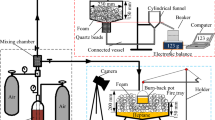
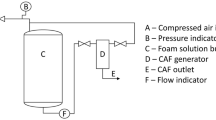
Abstract
Foams have been developed almost entirely from experimental work. Although the technologies are rather mature, no fundamental explanations of foam extinguishment performance have been developed based on first principles. As a result, foams are characterized by (1) fire tests for which there is no general international agreement and (2) physical and chemical properties that may or may not correlate with empirical results. This chapter reviews the important parameters associated with foam agents, test methods used to evaluate foams, and relevant data in the literature that can be used to evaluate foam system designs. Because of their superior performance in extinguishing certain types of hydrocarbon liquid fuel fires, the emphasis is on film-forming foams and thin pool fires (e.g., from spills). Situations involving fuels “in depth” are limited to a discussion on foam modeling and small-scale tests to assess oil and petrochemical industry hazards.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Foams have been developed almost entirely from experimental work. Although the technologies are rather mature, no fundamental explanations of foam extinguishment performance have been developed based on first principles. As a result, foams are characterized by (1) fire tests for which there is no general international agreement and (2) physical and chemical properties that may or may not correlate with empirical results. This chapter reviews the important parameters associated with foam agents, test methods used to evaluate foams, and relevant data in the literature that can be used to evaluate foam system designs. Because of their superior performance in extinguishing certain types of hydrocarbon liquid fuel fires, the emphasis is on film-forming foams and thin pool fires (e.g., from spills). Situations involving fuels “in depth” are limited to a discussion on foam modeling and small-scale tests to assess oil and petrochemical industry hazards.
Fire-fighting foam consists of air-filled bubbles formed from aqueous solutions. The solutions are created by mixing a foam concentrate with water in the appropriate proportions (typically 1, 3, or 6 % concentrate to water). The solution is then aerated to form the bubble structure. Some foams, notably those that are protein-based, form thick, viscous foam blankets on liquid hydrocarbon fuel surfaces. Other foams, such as film-formers, are much less viscous and spread rapidly on the fuel surface. The film-formers are capable of producing a vapor-sealing film of surface-active water solution on most of the hydrocarbon fuels of interest.
Because the foam is lighter than the aqueous solution that drains from the bubble structure, and lighter than flammable or combustible liquids, it floats on the fuel surface. The floating foam produces an air-excluding layer of aqueous agent, which suppresses and prevents combustion by halting fuel vaporization at the fuel surface, and preventing air from reaching the combustion zone. If the entire surface is covered with foam, the fuel vapor will be completely separated from air, and the fire will be extinguished. Low-expansion foams (i.e., foam volume-solution volume of ≤10:1) are quite effective on two-dimensional (pool) flammable and combustible liquid fires, but not particularly effective on three-dimensional fuel fires. This is particularly true of three-dimensional fires involving a low flashpoint fuel. Typically, an auxiliary agent, such as dry chemical, is used with foam where a three-dimensional fire (running fuel or pressurized spray) is anticipated. In enclosed hazard areas, other extinguishing media may be used, such as water mist or high-expansion foam. These agents generally require total flooding of the hazard volume.
Description of Foam Agents
There are no universally agreed-on definitions of foam agents or terms associated with fire-fighting foam. For example, where foam is referenced in NFPA standards, definitions vary from document to document. Because foams vary in performance, in terms of application rates and quantities required for extinguishment, agent definitions can be cast to accentuate positive attributes, such as “rapid knockdown” or “superior burnback resistance.” Geyer et al. have described the composition of various foam agents, paraphrased as follows [1].
-
1.
Protein foam. Protein foam is a “mechanical” foam produced by combining (proportioning) foam concentrate and water and discharging the resulting solution through a mixing chamber. The mixing chamber introduces (aspirates) air, which expands the solution to create foam bubbles. The liquid concentrate consists primarily of hydrolyzed proteins in combination with iron salts. Hoof and horn meal and hydrolyzed feather meal are examples of proteinaceous materials used in protein-foam concentrates. No aqueous film is formed on the fuel surface with this type of agent.
-
2.
Fluoroprotein. These agents are basically protein foams with fluorocarbon surface-active agents added. The varying degrees of performance are achieved by using different proportions of the base protein hydrolyzates and the fluorinated surfactants. Although fluoroprotein foams generally have good fuel shedding capabilities and dry chemical compatibility, the solution that drains out from the expanded foam does not form a film on hydrocarbon fuels. However, the addition of the fluorinated surfactants may act to reduce the surface tension of the solution. This reduction may, in turn, decrease the viscosity of the expanded solution, thus promoting more rapid fire control when compared to protein foams.
-
3.
Aqueous film-forming foam (AFFF). These agents are synthetically formed by combining fluorine-free hydrocarbon foaming compounds with highly fluorinated surfactants. When mixed with water, the resulting solution achieves the optimum surface and interfacial tension characteristics needed to produce a film that will spread across a hydrocarbon fuel. The foam produced from this agent will extinguish in the same air-excluding fashion as other foams. Further, the solution that results from normal drainage or foam breakdown produces an aqueous “film” that spreads rapidly and is highly stable on the liquid hydrocarbon fuel surface. It is this film formation characteristic that is the significant distinguishing feature of AFFF as it actually results in a seal significantly mitigating the emission of vapors from the liquid.
These definitions are by no means all-inclusive. For example, film-forming fluoroprotein (FFFP) foam is an agent that is produced by increasing the quantity and quality of the surfactants added to a protein hydrolyzate. By doing this, the surface tension of the resulting solution, which drains from the expanded foam, is reduced to the point where it may spread across the surface of a liquid hydrocarbon fuel. An alcohol-resistant concentrate is formulated to produce a floating polymeric skin for foam buildup on water-miscible fuels. This polymeric skin protects the foam from breakdown by polar solvents, for example, acetone, methanol, and ethanol. Hybrid AFFFs are being formulated to reduce or eliminate fluorosurfactants, which may have an adverse environmental impact.
A potential new class of foams, fluorosurfactant-free foam, has been developed in response to the environmental impact of fluorosurfactants (see section on “Foam Environmental Considerations”). This is neither a film forming or protein based foam. Underwriters Laboratories (UL) classifies these foams generically as “synthetic” foams. UL defines synthetic foams as those having as its base other than a fluorinated surfactant or hydrolyzed protein.
