Abstract
As the essential component(s), long-chain perfluorinated or short-chain perfluorinated ionic surfactants are required for effective aqueous film-forming foam (AFFF); nevertheless, the associated qualities of persistent pollution and toxicity have raised significant concerns. It has become critical to develop alternatives to the present fluorine component for AFFF to offset the negative effects. In this study, a short-chain perfluorinated nitrogen-heterocyclic nonionic amine oxide surfactant was combined with hydrocarbon surfactants and additives to prepare an AFFF concentrate. A laboratory technique was developed to evaluate the influence of ingredients on the performance of a 6% AFFF diluent, resulting in an improved AFFF formulation. The performance parameters for pool fire extinguishment and fire resistance of the AFFF formulation were encouraging, including a spreading coefficient of 5.4, foam expansion of 8.11, 25% drainage time of 4.6 min, extinguishing times for forceful application of 58 s, and fire burnback time of 18.6 min. In addition, the AFFF concentrate showed significant freezing resistance when stored at − 20 °C for an extended period of time. The formulation outperformed the technical standard criteria and has the potential to be used as a novel AFFF agent.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fires often pose a significant threat to the safety of chemical and process industries. The most common type of fire incident in these settings is a Class B fire, also referred to as a hydrocarbon liquid pool fire, caused by ignition of flammable liquids. The flames resulting from such flammable liquids are known to be extremely explosive, high temperature, and radiating, spread over a large area, prone to reignition and splashing, making them notoriously challenging to extinguish (Kang et al. 2019). As these fires rage, unburned hydrocarbons and dangerous gases can escape, leading to air pollution and a range of other unfavorable effects that can put lives and property at risk (Rengel et al. 2018). Hence, there is an urgent need for an effective fire extinguisher that is cost-efficient, practical, and capable of quickly extinguishing hydrocarbon liquid pool fires, preventing injury and reignition. To this end, firefighting foams have been widely explored and found to be practical and effective for mitigating hazardous liquid fires because of its remarkable cooling and covering isolation capacities, providing resistance against heat and mass transmission (Ananth et al. 2019; Yu et al. 2021).
Aqueous film-forming foam (AFFF) is a type of firefighting foam that has been proven to be the most effective in extinguishing hydrocarbon fuel fires in various settings, including military, aviation, municipal, and industrial applications. This is due to its ability to generate both a thick aqueous film and a foam layer, making it a dual-action solution (Zaggia et al. 2010; Han et al. 2011). In terms of preventing the burnback of fuel and solvents, AFFF relies heavily on fluorinated surfactants, which serve as the major fire-quenching element and vapor suppressants (Lattimer et al. 2003; Laundess et al. 2011; Xu et al. 2020). Long-chain fluorinated surfactants, such as perfluorooctanoic acid and perfluorooctane sulfonate (PFOA/PFOS), were previously used widely in AFFF due to their exceptional performance and effective fire extinguishing properties (Moody et al. 2000). However, perfluorooctyl, a derivative of these surfactants, was found to be toxic, bioaccumulative, and persistent, causing significant harm to the environment and human health (Gao et al. 2019; Ghisi et al. 2019). As a result, under the Stockholm Convention, PFOA/PFOS were declared as persistent organic pollutants, and their usage was restricted. Two alternatives to PFOA/PFOS-based AFFF are available, including fluorotelomer-based firefighting foams and fluorine-free firefighting foams (Sheng et al. 2018a, b). Nonetheless, fluorine-free solutions have poor film-forming properties due to their high surface tension, while fluorotelomer-based surfactants can be contaminated by long-chain perfluorinated compounds during production (Hetzer et al. 2014; Sontake et al. 2014).
