine) containing a few drops of Tween 80, and then rinsed three times with sterile distilled water. Leaf explants (size approx. 0.5 × 0.5 cm) were excised either from the lamina. These were placed in 9-cm Petri dishes containing 25 ml MS basal medium supplemented with, 1 mg NAA l−1, 0.05 mg KT l−1, 3% (w/v) sucrose and solidified with 2.5% (w/v) agar (Hao et al. 2009a). Cultures were kept in the light (50 μmol m−2 s−1) with a 16/8 h light/dark photoperiod at 22 ± 1°C. Callus formation was monitored over 1 month and subsequent growth (in terms of fresh weight) was followed until the stationary phase was reached. Once established, callus [1 g cell fresh weigh (FW)/20 ml medium] was transferred into MS liquid medium supplemented with 3% (w/v) sucrose and 1 mg NAA l−1, 0.05 mg KT l−1 to induce proliferation. The cell suspension cultures were shaken (100 rpm) in dark and on a incubator maintained at 25°C.
Preparation of fungal endophyte elicitor
Sphaeropsis sp B301 was screened from the inner bark of the G. biloba and maintained in our laboratory. The elicitor was prepared from the liquid culture of the isolate of Sphaeropsis sp B301. Liquid cultures were initiated from 7 days potato/dextrose/agar (PDA) by transferring 2 cm3 squares of medium to 150 ml PDA liquid medium. The flasks were incubated in the dark at 120 rpm and 25°C for 6 days. The mycelia were collected by filtration, and the elicitor was prepared using method described by Zhang et al. (2000). The elicitor dose was measured by the total carbohydrate content of the fungal homogenate, which was determined by the phenol/sulfuric acid method using glucose as the standard.
Measurement of G. biloba cell growth
Ginkgo biloba cell suspension culture material weighing 1 g FW was transferred into a 100 ml conical flask containing 20 ml culture medium. Cell growth was monitored at every 3rd day (up to 30 days) by determining the fresh weight. Briefly the cell cultures were harvested on every 3rd day to collect cells by filtration. The fresh weight of the cells was recorded with the help of a physical balance. Alternatively cell growth was measured, by sedimented cell volume (SCV) measurement. All data are expressed as an average of three separate experiments.
Treatments of G. biloba suspension cells with fungal endophyte elicitor and various chemical compounds
The following chemical compounds have been used in treating G. biloba suspension cells. ABA and Fluridone were purchased from Sigma-Aldrich. Fluridone was used as an inhibitor of abscisic acid biosynthesis. These reagents were dissolved in water or methanol were sterilized by filtration and added to the fresh autoclaved medium to give the 20–120 μM ABA, 0.1 mM Fluridone and 80 μM ABA + 0.1 mM Fluridone. The controls received solvent only. Fungal endophyte elicitor and various chemical compounds were added to 8-day old subcultured cells.
Measurement of ABA content
ABA contents was determined as described by Hao et al. (2009b). Briefly, 1 g ginkgo cells was suspended in 15 ml extraction solution containing 80% (v/v) methanol, 100 mg butylated hydroxytoluene/l, and 0.5 g citric acid monohydrate l−1. The suspension was stirred overnight at 4°C and centrifuged at 1000×g for 20 min. The supernatant was transferred to a new tube and dried under vacuum. The dry residue was dissolved with 100 μl methanol plus 900 μl Tris-buffered saline (50 mM Tris, 0.1 mM MgCl2·6H2O, and 0.15 M NaCl, pH 7.8). ABA concentration in the solution then was determined using the Phytodetek ABA immunoassay kit (Idetek, Inc., Sunnyvale, CA).
PAL activity assay
Collected cells (0.5 g FW) were homogenized with an extraction buffer (0.5 ml g−1) containing 50 mM Tris/HCl (pH 8.0), 10 mM 2-mercaptoethanol, 4 mM EDTA and 1 μM leupeptin. The homogenate was centrifuged at 10,000×g for 20 min, and the supernatant was collected for enzyme assay. PAL activity was determined based on cinnamic acid production according to method by Hao et al. (2009a). Briefly, 1 ml the extraction buffer, 0.5 ml 10 mM l-phenylalanine, 0.4 ml double distilled water and 0.1 ml enzyme extract were incubated at 37°C for 1 h. The reaction was terminated by the addition of 0.5 ml 6 M HCl, and the product was extracted with 15 ml ethyl acetate, followed by evaporation to remove the solvent. The solid residue was suspended in 3 ml 0.05 M NaOH and the cinnamic acid concentration wherein was quantified with the absorbance measured at 290 nm. PAL activity was calculated in nmol cinnamic acid mg−1 protein h−1.