The descriptions show that there are distinct chemical differences between protein-based foams and AFFF. In general, the surfactants used in aqueous foams are long-chained compounds that have a hydrophobic or hydrophilic (i.e., water repelling or water attracting, respectively) group at one end [2]. The molecular structure of a typical AFFF fluorinated surfactant is shown in Fig. 47.1 [3]. In this molecule, the perfluoroctyl group on the left is the hydrophobic group, while the propyltrimethylammonium group is the hydrophilic group. When these compounds are dissolved into solution with water, they will tend to group near the surface of the solution, aligned so that their hydrophobic ends are facing toward the air/solution interface. The advantage of this is that the perfluoroctyl group found in these compounds is also oliophobic (i.e., oil repelling) as well as hydrophobic [4].
Typical AFFF fluorosurfactant molecule [3]
AFFF concentrates also contain hydrocarbon surfactants. These compounds are less hydrophobic than those containing the perfluoroctyl group. However, they do provide greater stability once the solution is expanded into a foam. As a result, the surface tension of the solution is reduced below that of water; the expanded foam produced from the solution is resistive to breakdown from heat, fuels, or dry chemical extinguishing agents; and the solution that drains out from the expanded foam is able to form a film on hydrocarbon fuels.
The importance of both the film formation and foam bubble characteristics of AFFF, resulting from the combination of fluorocarbon and hydrocarbon surfactants, was evaluated in early work by Tuve et al. [5] When a highly expanded, stiff formulation of AFFF was used, these researchers found it difficult to obtain good fire extinguishment and vapor sealing characteristics. The foam resisted flow, and drainage of the aqueous solution (film) was slow. The drainage was corrected by expanding the foam to a lesser degree. This pioneering AFFF formulation, with an expansion ratio of 8:1 and 25 % drainage time of 6 min, appeared to offer the best compromise in characteristics. It provided a readily flowable foam that sealed up against obstructions, promoted the rapid formation of a surface-active film barrier on the fuel, and provided a sufficiently stable foam to resist burnback.
Fire Extinguishment and Spreading Theory
As noted in the Fire Protection Handbook ® review of suppression theory, the fundamental mechanisms of foam fire extinguishment on two-dimensional pool fires have not been developed [6]. Usually, fire extinguishment is described simply as a factor of the cessation of fuel vaporization at the fuel surface. As the area of fuel vapor production decreases due to the spreading foam, the size of the combustion zone decreases. When the area is totally covered, sufficient amounts of air cannot reach the liquid fuel and extinguishment occurs. As the fuel is covered, cooling must also occur to bring the vapor pressure of the fuel below that of its boiling point. Once the fuel is cooled, a layer of foam must continue to be applied either manually or by spreading to terminate combustion and prevent re-ignition. Hanauska et al. have proposed fundamental extinguishment parameters [7]. A similar foam extinguishment model has been proposed by Persson and Dahlberg [8]. Bench-scale experiments have been combined with correlation/modeling techniques.
Foam Loss Mechanisms
Fire extinguishment by foams can be summarized as shown in Fig. 47.2. Foam having a temperature, T i , and depth, h, spreads at a rate of V s along a fuel of temperature, T s , and vapor pressure, P v . Fuel is volatized by the fire at a rate of ṁ fuel, which is a function of the radiative feedback, \( {\dot{q}}_{\mathrm{rad}} \). The foam is added by the discharge application, ṁ add, and lost through evaporation, ṁ vap, and drop-through, ṁ drop.
The total mass loss of the foam is a function of the loss due to drop-through and the mass loss due to vaporization. The mass loss due to drop-through is at least partially dependent on the drainage of liquid from the foam. Evaporation of the liquid results primarily from radiant energy from the fire. Assuming that most of the radiation results in direct evaporation of the foam, the evaporation of foam can be characterized by
where ΔH v is the combined latent and sensible heats of vaporization. Using a rough estimate of \( {\dot{q}}^{{\prime\prime} } \) from large pool fires of 45–185 kW/m2 yields an evaporation rate of 18–72 g⋅m2/s, assuming a heat of vaporization of 2563 kJ/kg. To account for reflective and absorbed losses, Persson [9] has proposed a calculation method
where k e is an experimentally derived constant using different fluxes from a radiant exposure. For \( {\dot{q}}_{\mathrm{rad}}^{{\prime\prime} } \) values of 45 and 185 kW/m2, Equation 47.2 yields values for\( {\dot{m}}_{\mathrm{vap}}^{{\prime\prime} } \)of 11 and 46 g⋅m2/s, respectively. Because the estimated\( {\dot{m}}_{\mathrm{vap}}^{{\prime\prime} } \)values based on Equation 47.1 at the same heat fluxes were 18 and 72 g⋅m2/s, the experimental mass loss rate results are about 62 % lower than the theoretical loss. The difference between values is attributable to neglecting the reflected and absorbed losses in Equation 47.1. This indicates that about 48 % of the radiant flux to the foam surface is either reflected from or absorbed into the foam blanket. The division between these two heat transfer mechanisms is not clear and is an area for further study.
Foam loss can likewise be described theoretically, based on the downward force of gravity and the opposing forces due to surface tension and buoyancy. Alternately, a model mass loss due to drainage can be expressed as a time-averaged constant
where k d is an experimentally determined drainage coefficient. From the data of Persson, the drainage coefficient can be estimated to be 17–25 g⋅m2/s [9]. The drainage rate was found to be relatively independent of the radiant heat flux to the foam, but highly dependent on the expansion ratio. Foams with lower expansion ratios will drain faster. For example, decreasing the expansion ratio by about half (11.3–5.3) increased the drainage rate by a factor of about 2 (55–105 g/min). Decreasing the expansion ratio changes fundamental parameters of the foam, which allows it to drain faster.
Experimental work on the foam model, particularly with regard to the effects of incident heat flux on the foam blanket, has been performed in the United States and Europe. Lattimer et al. [10] designed a test apparatus that was used to measure the behavior of foam when exposed to irradiance levels of 0–50 kW/m2. The apparatus provided data on evaporation rate, drainage rate, foam destruction rate, foam temperature, heat penetration, and time to fuel ignition. The performance of a single AFFF formulation was characterized.