Research suggests that surfactants with short fluorocarbon chains or branched perfluoroalkyl chains offer similar surface activity to long fluorocarbon chains but have a lower environmental impact. These surfactants have not yet been classified under international law, but may be suitable substitutes for long-chain fluorosurfactants (Peshoria et al. 2020). Zhu et al. synthesized a class of short-chain fluorinated cationic surfactants and prepared three types of AFFF solutions (AFFFs-eth, AFFFs-pro, and AFFFs-but), with AFFFs-but being the most effective, consistent with China National Standard 15,308-2006, ICAO, and NFPA requirements (Zhu et al. 2022). Additionally, a perfluoro branched short-chain fluorocarbon cationic surfactant with high surface activity was produced and utilized to form three AFFF formulations (F-1, F-2, and F-3), with the F-3 formulation outperforming typical AFFF formulations (Yang et al. 2022). Furthermore, mixtures of cationic-anionic fluorinated surfactants with short fluoroalkyl chains were tested as an alternative to AFFF bioaccumulative products based on PFOA/PFOS. He et al. reported that an equimolar mixture of C4F9SO2NH(CH2)3N(CH3)3I and C3F7COONa in an aqueous solution had a high film spreading and sealability over fuels, suggesting its potential use in AFFF (He et al. 2019). Nevertheless, it is important to note that alternative surfactants with short fluorocarbon chains or branched perfluoroalkyl chains are ionic; research has shown that anionic and cationic surfactants are more harmful than nonionic varieties, with cationic types being the most toxic (Grant et al. 1992; Cserháti et al. 2002; Zhou et al. 2020).
Nonionic surfactants are known to be very safe in terms of toxicity. Previous research has explored the potential use of a short-chain perfluorinated nitrogen-heterocyclic nonionic amine oxide surfactant (F4MO) as an evaporation suppressor and in firefighting foams (Wu et al. 2021). This investigation utilized F4MO as the primary component to develop an AFFF concentrate, incorporating hydrocarbon surfactants and additives. A laboratory technique was developed to evaluate the various components’ impact on the performance of a 6% AFFF dilution and optimize the formulation of this product. Furthermore, the extinguishing potential of the 6% AFFF diluent, as well as its fire resistance capabilities, was assessed by Hubei Hongxin Fire Technology Development Co., Ltd. (Wuhan, China). Additionally, the concentrate’s ability to resist freezing was also evaluated using cryogenic methods.
Experimental
Materials
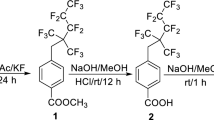
The fluorinated surfactant F4MO was self-synthesized (Fig. 1 shows the synthetic route), and the technique, structural characterization, and surface tension were previously published in our study (Wu et al. 2021). Sodium dodecyl sulfate (SDS, CP grade), 1,2-propanediol (CP grade), diethylene glycol monobutyl ether (DGME, CP grade), urea (AR, grade), xanthan gum (XG, USP grade), cyclohexane (CP grade) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Cocamidopropyl betaine (CAB, 98% purity) was purchased from Shandong West Asia Chemical Industry Co., Ltd. (Jinan, China). Alkyl polyglucoside (APG, 50% purity) was purchased from Shanghai Fakai Chemical Co., Ltd. (Shanghai, China). Imidazoline (90% purity) was purchased from Shanxi Rixin Petrochemical Co., Ltd. (Xian, China). All the reagents were used without further purification. Deionized water was used in laboratory tests, and tap water was used in pool fire suppression.
Preparation of AFFF concentrate
AFFF is a complex combination of fluorinated, hydrocarbons surfactants, solvents, and additives, which collectively provide necessary mechanical and chemical properties (Boone et al. 2019). Therefore, the first step is to select the appropriate components. Herein, the primary fluorinated surfactant utilized was F4MO, with hydrocarbon surfactants such as SDS, CAB, APG, and imidazoline following closely. Other essential ingredients include 1,2-propanediol as an antifreeze, XG acting as a foam stabilizer and thickener, DGME as an organic solvent, and water as the remaining component. To prepare AFFF concentrates, a solution comprising appropriate quantities of F4MO, SDS, CAB, APG, imidazoline, DGME, and 1, 2-propanediol was dissolved in deionized water. This was followed by the addition of a pre-dissolved mixture of xanthan gum and urea in the required amount to reach a total mass of 20 g. Finally, the solution was stirred until it was clear and transparent.