Flavonoids extraction and determination
The collected cells were immediately dried for 72 h at 35°C with forced ventilation. Dried ginkgo leaves (0.5 g) were ground to a powder with a mortar and pestle. The powder was extracted with methanol containing 1% (v/v) HCl, followed by addition of 2 M HCl (equal volume) and 1 h incubation at 90°C. Acid-hydrolyzed extracts were dried and resuspended in methanol (200 μl). Samples were injected onto an HPLC system connected with a Nucleosil 100-5 C18 column (5 μm, 150 × 2 mm, Agilent Technologies). The separation condition of HPLC for flavonoids was as follows: the mobile phase was methanol and 0.4% phosphoric acid (45:55 v/v) at 0.7 ml min−1, the column temperature was 25°C, and the sample volume was 20 μl. The UV detector was set at 370 nm. The extraction samples were prepared for HPLC analysis with each sample injected three times. The quercetin, kaempferol and isorhamnetin were selected as standards because most of the flavonoids in Ginkgo leaves are derivatives of this three flavonol aglycones (van Beek 2002). Based on the methods of flavonoids concentration described by van Beek (2002), flavonoid contents were calculated by multiplication of total content of quercetin, kaempferol and isorhamnetin by a factor 2.51 and were expressed as percentage.
Statistical analysis
Each experiment was repeated at least three times. Values were expressed as means ± SE. Data are analyzed using SigmaPlot software (version 8.0, SYSTAT Software Inc., Richmond, California, USA). All mean comparisons were subjected to a one-way analysis of variance (ANOVA). In all cases the confidence coefficient was set at P < 0.05 level.
Results
Effects of amount of fungal endophyte elicitor on growth and flavonoid production of G. biloba cells
Prepared fungal endophyte elicitor was added to 100 ml 8-day suspension cultures of G. biloba cells. As shown in Fig. 1, 4 days treatments with 25 and 50 μg glc equiv/ml fungal elicitor had no obvious effects on G. biloba cells growth (P > 0.05), but the biomass of cultures treated with 75 and 100 μg glc equiv/ml fungal elicitor was 40 and 48% lower than that of control cultures, respectively. Cultures treated with 100 μg glc equiv/ml fungal elicitor were visibly browned after 2 days. Highest yield of flavonoid was in cultures treated with 75 μg glc equiv/ml fungal elicitor, being 1.8-fold higher than that from control cells (2% DW for fungal endophyte elicitor versus 1.1% DW for the control cells). From these results, the optimal amount of fungal endophyte elicitor should be 75 μg glc equiv/ml fungal elicitor.
Effect of different fungal endophyte elicitor concentration treatment for 4 day on cell growth and flavonoid content of G. biolba suspension cells. Twenty-five, 50, 75, 100 μg glc equiv fungal elicitor/ml (25, 50, 75, 100 elicitor) add to 100 ml 8-day suspension cultures of G. biloba cells. Data are means ± SE, N = 3
Fungal endophyte elicitor-induced an increase in ABA content, PAL activation, and flavonoid synthesis in G. biloba suspension cells
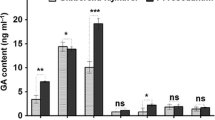
Accumulation of ABA is one of the common reactions of plant cells to pathogenic microorganism (Asselbergh et al. 2008). The results of present work showed that the ABA contents of the cells treated with the fungal elicitor is much higher than that in the control cells, showing that the fungal elicitor induces ABA biosynthesis of G. biloba suspension cells. The highest ABA level was observed about 6 h after elicitor treatment (Fig. 2), which is about 4.2 times that of the controlled cells (3.1 μg/g versus 0.7 μg/g fresh weight). As shown in Fig. 2, fungal endophyte elicitor also induced an increase of PAL activation and flavonoid synthesis of G. biloba suspension cells. Both PAL activity and flavonoid content increased mainly after ABA reached peak levels of 169 nmol mg−1 h−1 and 2% DW in about 15 and 24 h of treatment respectively (Fig. 2). These finding suggested that ABA production occurred earlier than fungal endophyte elicitor-induced activation of PAL and synthesis of flavonoids in G. biloba suspension cells.