Evaporation rates were measured primarily to be a function of irradiance, making it possible to predict evaporation using the irradiance from the fire and an effective heat of vaporization. The AFFF foam evaluated in this study was determined to have an effective heat of vaporization of 4.87 ± 0.75 MJ/kg. This result is slightly higher than that found by Ikasson and Persson [11], 4.0 MJ/kg. Different AFFF formulations may explain this difference.
Foam drainage rate was measured to be insensitive to the irradiance level or the presence of a fuel layer below the foam. This was consistent with the findings of the Swedish researchers. For foams with expansion ratios ranging from 6.0 to 9.7, drain rate was determined to be a function of foam mass per unit area. A single curve was developed to characterize the drain rate for all foams with a thickness equal to or less than 75 mm. The drainage rate was measured to be constant down to a foam mass per unit area of 3.0 kg/m2 and decreased linearly to zero by 1.5 kg/m2. The steady-state drain-rate level decreased from 40 g⋅m2/s to 28 g⋅m2/s by increasing the expansion ratios from 6.0 to 9.7, respectively.
The drainage rate of low-expansion ratio foams (3.3) was as much as 4–10 times higher than levels measured at higher expansion ratios. The high level was attributed to the fluidity of the foam, which is affected by solution density in foam, breaking and coalescing of bubbles, and solution viscosity. Measurements of foam fluidity for different AFFF foam expansion ratios and temperatures are necessary to further understand these trends in the data at low-expansion ratios.
Foam depletion rate was measured primarily to be a function of the irradiance level incident on the foam. As irradiance increased, the foam depletion rate increased. Foam depletion rate was independent of the initial foam height and expansion ratio.
Heat penetration through the foam was measured to be a function of foam height and foam mass. For all of the different tests where heat penetration was measured, the data indicate that heat begins penetrating through the foam when the foam becomes approximately 50 ± 7 mm thick and has a foam mass of 4.2 ± 1.2 kg/m2.
Ignition time in tests with JP-5 fuel layers was measured to be a function of both irradiance and initial foam height. Increases in irradiance and decreases in initial foam height were determined to decrease the time to ignition. This result was found to be independent of expansion ratio and initial fuel temperature. At ignition, nearly all of the AFFF (less than 0.8 kg/m2) had been lost from the fuel surface.
Additional small-scale testing needs to be performed to quantify the foam losses and foam spread characteristics of other foam concentrates. Foam loss and spread data are expected to be concentrate dependent, and these data are necessary to further validate the performance of the foam extinguishment model.
Foam drainage is a complicated phenomenon that is highly time dependent. Besides the forces associated with the bubble structure, drainage is dependent on the continual changing geometries of the cells and other variable conditions, such as collapsing cells. Even though all aspects of this problem cannot be fully detailed, simplified models have been created that predict the drainage rate for foams. Kraynik has developed one such model that considers the drainage from a column of persistent foam [12]. The model contains no empirical parameters and assumes the foam is dry with very thin walls such that the liquid contained in the cell walls is negligible. Additional modeling has been performed as described in the following paragraphs.
The focus of the tests on the foam by Lattimer et al. [10] was to quantify the evaporation and drainage loss mechanisms, and develop methods for using these data in suppression models. Drain rates were shown to be affected by both expansion ratio and initial foam height. Evaporation rates were primarily affected by the irradiance on the foam. Additional data analysis was conducted to develop methods for expressing the data in a form that could be used in modeling the losses of foam during a fire where the foam may be exposed to a range of irradiance levels. A simple model that monitors the mass of the foam was used to evaluate the proposed methods for predicting evaporation and drainage.
Solution drain rate from foams being heated is extremely difficult to model from first principles due to the complexities that arise from bubbles expanding, coalescing, and bursting. A simple approach for predicting solution drainage was sought for use in fire suppression modeling. Persson et al. [13] found that initial foam height affected drainage rate with time for a particular type and expansion ratio of foam. Empirical relations for drain rate were developed as a function of time and foam height.
In order to avoid having to rely on accurate predictions of foam height in fire suppression calculations, an alternative approach was developed. Through analysis of the data, the mass of the foam was found to be related to the drain rate. Plotting the drain rate versus foam mass essentially collapsed the data for tests with an expansion ratio (ER) of 6 and 10. The relation between drain rate and foam mass was found to be generally unaffected by irradiance or initial foam height.
The second mechanism by which foam will lose solution is through evaporation. The evaporation of solution from the foam surface was modeled as a Lagrangian thin film of solution at a constant temperature of 100°C. The evaporation rate was simplified to
where the \( {\upvarepsilon}_{h\kern-0.12em f\kern-0.12em g}=0.96 \) and the average heat flux is 97 % of the centerline heat flux, \( {q}_{h\kern-0.12em f\kern-0.12em g}^{{\prime\prime} }=0.97\kern0.5em {q}_{h\kern-0.12em f\kern-0.12em g, cl}^{{\prime\prime} } \), and the heat of vaporization is that of water at 100°C \( \left(\Delta {h}_v=2257\ \mathrm{kJ}/\mathrm{kg}\right) \). In the experiments, the mass evaporated was measured directly but the absorptivity of the foam was unknown. Incorporating the constants above, the absorptivity of the foam can be determined by
This pure radiation model does not account for other phenomena that may affect the evaporation rate such as bubbles bursting, foam density on the surface, and transient heating. Therefore, the absorptivity determined using experimental data would be an effective absorptivity that embodies the radiation properties of the foam and the other phenomena that affect the evaporation rate. This average effective absorptivity is shown in Table 47.1.
These methodologies were used to predict the foam mass drained and evaporated. The mass drained was predicted using a reference curve that related foam mass to drain rate. This function was developed from test data at an irradiance of 20 kW/m2 with a foam height of 75 mm. The mass evaporated was determined from using the effective absorptivity values provided in Table 47.1. Additional simulations were conducted to evaluate the sensitivity of the results to the range of effective absorptivity stated in Table 47.1.
Predictions of foam having an expansion ratio equal to six are shown in Figs. 47.3 and 47.4 for irradiance levels of 20 and 50 kW/m2, respectively. Also shown is the reference curve used to predict the drainage rate. The model predicts the masses quite well, particularly near the end of the test where mass of foam on the surface could be used to predict time of fuel ignition. Data from this study indicate that the fuel beneath the foam will ignite when the foam mass per unit area is approximately 0.8 kg/m2. With good agreement between the model and the data especially near the end of the test when ignition will occur, the model could be used to also predict fuel layer ignition. Also shown in Figs. 47.3 and 47.4 is the effect of varying the effective absorptivity. Because the evaporation represents a small portion of the mass loss, the results were not strongly affected by varying this parameter. Similar results were determined for foam at an ER = 10.