Preparation of 6% AFFF diluent
AFFF concentrates are typically utilized for storage and transportation purposes. When it comes to firefighting, they are usually diluted with water to either 6% or 3%. In the present study, AFFF concentrate was diluted to 6% concentration to ensure a strong fire suppression and resistance effect. To prepare a 6% AFFF diluent, 6 g of AFFF concentrate is mixed well with 94 g of deionized water.
Characterization techniques
Surface/interface tension was measured using the pendant drop method on the Contact Angle System (OCA 20) at room temperature (25 ± 0.5 °C) and recorded three times to verify repeatability for the AFFF solution.
Optimization of AFFF formulation by laboratory strategy
Table 1 displays the original formulation for AFFF, as provided by the cooperative. Each ingredient was given as a range value, and optimization was necessary to attain exact dosage of each component. In order to screen out the exact dosage visually and quickly, a visual fire extinguishing technique was utilized to analyze and optimize formulations. The following are the specific steps involved in this experimental procedure.
Firstly, 6 g of AFFF concentrate was prepared at a predetermined dosage and then mixed with 94 g of deionized water in a 600 mL plastic container to create a 6% AFFF dilution. The container was then securely capped and shaken vigorously for five seconds to observe the growth of foam. The expansion ratio was determined by checking whether the foam completely filled the plastic bottle without generating a distinct water sound when shaken. If the expansion ratio exceeded 5 times, the procedure continued to the next step. The drainage status of the solution was checked by placing the plastic bottle on a table and measuring the time taken for 25 g of liquid to emerge at the bottom. If the 25% drainage time was more than 30 s, the spreading experiment could be conducted. A disposable 1 mL plastic dropper was used to drop 0.05 mL of the drained liquid onto the center of the cyclohexane surface in a 5 cm-diameter petri dish at a height of approximately 5 mm. If the drained liquid spread quickly and produced a water film on the cyclohexane surface, the liquid was considered to have good sealability and was then tested for fire resistance and extinguishment. To test the extinguishing properties of the solution, 20 mL of cyclohexane was added to a 250 mL beaker and ignited. Once the cyclohexane combustion had stabilized for 60 s, 1 mL of fresh 6% AFFF was applied and its extinguishing time was recorded. After the fire was extinguished, an additional 3 mL of fresh 6% AFFF was added followed by a slight shake to break the water layer. Finally, the cyclohexane was immediately ignited to test its flammability. This process was repeated until the fire could no longer be extinguished automatically, and the number of replications was counted. The AFFF formulation with the shortest fire extinguishing time and the greatest number of duplicates was selected as the optimal formulation and used for pool fire extinguishing. The bench-scale anti-reburn experiment and the fire extinguishment technique were videotaped and submitted as attachments.
Pool fire extinguishment
A total of 10 L of AFFF concentrate was produced and tested by Hubei Hongxin Fire Technology Development Co., Ltd. (Wuhan, China), for use in extinguishing pool fires. To ensure accuracy, parameters such as foam expansion, spreading coefficient, and 25% drainage time were measured prior to starting the pool fire.
Calculation of spreading coefficient
Equation (1) is used to calculate the spreading coefficient (S), where \({\gamma }_{o}\) and \({\gamma }_{w}\) is the surface tension of cyclohexane and 6% AFFF, and \({\gamma }_{o/w}\) is the interfacial tension between cyclohexane and 6% AFFF.
Determination of foam expansion and 25% drainage time
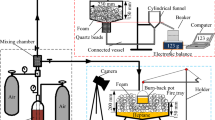
Figure 2a displays the results of measuring foam expansion and 25% drainage time using a specialized device designed to gauge low expansion foam drainage rates. The compressed foam fire extinguishing technique yielded substantial amounts of foam, as depicted in Fig. 2b. In order to create foam, a solution consisting of 3 L of AFFF concentrate and 47 L of tap water was added to the pressure tank. Next, the intake pressure of the foam gun was adjusted to 0.7 ± 0.03 MPa, which resulted in a flow rate of 0.75 ± 0.025 L/min. The foam receiving tank was dampened inside and then wiped down prior to being weighed (\({m}_{1}\)). To begin the measuring process, foam was sprayed for 30 s and then collected in the foam receiving tank, with the timing commencing simultaneously. Any excess foam present on the device was removed and weighed (\({m}_{2}\)). The weight of the 25% drained liquid (\({m}_{3}\)) was calculated employing Eq. (2).