Effects of exogenous ABA on PAL activity and flavonoid content of G. biloba suspension cells
As PAL activity and flavonoid content were highest 15 and 24 h, respectively, following fungal endophyte elicitor treatment, these two time points were selected for the next set of experiments. To determine whether exogenous ABA enhances PAL activity and flavonoid content, suspension cells were treated with different concentrations of ABA. Our results (Fig. 3) showed that both PAL activities and flavonoid content were enhanced by exogenous ABA under fungal endophyte elicitor treatment, and that ABA effects were dose-dependent. As shown in Fig. 3, the PAL activity and flavonoid content increased with the addition of ABA up to 80 μmol/l. Above 80 μmol/l, PAL activity and flavonoid content decreased. The PAL activity and flavonoid content of G. biloba cells treated with 80 μmol ABA/l were the highest at 194 nmol mg−1 h−1 and 2.5% DW, respectively. These data indicate that exogenous ABA assuredly enhanced both PAL activity and flavonoid content of G. biloba cells.
Effect of Fluridone on ABA release of G. biloba under fungal endophyte elicitor treatment
Fluridone, is an inhibitor of phytoene desaturase, which converts phytoene to phytofluene in the pathway of carotenoids biosynthesis (Bartels and Watson 1978; Fong and Schiff 1979). Carotenoids are the main precursors of ABA in plants (for review, Quatrano et al. 1997). Thus, the inhibition of carotenogenesis should also prevent the biosynthesis of ABA. Fluridone is also useful in such studies (Yoshiokal et al. 1998). As shown in Fig. 4, ABA production were remarkably decreased by Fluridone in combination with fungal endophyte elicitor treatment because Fluridone inhibited the phytoene desaturase, indicating that the inhibition of carotenogenesis in G. biloba cells also present the ABA biosynthesis. From Fig. 4, we can also see that exogenous ABA increased endogenous ABA production compared with the control cells with or without fungal endophyte elicitor treatment (Fig. 4, P < 0.05).
Effects of Fluridone on ABA generation under fungal endophyte elicitor treatment. Eight-day-old cells were treated with fungal endophyte elicitor (FE) or combined with 80 μM ABA (FE + ABA), 0.1 mM Fluridone (FE + Fluridone); 0.1 mM Fluridone (Fluridone), 80 μM ABA (ABA) alone without fungal endophyte elicitor treatment. These treated suspension cells were harvested to determine ABA generation after fungal endophyte elicitor treatment for 6 h. MS basal medium supplemented with 1 mg NAA l−1, 0.05 mg KT l−1 without fungal endophyte elicitor as Control. Data are means ± SE, N = 3. Within each set of experiments, bars with different letters are significantly different at P < 0.05
The enhancement of PAL activity and flavonoids biosynthesis in G. biolba suspension cells induced by fungal endophyte elicitor is reversed by Fluridone
As shown in Fig. 2, the PAL activity in the ginkgo cells increased rapidly by fungal endophyte elicitor, reaching a maximum in 15 h, which was about one fold of the control level (Figs. 2, 5a). Fungal endophyte elicitor stimulated flavonoid production, increasing flavonoid content in ginkgo cells by about 1.8-fold (2% DW for fungal endophyte elicitor versus 1.1% DW for the control cells) after 24 h of treatment (Figs. 2, 5b). To determine whether endogenous ABA is involved in fungal endophyte-induced PAL activity increase and flavonoid biosynthesis in G. biloba cells, cells were treated with Fluridone under fungal endophyte elicitor treatment. Results can be seen in Fig. 5 that exogenous ABA induced PAL activation and flavonoid biosynthesis in the absence of fungal endophyte elicitor, which indicates that ABA is sufficient for triggering PAL activation and flavonoid biosynthesis. ABA treatment also slightly but significantly enhanced fungal endophyte elicitor-induced PAL activity and flavonoid biosynthesis (FE + ABA versus FE) (Fig. 5a, b, P < 0.05). Figure 5 also showed that Fluridone did not significantly affect PAL activity and flavonoid biosynthesis of G. biloba leaves in the absence of fungal endophyte elicitor. However, fungal endophyte elicitor-induced PAL activity and flavonoid biosynthesis was significantly depressed by Fluridone (Fig. 5a, b, P < 0.05). These data strongly indicate the requirement of ABA action during fungal endophyte elicitor-enhanced PAL activation and flavonoid biosynthesis.