Predicted masses for foam at an ER = 3 are shown in Fig. 47.5 along with the reference curve used to predict the drainage rate. Due to the initial surge of drainage in the beginning of these tests, the model does not predict these masses as well in the initial part of the test. After approximately 150 s, the model is within 10 % of the data. Again, the predicted mass of foam near the end of the test agrees well with the data, which indicates that the time of ignition could be predicted using this model even for lower expansion foams.
Foam Spread over Liquid Fuels
In order to predict the extinguishment of a liquid pool fire by fire-fighting foam, it is necessary to describe the process of spreading the foam over the liquid fuel surface. This process of foam spread on a liquid fuel is similar to the spread of a less dense liquid (such as oil) on a more dense liquid (such as water). This phenomenological approach to the spread of foam on a liquid pool fire is appropriate to the extent that foam can be treated as a liquid. Kraynik characterizes foams macroscopically as being Bingham fluids with a finite shear stress and non-Newtonian viscosity [14]. That is, foam displays an infinite viscosity up to some initial shear rate above which it displays a shear-rate dependent viscosity.
Because fuels typically have low viscosities (especially compared to foam viscosities at relatively low shear rates), it may be appropriate to model foam spread across a fuel surface using models developed for oil spread on water. These models assume that the oil spreads as a fluid with a viscosity much higher than the water on which it is spreading. The process of oil spread on water has been described in detail by Fay [15], and Fay and Hoult [16]. Their phenomenologically based model describes three regimes of spread characterized by combinations of spreading forces and retarding forces. The first regime is the gravity-inertia regime, where the outward spread of the oil is driven by a gravity force and retarded by the inertia required to accelerate the oil. The second regime is the gravity-viscous regime, where the gravity-induced spreading is retarded by viscous dissipation in the water. Because the oil is much more viscous than the water, they assume that there is slug flow in the oil and that the viscous drag force is dominated by the velocity gradient in the water. The third regime is characterized by a surface-tension spreading force opposed by the viscous retarding force. By setting the spreading and retarding forces equal in each of the regimes, they developed equations to estimate the length of the spread as a function of time.
By treating the spread of foam on fuel as similar to the spread of oil on water, the equations developed by Fay and Hoult might be used to describe the spread of a foam blanket over a fuel pool as a function of time [15]. Because foam generally has a much higher viscosity than the fuel on which it is spreading, the assumption of slug flow made for the oil by Fay and Hoult should be reasonably valid for foam spread on fuel as well [16]. The equations are
where
-
l = Length of spread (cm)
-
\( \Delta =\left({\uprho}_{\mathrm{fuel}}-{\uprho}_{\mathrm{foam}}\right)/{\uprho}_{\mathrm{fuel}} \)
-
g = Acceleration of gravity (981 cm/s2)
-
V = Foam volume (cm3)
-
t = Time (s)
-
v = Kinematic viscosity of fuel (cm2/s)
-
σ = Spreading coefficient (dynes/cm)
-
ρ = Density of fuel or foam (g/cm3)
Equation 47.6 represents an untested theoretical model of foam spread. The equation includes the parameters that are known or suspected to affect foam spread. They are presented here as an initial effort to understand foam flow based on first principles. They are not yet developed for engineering use. The following discussion expands on this theory.
The transition from gravity-dominated spread to surface-tension-dominated spread can be shown to occur at a critical thickness of the foam layer, h c , given by
The transition from inertia- to viscous-dominated retarding force occurs when the foam thickness, h, is equal to the viscous boundary layer thickness, δ, of the fuel, with
The equations for length of spread can be used to generate preliminary estimates of the spread distance and area coverage for the placement of a volume of foam on a fuel surface. The equations are only estimates because they consider a force balance between just the dominant forces for each regime. All forces are actually present in each regime. Also, the densities of both fluids are considered to be very nearly equal for the development of the equation for the gravity-viscous regime. This is the case for oil spread on water, but may not be the case for foam on fuel.
Using approximations for fuel and foam characteristics, it can be shown that a positive spreading coefficient does not begin to affect the spread of foam until the foam layer has become very thin. For the placement of a volume of foam on a fuel, this may not occur until after significant time has passed, relative to the time scale for knockdown desired in many fire protection situations.
The equations for foam spread on fuel include many of the parameters known to be important to foam spread. However, the equations are independent of the foam viscosity. Observations indicate that low-viscosity nonrigid foams, such as AFFF, spread faster than high-viscosity rigid protein foams. The inclusion in the model of a term to account for this is desirable.
The equations for spread length so far have assumed that the foam spreads over the fuel as plug flow, with no relative movement within the foam itself. It is easy to conceive that the foam has the capability to flow over itself. The relative movement within the foam is equivalent to the foam flowing over a solid surface. The total foam flow might ultimately be modeled as the combination of the foam plug over the fuel and the flow within the foam layer itself.
According to Cann et al., several regimes exist for spread of a liquid on a solid that are similar to those described for spread of a liquid on a liquid [17]. Most of this spread occurs in a gravity-viscous force regime, where the spread is given by
where k is an empirically determined constant, and μ is the foam viscosity.
Thus, the spread of foam over fuel can be characterized by two scenarios: (1) high-viscosity liquid spreading over a low-viscosity liquid and (2) a liquid spreading over a “solid.” The spread of foam can be described by modifying Equation 47.6, as follows:
Kraynik describes foams as being characterized by a yield stress and shear thinning viscosity [14]. Thus, the foam viscosity in the equations above is not a constant but is a function of the shear rate. The stress in the foam is a result of the gravity-induced pressure gradient. As the foam flows out and becomes thin, the stress will be reduced. When the stress falls below the yield stress, the viscosity will become infinite and the second term, kt/μ, in the spread length equations will go to zero. The foam will flow simply as plug flow. Above the yield stress, the foam will have a finite viscosity, but this viscosity will be dependent on the yield stress.