Measurement of extinguishing time and burnback time
Fire suppression and fire resistance tests were conducted in a windless combustion chamber. To begin the experiment, 40 L of 120# gasoline (a gasoline with an octane rating of 0.015, a lead concentration of 0.002 g/L, and a moisture plus impurities content of 0.0012%) was added to a 1 m-diameter oil pan and pre-burned for one minute. Next, foam was directly sprayed onto the burning fuel surface, and the timer was started to measure the extinguishing time, which is the interval between the start of foam spraying and complete fire extinguishment. Following the extinguishment, foam application was continued for an additional three minutes to create a foam layer that would be challenged for reignition. In continuation, a 120 mm-diameter burning tank containing 1 L of 120# gasoline was placed in the center of the oil pan and ignited. The burnback time, which is the duration from the ignition of gasoline in the burning tank to complete re-burning of gasoline in the oil pan, was recorded. Throughout this pool fire extinguishment and anti-reburn experiment, the video footage was captured and uploaded as attachments.
Freezing resistance tests
For the purpose of evaluating potential stratification and heterogeneity in a 10 mL AFFF concentrate, the sample was subjected to a chilling process at − 20 °C for 24 h followed by storage at room temperature for 24 h. This procedure was repeated three times.
Results and discussion
The role of individual ingredients in the AFFF formulation
Fluorinated surfactants decrease the surface tension between air and water, while hydrocarbon surfactants regulate the interfacial tension between water and fuel. This allows the foam solution to spread uniformly over the hydrocarbon fuel surface (Zaggia et al. 2010; Kovalchuk et al. 2014). For long-term preservation of foam concentration, certain organic solvents are required to maintain it suitable for use (Peshoria et al. 2020). Additionally, antifreeze, foam stabilizer, and thickening chemicals are used to enhance foam function.
In this study, F4MO was chosen as the primary fluorinated surfactant due to its high surface activity (\({\gamma }_{CMC}\)=19.56 mN/m) and low CMC of 5.4 × 10–4 mol/L (Wu et al. 2021), which are essential qualities of a surfactant in specific solutions like AFFF (Czajka et al. 2015). Furthermore, F4MO was subjected to reflux in solutions with pH values of 3, 7, and 12 for 24 h. Thin layer chromatography monitoring revealed no decomposition products, and F4MO aqueous solution under varying pH conditions was desolvated and subsequently dried to obtain white solid samples. HRMS analysis confirmed that the molecular weight of the resulting solid samples matched that of F4MO, suggesting that F4MO possesses high water stability despite the presence of strong electron-withdrawing sulfonate groups. Hydrocarbon surfactants, such as SDS, CAB, APG, and imidazoline, not only reduce interfacial tension but also improve foamability, foam density packing, and antibacterial properties. The antifreeze used in this study was 1,2-propanediol, whereas xanthan gum (XG) was employed as a foam stabilizer and thickening agent (Sheng et al. 2016, 2018a, b). Although XG significantly improves the stability of the foam layer and water film, it absorbs water easily and can form a gel-like mass that impedes the passage of water molecules into the inner layer resulting in a reduced solubility. Thus, urea was incorporated as a dispersant to facilitate XG’s dispersion in an aqueous solution. To prevent agglomeration due to ionic interactions between surfactants and different ionic types, DGME was employed as an organic solvent. The formulation was refined after repeated testing, ultimately resulting in an optimized formulation, which is presented in Table 1.