The enhancement of PAL activity and flavonoids biosynthesis of G. biolba suspension cells induced by fungal endophyte elicitor is reversed by Fluridone. Eight-day-old cells were treated with fungal endophyte elicitor (FE) or combined with 80 μM ABA (FE + ABA), 0.1 mM Fluridone (FE + Fluridone); 0.1 mM Fluridone (Fluridone), 80 μM ABA (ABA) alone without fungal endophyte elicitor treatment. These treated suspension cells were harvested to determine PAL activity and flavonoid content after fungal endophyte elicitor treatment for 15 and 24 h, respectively. MS basal medium supplemented with 1 mg NAA l−1, 0.05 mg KT l−1 without fungal endophyte elicitor as control. Data are means ± SE, N = 3. Within each set of experiments, bars with different letters are significantly different at P < 0.05
Discussion
Symbiotically, many endophytes protect hosts from the attack of natural enemies such as herbivores and pathogenic microorganisms (Wilson 1993). Some studies showed that endophyte fungal may attack natural enemies by providing protective secondary metabolite induced by invading pathogens (e.g. antifungal, antibacterial) (Strobel et al. 1997). The roles of fungal endophytes and their plant hosts during accumulation of secondary metabolites are even more complex. Our results show that the accumulation of flavonoids in ginkgo suspension cell can be stimulated by an fungal endophyte elicitor. However, the signal mechanism of secondary metabolites biosynthesis induced by fungal endophytes in plants has not been clarified yet. Accumulating evidence suggests that ABA is an important signal molecule involved in the plant response to pathogens, and ABA also involved in plant secondary metabolite synthesis. Here, we provide the evidence that ABA generation is required, at least partly, for fungal endophyte-induced flavonoid biosynthesis in cell suspension cultures of G. biloba.
ABA is an important plant growth regulator involved in various physiological processes in plants (Zeevaart and Creelman 1988). In developing seeds, ABA is necessary for inducing the synthesis of storage proteins and lipids, as well as for the onset of seed dormancy and the acquisition of desiccation tolerance (Finkelstein et al. 2002). ABA also plays important roles in vegetative development in response to various environmental osmotic stresses such as drought and high-salinity conditions (Jensen et al. 1996; Machuka et al. 1999; Xiong et al. 2002). The involvement of ABA on secondary metabolism accumulation is an intriguing field of research, but the literature on this subject is still controversial, although some discrepancies might be easily explained taking into account the different plant species and tissues analysed. With respect to anthocyanin synthesis, ABA application promoted anthocyanin synthesis in leaf discs of Vitis vinifera (Pirie and Mullins 1976) and excised axes of Phaseolus vulgaris (Walton and Sondheimer 1968). The highest paclitaxel production was obtained after exogenous ABA application to Taxus chinensis suspension cultured cells (Luo et al. 2001). Reversely, Sun et al. (2007) reported that exogenous supplements of ABA to M9 medium significantly decreased the formation of shikonin in the cultured cells during the entire course of culturing.
The present study revealed that fungal endophytes-induced an increase in ABA production, PAL activation, and flavonoid synthesis in suspecion cell of G. biloba (Fig. 2). This ABA generation was detected earlier than the increases in PAL activity and in flavonoid content (Fig. 2), thus suggesting that ABA production was localised upstream of fungal endophytes-induced activation of PAL and synthesis of flavonoids in G. biloba cells. From all results in this study, it is clear that the suspension cells of G. biloba under conditions in which flavonoid synthesis was induced exhibited high endogenous ABA levels on the medium containing endophyte fungal elicitor (Figs. 2, 4). Suspension cultured cells under conditions in which flavonoid synthesis was not induced showed low endogenous ABA levels (Figs. 2, 4). In addition, both fungal endophytes-induced ABA release and enhancement of PAL activity and flavonoid biosynthesis were substantially suppressed in the presence of the ABA biosynthesis inhibitor Fluridone (Figs. 4, 5). This suggests that endogenous ABA plays an important role in the induction of flavonoid synthesis in this system. Similar observations have been reported in maize and arabidopsis. The anthocyanin pathway in maize was blocked in the viviparous-1 (vp1) mutant, an abscisic acid insensitive mutant (McCarty et al. 1989; Paek et al. 1997). In recent studies, Arabidopsis seedlings of an ABA-biosynthetic mutant and ABA-insensitive mutants (aba2, abi3, abi4, and abi5) accumulated a lower amount of anthocyanin than that in the wild type (Huijser et al. 2000; Finkelstein et al. 2002).