An AFFF agent that is very free flowing will have a relatively small yield stress and will retain the second term in the spread length equations until it has flowed out to a very thin layer. A protein foam that is relatively stiff will have a large yield stress, and the second term will go to zero before the foam has spread very far. Above the yield stress, the viscosity of the AFFF will be lower than that of a protein foam, and the second term will provide a greater contribution to foam spread. The rheological properties described appear to have a significant impact on foam spread; however, the properties are not a part of any current specification and are rarely measured.
Foam Extinguishment Modeling
At present, modeling of foam extinguishment cannot be performed because of the large number of remaining uncertainties. A model would have to take into account the addition of foam to the fuel surface, the spread of foam on the fuel surface, and the foam loss mechanisms of evaporation and drop-through. The foam spread length equations can be used to estimate the area of foam coverage at a specific time and for a specific quantity of foam. Modeling at this time is limited because of the lack of established values for k e (Equation 47.2) and k d (Equation 47.3). Also, the yield stress and viscosity relationships for fire-fighting foams have not been quantified. Experimental work is needed to complete this modeling effort. Also, the actual method of application (e.g., from a handline nozzle or fixed device such as a sprinkler) must ultimately be taken into account. Even so, preliminary calculations using this methodology are encouraging and support continued development [7].
An attempt has been made to model large tank fires [13]. This included modeling of foam spread with gentle and over-the-top application. The models were based on the assumption that a driving force caused by hydrostatic pressure differences in the foam and a resisting force due to viscous friction between the foam and the fuel is governing the foam spread. In case of foam flow in a channel, there is also a resisting force due to friction between the foam and the sidewalls. The models take into account ordinary drainage, radiation-induced drainage, and evaporation. Friction data for the models were obtained from cold foam flow tests in laboratory scale. In general, the models for gentle application agree well with the experiments. Due to lack of data, it was not possible to incorporate the destruction of foam at the foam front when it starts to dry out. The effect of this is that the models generally predicted spreading times that were too short. A remaining uncertainty in the models is how to scale the friction data when increasing the length scale by orders of magnitude (e.g., to tank diameters 100–120 m). This is because detailed large-scale data are lacking. Obtaining better experimental observations of large tank fires was recommended.
The model was compared with a few full-scale tank fires ranging from 40 to 80 m in diameter where detailed observations were available. In general, the predicted time to cover is in the range of 10–20 min shorter than the observed time to knockdown. This is because some effects are not included in the model, such as the initial destruction of foam when the foam plunges into the burning fuel, fuel pickup, and foam destruction at the front due to drainage and evaporation. It was concluded that further work is needed to incorporate the destruction of foam at the front, quantify the initial delay phase caused by foam destruction, determine how to scale the friction data when increasing the length scale by orders of magnitude, and obtain more accurate data on foam properties generated by various types of large-capacity foam nozzles.
Surface Tension and Spreading Coefficient
Film-forming foams are defined by the ability of the aqueous solution draining from the foam to spread spontaneously across the surface of a hydrocarbon fuel. The fundamental relationship used to describe the spreading coefficient is
where
-
S a/b = Spreading coefficient (dynes/cm)
-
γ b = Surface tension of the lower liquid phase of a hydrocarbon fuel (dynes/cm)
-
γ a = Surface tension of the upper layer of liquid using AFFF solution (dynes/cm)
-
γ l = Interfacial tension between liquids a and b (dynes/cm)
Surface tension and interfacial tension can be measured using methods such as those described in ASTMD-1331, Standard Test Methods for Surface and Interfacial Tension of Solutions of Surface-Active Agents. Reagent-grade cyclohexane is typically used as a reference fuel. A du Nouy tensiometer, having a torsion balance with a 4- or 6-cm-circumference platinum-iridium ring, is lowered into the liquid and slowly pulled out until the liquid detaches from the ring’s surface. The force recorded at the point where this separation occurs is the surface tension (dynes/cm) of the pure liquid. Similarly, the interfacial tension is the measurement of tension when the ring is pulled through the boundary layer between two liquids.
The Naval Research Laboratory developed some of the earliest quantitative data on the spreading coefficient of AFFF on hydrocarbons, as shown in Tables 47.2 and 47.3 [18]. As fuel temperature increases, the surface tensions of both the fuel and the solution decrease. The spreading coefficient may go to zero or go negative [18, 19].
Although it has been shown that film-forming foams are superior fire extinguishing agents compared to other foams, there are no one-to-one correlations between bench-scale surface-tension/spreading coefficient data and fire control, extinguishment, and burnback resistance times. Both Scheffey et al. [20] and Geyer [21] have demonstrated that there is no direct correlation between fire extinguishment and spreading coefficient. As such, spreading coefficient data alone cannot be used as a relative predictor of fire performance.
Because the surface tensions of most AFFFs are approximately equal, there must be a balance between the surface tension of the fuel and the interfacial tension of the two liquids to create a positive spreading coefficient. It can be seen then that, while both the surface tension of the foam solution and the interfacial tension between the liquids have an impact on the spreading coefficient, the interfacial tension is usually the determining factor. For fuels, such as avgas or n-heptane, which have surface tensions in the range of 19–20 dyn/cm, either the foam surface tension or the interfacial tension, or both, must be reduced. Normally, the changes resulting from a modification of the formulation will be more significant for the interfacial-tension value than they will be for the foam surface-tension value. Still, a relationship between the two values does exist. [4] Therefore, in reducing the sum of the values to obtain a positive spreading coefficient, a delicate balance must be maintained.
Maintaining this balance and achieving a positive spreading coefficient is accomplished by controlling the amount and type of fluorinated surfactants used to formulate the agent. This at first seems beneficial, because a positive number on a low surface-tension fuel will ensure an even larger value with higher surface-tension fuels (e.g., JP-5 or motor gasoline). But, in reducing the interfacial tension, the foam may lose some of its fuel-shedding capabilities. The effects of adding too much fluorosurfactant to an aqueous solution and the result on foam bubble stability are described by Rosen [4] and Aubert et al. [2] This could be a problem that manifests itself only during actual fire testing. The type and amount of fluorosurfactants also affect the spreading coefficient [20].
Despite the lack of one-to-one correlations between surface-tension spreading coefficient data and fire control, extinguishment, and burnback results, these criteria are useful in categorizing film-forming agents. The spreading coefficient test is used internationally as a standard indicator of aqueous film-forming foams. Although undocumented, it is believed that film formation results in improved viscosity (or associated mechanisms that improve spreading), ultimately resulting in superior extinguishing performance.