Spreading coefficient
For an AFFF to effectively spread over the surface of a hydrocarbon liquid, it is essential to have a positive spreading coefficient (Hinnant et al. 2020). Notably, a larger spreading coefficient results in a faster formation of the AFFF solution film (Pabon et al. 2002). However, several kinetic variables, such as inertia, gravity force, and viscous drag force, may hinder the AFFF solution from properly diffusing over the hydrocarbon liquid surface (Fay 1971). To establish the 6% AFFF diluent spreading capacity, the observation approach was utilized during formulation screening studies (He et al. 2019). An AFFF solution with a 6% concentration, formulated using a unique approach, was determined to possess the lowest interfacial tension of 2.26 mN/m, as well as the lowest surface tension of 17.90 mN/m, resulting in the highest spreading coefficient of 5.40 (Table 2). Interestingly, the calculated spreading coefficient agreed with observed data, indicating that the 6% AFFF diluent had a higher capacity for spreading over hydrocarbon surfaces.
Foam expansion ratio and 25% drainage time
When describing foam quality, two important metrics to consider are foam expansion and 25% drainage time. The foam layer, coupled with the water film, has a dual function of screening heat radiation and sealing the surface of the hydrocarbon liquid to reduce evaporation while blocking oxygen. Additionally, the water that drains from the foam layer can replace water loss from the water film to maintain its completeness and stability (He et al. 2019). In regard to low expansion AFFF, AFFF formulations that are highly expanded can have poor flowability and slow drainage rates, which can cause delays in supplementing water loss and degrade AFFF performance (Scheffey 2016). As a result, AFFF that has an appropriate foam expansion and 25% drainage time is beneficial when extinguishing a pool fire.
Numerous organizations have put forward standardized specifications for assessing foam performance. According to China National Standard (GB15308-2006), the foam expansion rate ranged from 5 to 20, with a minimum 25% drainage time of 2.5 min. Alternatively, US Military Specification (MIL-F-24385F) requires foam expansion and 25% drainage times to exceed 6 and 2.5 min, respectively (Specification 1992). The National Fire Protection Association (NFPA 412) states a minimum foam expansion rate of 5 and a 25% drainage time of 2.25 min (American National Standards Institute 1993). Analysis of the data in Table 3 reveals a foam expansion rate of 8.11 and a 25% drainage time of 4.6 min. These results align with the previously mentioned international standards, indicating exceptional foam performance for the 6% AFFF diluent.
Extinguishing time and burnback time
Extinguishing time and burnback time are two crucial variables that directly reflect the extinguishing and anti-reburn performance of 6% AFFF diluent. The fire extinguishing performance is better when the extinguishing time is shorter and the burnback time is longer.
Firefighting in a pool involves two distinct methods: gentle application and forceful application. Gentle application involves adding foam indirectly to the surface of hydrocarbon liquids through a baffle or tank wall, while forceful application involves the direct addition of foam to the hydrocarbon liquid surface (Zhang and Liao 2008). The distinction between the two approaches is outlined as follows (Li et al. 2012): (1) When utilizing the forceful application technique, a substantial volume of foam is ejected into the oil pan; (2) the temperature field surrounding the hot fuel fluctuates considerably, causing the foam to be unable to access the fuel surface due to the impact of the fire plume velocity. These two factors contribute to a loss of foam, resulting in a longer extinguishing time for a forceful application than for a gentler approach.
To achieve cost savings, the forceful application method was utilized for extinguishing a pool fire. As depicted in Fig. 3, after thoroughly pre-combustion the fuel for 1 min (Fig. 3a), a quantity of foam was sprayed directly onto the burning fuel surface at 52″ (Fig. 3b). Over time, the fire gradually weakened (Fig. 3c) and finally totally extinguished at 1′50″ following 58 s of continuous foam injection (Fig. 3d), as demonstrated by exceptional extinguishing effectiveness.
Figure 4 illustrates the pool fire resistance test that was conducted. The assessment involved the continuation of foam spraying for a duration of three minutes after the fire had been successfully extinguished (Fig. 4a). Next, the procedure involved the placement of a flammable tank, carrying 1 L of 120# gasoline, at the center of the oil pan, followed by its ignition to examine the reignition scenario, as illustrated in Fig. 4b. Interestingly, the area did not experience any reignition, except for a minor reduction in foam volume around the flaring tank over time. Subsequently, the foam volume continued to decrease past the 12 min mark, and the fire eventually burned only in the burning tank, as illustrated in Fig. 4c. Roughly six minutes later, the fire spread to the surrounding oil pan, as shown in Fig. 4d. At 18′36", the reignition area reached 90%, which is indicative of excellent burnback performance. It is pertinent to highlight that during the latter stages of the anti-burning experiment, there was a significant reduction in the foam presence. Despite a portion of the gasoline surface being exposed, no reignition occurred, thereby further substantiating the AFFF’s dual fire extinguishing mechanism of foam and water film.