Exogenous supplements of ABA could also induce flavonoid synthesis on medium without fungal endophytes. Moreover, direct treatment with ABA with fungal endophytes also induced the activation of PAL and synthesis of flavonoids, indicating that ABA was sufficient for triggering PAL activation and flavonoid synthesis in G. biloba cells. Our preliminary experiment revealed that the flavonoid content in the cell suspension cells culture with 80 μM ABA with fungal endophytes in the light for 24 days was 2.5% DW, which was about two times higher than that of cells cultured with 80 μM ABA, but without fungal endophytes elicitor (1.5% DW).
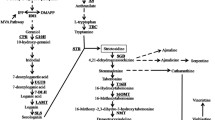
Some experiments provided evidence that ABA generated in plant is involved in defence response to pathogens. The effects of ABA on flavonoid synthesis of G. biloba cells induced by fungal endophyte elicitor may be associated with its functions in activating the defense responses of plant cells. The activation of phenylpropanoid pathway as a response to a wide diversity of stress-factors has led to its use as a genetic marker for the induction of plant defense responses (Pellinen et al. 2002). Phenylalanine ammonia-lyase (PAL) catalyzes the first step in phenylpropanoid biosynthetic pathway (Jones 1984). In this study, it is apparent that ABA is an essential signal mediating defense responses to fungal endophytes, and that fungal endophyte elicitor enhances PAL activity and biosynthesis of flavonoids (Fig. 2). Moreover, fungal endophytes-induced PAL activity and flavonoid production are enhanced by the exogenous ABA, but they are inhibited by the ABA biosynthesis inhibitor Fluridone (Fig. 5).This suggests that the activation of PAL and flavonoid biosynthesis may be regulated by the endogenous ABA. Thus, ABA is a signal molecule within the signaling cascade leading to fungal endophytes-induced PAL activation and flavonoid synthesis, and that ABA is a prerequisite for fungal endophytes-induced flavonoid synthesis in G. biloba cells.
Plants respond to pathogens infection by activating a large variety of defense responses. Multiple elements, including cell surface receptor (Hulbert et al. 2001), Ca2+ influx (Errakhi et al. 2008), ROS (Scheel. 1998), JA (Duval et al. 2005), SA (Duval et al. 2005), and MAPK (Errakhi et al 2008) have frequently been discussed as putative components involved in pathogens stress signal transduction chain(s). ABA was reported to play an ambivalent role in pathogen defense and several putative mechanisms were proposed (Mauch-Mani and Mauch 2005).
Mounting evidence suggests that ABA plays an ambivalent role in defense responses to pathogens, acting as both a positive and negative regulator of disease resistance by interfering at multiple levels with biotic stress signaling. In this context, a wide range of putative mechanisms underpinning the beneficial and detrimental effects of ABA on plant defense have been proposed, including the suppression of SA- and ET/JA-dependent basal defenses, synergistic cross-talk with JA signaling, suppression of reactive oxygen species (ROS) generation, induction of stomatal closure, and stimulation of callose deposition (Asselbergh et al. 2008). For instance, in Arabidopsis, ABA-regulated stomatal closure is a key element of preinvasion SA-regulated innate immunity to Pseudomonas syringae (Melotto et al. 2006), whereas post-penetration virulence of the same pathogen depends on ABA-mediated suppression of several basal defense responses (de Torres-Zabala et al. 2007; Mohr and Cahill 2003, 2007). Adie et al. (2008) have reported that ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. A biologically very relevant direct positive effect of ABA signaling on pathogen defense is by closing stomata to prevent pathogen invasion. The fungal necrotrophic pathogen Sclerotinia sclerotiorum uses the virulence factor oxalate to prevent ABA-induced stomatal closure during infection (Guimarães and Stotz 2004). Another positive effect of ABA on pathogen defense is its ability to stimulate callous deposition. Both ABA signaling and callous formation are prerequisites for β-amino butyric acid (BABA)-triggered induced resistance to Plectosphaerella cucumerina and Alternaria brassicicola in Arabidopsis (Ton and Mauch-Mani 2004). Treatment with exogenous ABA could mimic the effect of BABA and resulted in priming for callose and resistance to P. cucumerina.