Assessment of Fire Extinguishing and Burnback Performance
Standard Test Methods
Because a fundamental model of foam spreading has not been developed, performance of foams is measured using fire tests. The use of bench-scale burning fuel trays (e.g., less than 1 m diameter) results in varying fuel burning rates for the same fuel. This was observed by Chiesa and Alger when they attempted to use a 15-cm by 45-cm pan for foam performance evaluation [22]. Data from their experiments are shown in Fig. 47.6, which correlates control times observed when foam samples were tested using bench-scale apparatus (laboratory) and 4.6 m2 (50 ft2) fire tests (field method). Equal control times correspond to a 45° line. Because the majority of the points fall below this line, the laboratory test is more severe (about 35 %) than the field test.
Correlation of control times observed in laboratory and field tests of foam [22]
Fire test methods used by regulatory authorities for certification are usually on the order of 2.6 to 9.3 m2 (28 to 100 ft2). Foams must also meet additional test parameters related to storage, proportioning, and equipment factors.
Underwriters Laboratories Standard 162
Underwriters Laboratories (UL) 162, Standard for Foam Equipment and Liquid Concentrates, is the principal test standard for the listing of foam concentrates and equipment in the United States. Test procedures outlined in this standard have been developed to evaluate specific agent/proportioner/discharge device combinations. When a foam concentrate is submitted for testing, it must be accompanied by the discharge device and proportioning equipment with which it is to be listed. Listed products, including the foam concentrate, discharge device, and proportioner, are then described in the UL Fire Protection Equipment Directory.
Listed with a system, foam liquid concentrates are associated with discharge devices classified as Type I, II, or III. Type I devices deliver foam gently onto the flammable liquid fuel surface, for example, a foam trough along the inside of a tank wall. These devices are no longer evaluated in UL 162. Type II discharge devices deliver foam onto the liquid surface in a manner that results in submergence of the foam below the fuel surface, and restricted agitation at the fuel surface. Examples include subsurface injection systems, tank wall–mounted foam chambers, and applications where foam is bounced off the wall of a tank. Type III discharge devices deliver foam directly onto the liquid surface and cause general agitation at the fuel surface, for example, by using handheld nozzles. The flammable liquid fire tests in UL 162 include methods for sprinklers, subsurface injection, and topside discharge devices, including nozzles.
Class B fire test requirements for Types II and III discharge devices and sprinklers are shown in Table 47.4. Commercial grade n-heptane is placed in a square test pan. The area of the pan is a minimum of 4.6 m2 (50 ft2). The application rates (“densities” in UL 162, Standard for Foam Equipment and Liquid Concentrates) for various concentrates are outlined in Table 47.4.
In the test fire, the fuel is ignited and allowed to burn for 60 s. Foam is then discharged for the duration specified in Table 47.4. The foam blanket resulting from the foam discharge must spread over and completely cover the fuel surface, and the fire must be completely extinguished before the end of the foam discharge period.
After all the foam is discharged, the foam blanket formed on top of the fuel is left undisturbed for the period specified in Table 47.4. During the time the foam blanket is left undisturbed, a lighted torch is passed approximately 25.4 mm (1 in.) above the entire foam blanket in an attempt to reignite the fuel. The fuel must not reignite, candle, flame, or flash over while the torch is being passed over the fuel. However, candling, flaming, or flashover that self-extinguishes is acceptable, provided that the phenomenon does not remain in one area for more than 30 s.
After the attempts to reignite the fuel with the lighted torch are completed, a 305-mm (12-in.) diameter section of stovepipe is lowered into the foam blanket. The portion of the foam blanket that is enclosed by the stovepipe is removed with as little disturbance as possible to the remaining blanket outside the stovepipe. The cleared fuel area inside the stovepipe is ignited and allowed to burn for 1 min. The stovepipe is then slowly removed from the pan while the fuel continues to burn. After the stovepipe is removed, the foam blanket must either restrict the spread of fire for 5 min to an area not larger than 0.9 m2 (10 ft2) or flow over and reclose the burning area.
When the UL 162 test is passed, the agent, proportioning device, and discharge device become listed together. The fact that foam concentrate has a UL label does not mean it has been tested under all potential end-use conditions. The UL Fire Protection Equipment Directory must be referenced to determine with what equipment the concentrate has been tested and approved.
UL 162, Standard for Foam Equipment and Liquid Concentrates, is not an agent specification; therefore, there are no requirements for physical properties, such as film formation and sealability and corrosion resistance. Neither are there any provisions to test, on a large scale, the degree of dry chemical compatibility of an agent, or the effects of aging or mixing with agents of another manufacturer. Requirements for a positive spreading coefficient (greater than zero using cyclohexane) for film-forming foams recently have been implemented [23].
As a result of environmental issues related to AFFF, and the removal of products from the marketplace, the U.S. oil industry conducted a series of fire-fighting foam tests [24]. The purpose of the tests was to provide updated data on suitable Class B fire-fighting foam concentrates for use by the oil and petrochemical industry. The foam is used to extinguish large, in-depth flammable liquid fires in both hydrocarbon and polar solvent fuels. These tests were conducted using UL 162 as a guide. The tests were conducted using normal heptane as a baseline model, along with 93 octane gasoline, 93 octane gasoline with 10 % ethanol blend, and isopropanol anhydrous. The objective of the testing was to provide the oil industry with an updated list of foam concentrates that have passed the UL protocol with fuels more commonly found than heptane used in the test standard. This information can then be used to select foams for use at petrochemical facilities and to verify claims by different foam concentrate manufacturers regarding use of their products as suitable for all flammable liquids, including both hydrocarbons and polar solvents, found in the petrochemical industry today. The data from the Chevron Foam Concentrate Team [24] provide comparative results when the UL 162 method is used with different fuel substrates and a range of different concentrates.
U.S. Military Specification
The U.S. Military Specification, MIL-F-24385, is the AFFF procurement specification for the U.S. military and federal government. The U.S. military, in all likelihood, is the largest user of foam in the world. It is important to recognize that MIL-F-24385 is a procurement specification as well as a performance specification. Hence, there are requirements for packaging, initial qualification inspection, and quality conformance inspection, in addition to fire performance criteria. Equipment designs unique to the military, in particular U.S. Navy ships, also impact on the specification requirements (e.g., use of seawater solutions and misproportioning-related fire tests). These requirements have been developed based on research and testing at the Naval Research Laboratory and actual operational experience with protein and film-forming foams.