Table 4 presents the results of an experiment in which the extinguishing time and burnback time were measured as 58 s and 18.6 min, respectively. The China National Standard (GB 15308-2006) and European Standard (EN 1568-3 2008) require that low expansion foams have an extinguishing time of no more than 180 s and a burnback period of no less than 10 min. It is also noted by the International Civil Aviation Organization (ICAO) that the extinguishing time for kerosene fuel should not exceed 60 s. These standard requirements are surpassed by our experimental data, demonstrating the outstanding effectiveness of 6% AFFF diluent in extinguishing fires.
Freezing resistance performance of AFFF concentrate
Ensuring freezing resistance is crucial for AFFF concentrate to withstand low temperatures and meet long-term storage requirements. The AFFF concentration exhibited fluidity and foamability before treatment (Fig. 5a). However, after treatment, no heterogeneous phenomena or clear stratification was observed (Fig. 5b). Moreover, the AFFF concentrate always maintains foamability after treatment, indicating a superior freezing resistance of the AFFF formulation.
Conclusions
In summary, AFFF concentrate containing hydrocarbon surfactants and additives was prepared using a short-chain perfluorinated nitrogen-heterocyclic nonionic amine oxide surfactant as a major component. The objective was to investigate the performance of a 6% AFFF diluent by examining the influence of various components. A self-developed laboratory technique was utilized to filter out a compelling AFFF formulation. The results demonstrate that the AFFF agent produced from the formulation is highly effective in suppressing pool hydrocarbon liquid fires. The characteristics of the AFFF formulation were evaluated based on several key performance indicators including spreading coefficient, foam expansion, 25% drainage time, extinguishing time for forceful application, and fire burnback time. The respective values for these characteristics were found to be 5.4, 8.11, 4.6 min, 58 s, and 18.6 min, respectively. These values are consistent with established standard specifications in China, America, and Europe. Furthermore, the AFFF concentrate exhibited high freezing resistance, as evidenced by the absence of significant stratification or heterogeneous phenomena after prolonged storage at − 20 °C. This characteristic makes the short-chain perfluorinated nitrogen-heterocyclic nonionic amine oxide surfactant a promising option as a replacement for PFOA/PFOS in reservoir fire suppression.
References
Ananth R, Snow AW, Hinnant KM, Giles SL, Farley JP (2019) Synergisms between siloxane-polyoxyethylene and alkyl polyglycoside surfactants in foam stability and pool fire extinction. Colloids Surf A 579:123686. https://doi.org/10.1016/j.colsurfa.2019.123686
Association NFP (2008) Standard for evaluating aircraft rescue and fire-fighting foam equipment. National Fire Protection Association.