All these findings suggested that ABA acts as a signal molecule to active defense responses in plants, but ABA is not the sole decisive factor. The present study shows that the application of ABA enhanced PAL activity and flavonoid content in G. biloba cells (Fig. 5), but increases were much lower than those induced by fungal endophyte elicitor, which suggests that ABA is an important element for fungal endophyte elicitor-induced PAL activation and flavonoid synthesis in G. biloba cells, but it is not the sole signal component (pathway). Moreover, the effects of Fluridone on fungal endophytes-induced the enhancement of PAL activity and flavonoid biosynthesis in ginkgo cells are only partial, suggesting that there are ABA independent pathways in fungal endophytes signal networks. Other signal elements or pathways may also be involved in the fungal endophytes-induced PAL activation and flavonoid synthesis, and complex relationship between ABA and other signal molecules may exist in G. biloba cells. This means cross-talk between signaling molecules may be already existent in the ancient seed plant.
Taken together, our findings indicate that ABA is an essential signaling molecule for triggering the fungal endophytes-induced PAL activation and flavonoid synthesis in G. biloba cells. These findings will no doubt help us in gaining further insights into the fungal endophytes signaling pathway of secondary metabolite biosynthesis in plants. However, little is known about the complex molecular network operating during flavonoid synthesis triggered by fungal endophytes elicitor. The relationship between ABA and other signaling molecules requires further investigation.
References
Adie BA, Pérez-Pérez J, Pérez-Pérez MM et al (2007) ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 19:1665–1681
Asselbergh B, De Vleesschauwer D, Höfte M (2008) Global switches and fine-tuning-ABA modulates plant pathogen defense. Mol Plant Microbe Interact 21:709–719
Bartels PG, Watson CW (1978) Inhibition of carotenoid synthesis by fluridone and norflurazon. Weed Sci 26:198–203
Cao YH, Chu QC, Fang YZ et al (2002) Analysis of flavonoids in Ginkgo biloba L. and its phytopharmaceuticals by capillary electrophoresis with electrochemical detection. Anal Bioanal Chem 374:294–299
Clinton CS, Erik BGF, Angela H et al (1998) ‘Hicksii’ yews as a sustainable source of anticancer compounds. http://www.cropinfo.net/AnnualReports/1997/yew.alt.html
de Torres-Zabala M, Truman W, Bennett MH et al (2007) Pseudomonassyringae pv. tomato hijacks the Arabidopsis abscisic acid signaling pathway to cause disease. EMBO J 26:1434–1443
Dörnenburg H, Knorr D (1995) Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzyme Microbiol Technol 17:674–684
Duval I, Brochu V, Simard M et al (2005) Thaxtomin A induces programmed cell death in Arabidopsis thaliana suspension-cultured cells. Planta 222:820–831
Errakhi R, Dauphin A, Meimoun P et al (2008) An early Ca2+ influx is a prerequisite to thaxtomin A-induced cell death in Arabidopsis thaliana cells. J Exp Bot 59:4259–4270
Faeth SH, Fagan WF (2002) Fungal endophytes: common host plant symbionts but uncommon mutualists. Integr Comp Biol 42:360–368
Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14(Suppl):15–45
Fong F, Schiff JA (1979) Blue-light-induced absorbance changes associated with carotenoids in Euglena. Planta 146:119–127
Guimarães RL, Stotz HU (2004) Oxalate production by Sclerotinia sclerotiorum deregulates guard cells during infection. Plant Physiol 136:3703–3711
Hao GP, Zhang XH, Wang YQ et al (2009a) Nucleotide variation in NCED3 Region of Arabidopsis thaliana and its association study with abscisic acid content under drought stress. J Integr Plant Biol 51:175–183
Hao GP, Du XH, Zhao FX et al (2009b) Role of nitric oxide in UV-B-induced activation of PAL and stimulation of flavonoid biosynthesis in Ginkgo biloba callus. Plant Cell Tissue Organ Cult 97:175–185
Huijser C, Kortstee A, Pego J et al (2000) The Arabidopsis sucrose uncoupled-6 gene is identical to abscisic acid insensitive-4: involvement of abscisic acid in sugar responses. Plant J 23:577–585
Hulbert SH, Webb CA, Smith SM et al (2001) Resistance gene complexes: evolution and utilization. Annu Rev Phytopathol 39:285–312