Table 47.5 summarizes the important fire extinguishment, burnback resistance, film formation, and foam quality requirements established by MIL-F-24385. The fire tests are conducted using 2.6 m2 (28 ft2) and 4.6 m2 (50 ft2) circular fire test pans. There are specific requirements to conduct a fire test of the agent after it has been subjected to an accelerated aging process (simulating prolonged storage) and after intentionally misproportioning the concentrate with water. In particular, the requirement to conduct a fire test of the agent at one-half of its design concentration is one of the most difficult tests. The 2.6 m2 (28 ft2) half-strength fire test must be extinguished in 45 s, only 15 s greater than allowed when the full-strength solution is used.
The physical and chemical properties evaluated for MIL-F-24385 agents are outlined in Table 47.6, along with the rationale for each test. These procedures have been developed based on experience and specific military requirements. For example, MIL-F-24385 requires that the agent be compatible with dry chemical agents. Dry chemical agents may be used as “secondary” agents in aviation and shipboard machinery space fires, for example, to combat three-dimensional fuel fires, where AFFF alone may have limited effectiveness. MIL-F-24385 requires that an agent’s compatibility with potassium bicarbonate dry chemical agent (PKP) be demonstrated. The burnback time of the foam in the presence of the dry chemical is measured. Also, the concentrate of one manufacturer must be compatible with concentrates of the same type furnished by other manufacturers, as determined by fire tests and accelerated aging tests.
Standards Outside the United States
The number of standards developed for foams outside the United States is quite substantial. A brief review of the literature yielded over 17 different standards and test methods [25]. Developments in the European community are reviewed here to provide examples of differences in test standards.
The International Civil Aviation Organization (ICAO) develops crash fire-fighting and rescue documents for its member bodies. The ICAO Airport Services Guide, Part 1, “Rescue and Firefighting,” describes airport levels of protection to be provided and extinguishing agent characteristics. Minimum usable amounts of extinguishing agents are based on three levels of performance: Level A and Level B. A performance Level C has been adopted. The amounts of water specified for foam production are predicated on an application rate of 8.2 L/min/m2 (0.20 gpm/ft2) for Level A, and 5.5 L/min/m2 (0.13 gpm/ft2) for Level B. Agents that meet performance Level B require less agent for fire extinguishment. ICAO foam test criteria are described in Table 47.7. Foams meeting performance Level B have an extinguishment application density of 2.5 l/m2 (0.061 gal/ft2) and 1.75 l/m2 (0.043 gal/ft2) for Level C. There are no surface-tension, interfacial-tension, and spreading coefficient requirements.
The International Organization for Standardization (ISO) has issued a specification for low-expansion foams, EN 1568–3 [26]. The specification includes definitions for protein, fluoroprotein, synthetic, alcohol resistant, AFFF, and FFFP concentrates. A positive spreading coefficient is required for film-forming foams when cyclohexane is used as the test fuel. There are toxicity, corrosion, sedimentation, viscosity, expansion, and drainage criteria. The fire test uses a 2.4-m (8-ft) diameter circular pan with heptane as the fuel. The UNI 86 foam nozzle is used for either a “forceful” or “gentle” application method at a flow rate of 11.4 L/min (3 gpm). The application rate is 2.4 L/min/m2 (0.06 gpm/ft2). For the greatest performance level, a 3 min extinguishment time is required. This extinguishment time results in an extinguishment application density of 7.6 l/m2 (0.19 gal/ft2).
The proposed ISO/EN requirements for extinguishing and burnback are summarized in Table 47.8. There are three levels of extinguishment performance and four levels of burnback performance. For extinguishing performance, Class I is the highest class and Class III the lowest class. For burnback resistance, Level A is the highest level and Level D is the lowest level.
Typical performance classes and levels for different concentrates are provided. Typical anticipated performance for AFFF is noted as Level IC, and Level IB for alcohol-type AFFF. For a fluoroprotein foam, performance is expected to be Level IIA for both alcohol-type and hydrocarbon-only concentrates.
Comparison of Small-Scale Tests
Table 47.9 outlines the large number of variables associated with foam performance and testing. These include factors such as foam bubble stability and fluidity, actual fire test parameters (e.g., fuel, foam application method and rate), and environmental effects. Even the fundamental methods of measuring foam performance (i.e., knockdown, control, and extinguishment) vary. For example, Johnson reported that FFFP fails the proposed ISO/EN gentle application tests because small flames persist along a small area of the tray rim [27]. As a result, the foam committees have proposed redefining extinction to include flames.
Given the variations and lack of fundamental foam spreading theory, it follows that tests and specifications for various foams and international standards have different requirements. The differences are reflected in Table 47.10, which compares four key parameters of MIL-F-24385, UL 162, ICAO, and ISO/EN standards for manual application (e.g., handline or turret nozzles). There is no uniform agreement among test fuel, application rate, the allowance to move the nozzle, and the extinguishment application density for AFFF. There is a factor of six difference between the lowest permitted extinguishment application density (MIL-F-24385) and the highest (ISO/EN). This significant difference is attributed, at least in part, to the fixed nozzle requirement in the ISO/EN specification.
No study has been performed to correlate test methods; given the significant differences in performance characteristics and requirements, it is unlikely that correlation between these test methods could be established, even when considering AFFF only. An AFFF that meets the ICAO standard could not be said to meet MIL-F-24385 without actual test data. The problem of correlating differences in small-scale tests was demonstrated by UL in a comparison of UL, MIL-F-24385, O-F-555B (U.S. government protein foam specification), and U.K. test methods [28]. In those tests, differences between different classes of agents (protein vs. AFFF) and between agents within a class (e.g., AFFF) were demonstrated. No correlations between test standards could be established.