Boone JS, Vigo C, Boone T, Byrne C, Ferrario J, Benson R, Donohue J, Simmons JE, Kolpin DW, Furlong ET (2019) Per-and polyfluoroalkyl substances in source and treated drinking waters of the United States. Sci Total Environ 653:359–369. https://doi.org/10.1016/j.scitotenv.2018.10.245
Cserháti T, Forgács E, Oros G (2002) Biological activity and environmental impact of anionic surfactants. Environ Int 28:337–348. https://doi.org/10.1016/S0160-4120(02)00032-6
Czajka A, Hazell G, Eastoe J (2015) Surfactants at the design limit. Langmuir 31:8205–8217. https://doi.org/10.1021/acs.langmuir.5b00336
Fay JA (1971) Physical processes in the spread of oil on a water surface. Int Oil Spill Conf 1971:463–467. https://doi.org/10.7901/2169-3358-1971-1-463
Gao S, Cao Z, Niu Q, Zong W, Liu R (2019) Probing the toxicity of long-chain fluorinated surfactants: interaction mechanism between perfluorodecanoic acid and lysozyme. J Mol Liq 285:607–615. https://doi.org/10.1016/j.molliq.2019.04.134
Ghisi R, Vamerali T, Manzetti S (2019) Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: a review. Environ Res 169:326–341. https://doi.org/10.1016/j.envres.2018.10.023
Grant RL, Yao C, Gabaldon D, Acosta D (1992) Evaluation of surfactant cytotoxicity potential by primary cultures of ocular tissues: I Characterization of rabbit corneal epithelial cells and initial injury and delayed toxicity studies. Toxicology 76:153–76. https://doi.org/10.1016/0300-483X(92)90162-8
Han Y, Qin J (2011) Development and application status of foam extinguishing agent. Fire Saf Sci 20:235–240. https://doi.org/10.1007/s11460-011-0118-2
He Y-H, Sun Q, Xing H, Wu Y, Xiao J-X (2019) Cationic-anionic fluorinated surfactant mixtures based on short fluorocarbon chains as potential aqueous film-forming foam. J Dispersion Sci Technol 40:319–331. https://doi.org/10.1080/01932691.2018.1468262
Hetzer R, Kümmerlen F, Wirz K, Blunk D (2014) Fire testing a new fluorine-free AFFF based on a novel class of environmentally sound high performance siloxane surfactants. Fire Saf Sci 11:1261–1270. https://doi.org/10.3801/IAFSS.FSS.11-1261
Hinnant K, Giles S, Smith E, Snow A, Ananth R (2020) Characterizing the role of fluorocarbon and hydrocarbon surfactants in firefighting-foam formulations for fire-suppression. Fire Technol 56:1413–1441. https://doi.org/10.1007/s10694-019-00932-7
Kang W, Yan L, Ding F, Guo X, Xu Z (2019) Experimental study on fire-extinguishing efficiency of protein foam in diesel pool fire. Case Stud Therm Eng 16:100557. https://doi.org/10.1016/j.csite.2019.100557
Kovalchuk N, Trybala A, Starov V, Matar O, Ivanova N (2014) Fluoro-vs hydrocarbon surfactants: why do they differ in wetting performance? Adv Colloid Interface Sci 210:65–71. https://doi.org/10.1016/j.cis.2014.04.003
Lattimer BY, Hanauska CP, Scheffey JL, Williams FW (2003) The use of small-scale test data to characterize some aspects of fire fighting foam for suppression modeling. Fire Saf J 38:117–146. https://doi.org/10.1016/S0379-7112(02)00054-1
Laundess AJ, Rayson MS, Dlugogorski BZ, Kennedy EM (2011) Small-scale test protocol for firefighting foams DEF (AUST) 5706: effect of bubble size distribution and expansion ratio. Fire Technol 47:149–162. https://doi.org/10.1007/s10694-009-0136-2
Li Q-X, Zhang G-H, Li Z-H, Yang H-L, Chen R-Q (2012) Forceful application of mass loss model and simulation of aqueous film-forming foam. J Nav Univ Eng 24:95–99+103. https://doi.org/10.7495/j.issn.1009-3486.2012.06.019
Moody CA, Field JA (2000) Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ Sci Technol 34:3864–3870. https://doi.org/10.1021/es991359u
Pabon M, Corpart J (2002) Fluorinated surfactants: synthesis, properties, effluent treatment. J Fluorine Chem 114:149–156. https://doi.org/10.1016/S0022-1139(02)00038-6
Peshoria S, Nandini D, Tanwar R, Narang R (2020) Short-chain and long-chain fluorosurfactants in firefighting foam: a review. Environ Chem Lett 18:1277–300. https://doi.org/10.1007/s10311-020-01015-8