Jensen AB, Busk PK, Figueras M et al (1996) Drought signal transduction in plants. Plant Growth Regul 20:105–110
Jones DH (1984) Phenylalanine ammonia-lyase: regulation of its induction, and its role in plant development. Phytochemistry 23:1349–1359
Li YC, Tao WY (2009) Paclitaxel-producing fungal endophyte stimulates the accumulation of taxoids in suspension cultures of Taxus cuspidate. Sci Hortic 121:97–102
Luo J, Liu L, Wu CD (2001) Enhancement of paclitaxel production by abscisic acid in cell suspension cultures of Taxus chinensis. Biotechnol Lett 23:1345–1348
Machuka J, Bashiardes S, Ruben E et al (1999) Sequence analysis of expressed sequence tags from an ABA-treated cDNA library identifies stress response genes in the moss Physcomitrella patens. Plant Cell Physiol 40:378–387
Mauch-Mani B, Mauch F (2005) The role of abscisic acid in plant–pathogen interactions. Curr Opin Plant Biol 8:409–414
McCarty DR, Carson CB, Stinard PS et al (1989) Molecular analysis of viviparous-1: an abscisic acid-insensitive mutant of maize. Plant Cell 1:523–532
Melotto M, Underwood W, Koczan J et al (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126:969–980
Mohr PG, Cahill DM (2003) Abscisic acid influences the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica. Funct Plant Biol 30:461–469
Nagira Y, Ikegami K, Koshiba T et al (2006) Effect of ABA upon anthocyanin synthesis in regenerated torenia shoots. J Plant Res 119(2):137–144
Paek NC, Lee B-M, BVai DG et al (1997) Regulatory roles pf abscisic acid for anthocyanin synthesis in maize kernels. Maydica 42:385–391
Pellinen RI, Korhonen MS, Tauriainen AA, Palva ET, Kangasjärvi J (2002) Hydrogen peroxide activates cell death and defense gene expression in birch. Plant Physiol 130:549–560
Pirie A, Mullins M (1976) Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate, and abscisic acid. Plant Physiol 58:468–472
Quatrano RS, Bartels D, Ho TD et al (1997) New insight into ABA-mediated processes. Plant Cell 9:470–475
Scheel D (1998) Resistance response physiology and signal transduction. Curr Opin Plant Biol 1:305–310
Strobel GA, Torczynski R, Bollon A (1997) Acremonium sp.—a leucinostatin A producing endophyte of European yew (Taxus baccata). Plant Sci 128:97–108
Sun DY, Yin ZJ, Wu SJ et al (2007) Effects of abscisic acid on the secondary metabolism of cultured Onosma paniculatum cells. Russ J Plant Physiol 54:530–535
Ton J, Mauch-Mani B (2004) Beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J 38:119–130
Van Beek TA (2002) Chemical analysis of Ginkgo biloba leaves and extracts. J Chromatogr A 967:21–55
Walton DC, Sondheimer E (1968) Effects of abscisin II on phenylalanine ammonia-lyase activity in excised bean axes. Plant Physiol 43:467–469
Wilson D (1993) Fungal endophytes: out of sight but should not be out of mind. Oikos 68:379–384
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14(Suppl):165–183
Yoshiokal T, Endo T, Satoh S (1998) Restoration of seed germination at supraoptimal temperatures by fluridone, an inhibitor of abscisic acid biosynthesis. Plant Cell Physiol 39:307–312
Yukimune Y, Tabata H, Higashi Y et al (1996) Methyl jasmonate-induced over-production of paclitaxel and baccatin III in taxus cell suspension cultures. Nat Biotechnol 14:1129–1132
Zeevaart J, Creelman R (1988) Metabolism and physiology of abscisic acid. Ann Rev Plant Physiol Plant Mol Biol 39:439–473
Zhang CH, Mei XG, Liu L (2000) Enhanced paclitaxel production induced by the combination of elicitors in cell suspension cultures of Taxus chinensis. Biotechnol Lett 22:1561–1564
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Acknowledgments
This work is supported by the research fund for the doctoral degree scholars of Taishan Medical University (No. 2005-04).
Author information
Authors and Affiliations
Corresponding author
Additional information
Gangping Hao and Xihua Du contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Hao, G., Du, X., Zhao, F. et al. Fungal endophytes-induced abscisic acid is required for flavonoid accumulation in suspension cells of Ginkgo biloba . Biotechnol Lett 32, 305–314 (2010). https://doi.org/10.1007/s10529-009-0139-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-009-0139-6