Rengel B, Mata C, Pastor E, Casal J, Planas E (2018) A priori validation of CFD modelling of hydrocarbon pool fires. J Loss Prev Process Ind 56:18–31. https://doi.org/10.1016/j.jlp.2018.08.002
Scheffey JL (2016) Foam agents and AFFF system design considerations. SFPE handbook of fire protection engineering. Springer, New York, pp 1646–706. https://doi.org/10.1007/978-1-4939-2565-0_47
Sheng Y, Lu S, Xu M, Wu X, Li C (2016) Effect of Xanthan gum on the performance of aqueous film-forming foam. J Dispersion Sci Technol 37:1664–1670. https://doi.org/10.1080/01932691.2015.1124341
Sheng Y, Jiang N, Lu S, Li C (2018a) Fluorinated and fluorine-free firefighting foams spread on heptane surface. Colloids Surf A 552:1–8. https://doi.org/10.1016/j.colsurfa.2018.05.004
Sheng Y, Lu S, Jiang N, Wu X, Li C (2018b) Drainage of aqueous film-forming foam stabilized by different foam stabilizers. J Dispersion Sci Technol 39:1266–1273. https://doi.org/10.1080/01932691.2017.1393432
Sontake AR, Wagh SM (2014) The phase-out of perfluorooctane sulfonate (PFOS) and the global future of aqueous film forming foam (AFFF), innovations in fire fighting foam. Fire Eng 39:19–23
Specification M (1992) Fire extinguishing agent, aqueous film-forming foam (AFFF) liquid concentrate, for fresh and seawater, Report No. MIL-F-24385F.
Wu W, Wang J, Zhou Y, Sun Y, Zhou X, Zhang A (2021) Design, synthesis and application of short-chained perfluorinated nitrogenous heterocyclic surfactants for hydrocarbon subphases. J Fluorine Chem 252:109919. https://doi.org/10.1016/j.jfluchem.2021.109919
Xu Z, Guo X, Yan L, Kang W (2020) Fire-extinguishing performance and mechanism of aqueous film-forming foam in diesel pool fire. Case Stud Therm Eng 17:100578. https://doi.org/10.1016/j.csite.2019.100578
Yang Y, Peng M, Sha M, Fang J, Zhang D, Pan RM, Jiang B (2022) Study on aqueous film-forming foam extinguishing agent based on fluorocarbon cationic-hydrocarbon anionic surfactants mixture system. J Surfactants Deterg 25:205–216. https://doi.org/10.1002/jsde.12554
Yu X, Li F, Fang H, Miao X, Wang J, Zong R, Lu S (2021) Foaming behavior of fluorocarbon surfactant used in fire-fighting: the importance of viscosity and self-assembly structure. J Mol Liq 327:114811. https://doi.org/10.1016/j.molliq.2020.114811
Zaggia A, Conte L, Padoan G, Bertani R (2010) Synthesis and application of perfluoroalkyl quaternary ammonium salts in protein-based fire-fighting foam concentrates. J Surfactants Deterg 13:33–40. https://doi.org/10.1007/s11743-009-1136-4
Zhang Y, Liao GX (2008) Experimental study on the characters and fire extinguishing properties of a new high spreading aqueous film forming foam (AFFF). Fire Saf Sci 1:1–7. https://doi.org/10.3969/j.issn.1004-5309.2008.01.001
Zhou C, Wang Y (2020) Structure-activity relationship of cationic surfactants as antimicrobial agents. Curr Opin Colloid Interface Sci 45:28–43. https://doi.org/10.1016/j.cocis.2019.11.009
Zhu X, Jia X, Zhang Y (2022) The physicochemical and fire extinguishing performance of aqueous film-forming foams based on a class of short-chain fluorinated surfactants. J Surfactants Deterg 25:193–204. https://doi.org/10.1002/jsde.12550
Acknowledgements
The authors gratefully acknowledge the financial supports from the Scientific Research Project of Hubei Provincial Education Department (Q20213104), the Hubei Provincial Natural Science Foundation of China (2022CFB854), and the National Natural Science Foundation of China (22277038).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 381380 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, WH., Wang, JL., Zhou, YQ. et al. Formulation and performance of aqueous film-forming foam fire extinguishing agent composed of a short-chain perfluorinated heterocyclic surfactant as the key component. Chem. Pap. 77, 6763–6771 (2023). https://doi.org/10.1007/s11696-023-02975-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-02975-1