The problem of correlation is compounded when a single test method is used in an attempt to assess different classes of foam (e.g., protein and AFFF). Attempts to use a single test method are problematic because of the inherent difference between these foams. That is, protein foams require air aspiration so that the foam floats on the fuel surface. This stiff, “drier” foam is viscous and does not inherently spread well without outside forces (e.g., nozzle stream force). AFFF, because of its film-formation characteristics, does not require the degree of aspiration that protein foams require. This heavier, “wetter” foam is inherently less viscous, which contributes to improved spreading and fluidity on fuel surfaces. This is related, at least in part, to the degree of aspiration of the foam. A more exact description of foam aspiration is appropriate. Thomas has described two levels of foam aspiration: (1) primary aspirated and (2) secondary aspirated [29]. Primary aspirated foam occurs when a foam solution is applied by means of a special nozzle designed to mix air with the solution within the nozzle. The consequence is foam bubbles of general uniformity. Air-aspirated foam refers to this primary aspirated foam. Secondary aspirated foam results when a foam solution is applied using a nozzle that does not mix air with the solution within the nozzle. Air is, however, drawn into the solution in-flight or at impact at the fire. Secondary aspirated foam is more commonly referred to as non-air-aspirated foam.
The correlation between foam solution viscosity and extinguishment time has been shown by Fiala, but the entire foam spreading and extinguishment theory has yet to be demonstrated based on first principles [30]. Thus, the test standards reference bench-scale methods that measure a factor of foam fluidity (e.g., spreading coefficient), but fail to recognize the total foam spreading system, including viscous effects. Fundamental understanding of foam mechanisms would promote the development of bench- and small-scale test apparatuses that potentially have greater direct correlation for predicting large-scale results.
There has been some criticism of the human element involved in many of the test methods. The human factor occurs when an operator is allowed to apply foam from a handheld nozzle onto the burning test fire. Personnel are also called on in some tests to qualitatively assess the percentage of fire involvement in the test pan during the burnback procedure. Using a fixed nozzle during a specification test eliminates the human element during extinguishment. For sprinkler applications, using a fixed nozzle is entirely appropriate and should yield results comparable to actual installations. For applications where movement is actually involved (e.g., fire-fighting handlines, crash-rescue truck turrets, and movable monitors on ships and at petrochemical facilities), the extinguishment densities in the fixed test application will generally exceed the densities found in actual applications in the field. (See Table 47.10 for differences in extinguishing densities for manual versus fixed applications.) Removal of the human element is certainly advisable from a test repeatability standpoint. However, removing the human element from approval fire tests has proved difficult. Both U.S. and Canadian military authorities have investigated the use of fixed nozzles. Both organizations concluded that tests with human operators resulted in better correlation with large fires and overall repeatability.
Quantitative methods for evaluating burnback performance have been described by Scheffey et al. [20] and been adopted in ISO/EN and Scandinavian (NORDTEST) test methods. These methods involve the use of radiometers to establish a heat flux during full test-pan involvement. After extinguishment, the radiometers measure the increasing flux as the burnback fire grows. This increasing flux due to burnback is compared against the original flux. A cutoff is established so that the maximum burnback time is the time for the burnback flux to reach some percentage (e.g., 25 %) of the original full-burning flux.
Critical Application Rates and Correlations Between Small- and Large-Scale Tests
The previous section described the application rate differences in standard test methods between AFFF, fluoroprotein, and protein foams. These application rate differences were established based on full-scale testing. For sprinklers, much of the fundamental application rate differences were established during testing conducted by Factory Mutual Research Corporation (FMRC). (See section on “Foam-Water Sprinkler Systems.”) For manual applications, tests in the aviation fire protection field provide the basis for the fundamental application rates. The application rates specified in test standards are usually rates lower than those used in actual practice (see Table 47.4). There are two reasons for this: (1) a factor of safety is used when specifying rates in actual practice and (2) differences between individual foam agents are more readily apparent at critical application rates. To demonstrate how application rates are developed and how specification tests correlate with large-scale results, an example from aviation fire tests will be used. This example is based on a review of foam fire test standards performed by Scheffey et al. for the Federal Aviation Administration (FAA) [25].
Tests were conducted by the FAA to determine application rates for a single-agent attack to achieve fire control (e.g., 90 % extinguishment of a fire area) within 1 min under a wide variety of simulated accident conditions. Two factors are important in addition to the application rate required for 1-min fire control: (1) the critical application rate, below which fires will not be extinguished independent of the amount of time an agent is applied; and (2) application density, which is the amount of foam per unit area to control or extinguish a fire.
Minimum application rates were originally developed by Geyer in tests of protein and AFFF agents [31]. These tests involved “modeling” tests with JP-4 pool fires of 21-, 30-, and 43-m (70-, 100-, and 140-ft) diameter. Large-scale verification tests with a B-47 aircraft and simulated shielded fires (requiring the use of secondary agents) were conducted with 34- and 43-m (110- and 140-ft) JP-4 pool fires. All tests were conducted with air-aspirating nozzles. The protein foam conformed to the U.S. government specification, O-F-555b, while the AFFFs used were in nominal conformance with MIL-F-24385 for AFFF. These tests were being performed at the time when the seawater-compatible version of MIL-F-24385 had just been adopted based on large-scale tests.
Figure 47.7 illustrates the results of the modeling experiments. The results show that, for a fire control time of 60 s, the application rate for AFFF was on the order of 1.6 to 2.4 L/min/m2 (0.04 to 0.06 gpm/ft2), whereas the application rate for protein foam was 3.3 to 4.1 L/min/m2 (0.08 to 0.10 gpm/ft2). The data indicated that the application rate curves become asymptotic at rates of 4.1 L/min/m2 (0.1 gpm/ft2) and 8.2 L/min/m2 (0.2 gpm/ft2) for AFFF and protein foam, respectively. Above these rates, fire control times are not appreciably improved. Likewise, critical application rates for fire control are indicated when control times increase dramatically. The single test with a fluoroprotein agent indicated that this agent, as expected, fell between AFFF and protein foam.
Fire control time as a function of solution application rate using protein foam and AFFF on JP-4 pool fires [31]
Large-scale auxiliary agent tests were conducted to identify increases in foam required when obstructed fires with an actual fuselage were added to the scenario. The results indicated that fire control times increased by a factor of 1 to 1.9 for AFFF and 1.5 to 2.9 for protein foams. It was estimated that the most effective foam solution application rates were 4.9 to 5.7 L/min/m2 (0.12 to 0.14 gpm/ft2) for AFFF and 7.5 to 9 L/min/m2 (0.18 to 0.22 gpm/ft2) for protein foam. This is the original basis of the recommendations adopted by ICAO of 5.5 L/min/m2 (0.13 gpm/ft2) for AFFF and 8.2 L/min/m2 (0.20 gpm/ft2) for protein foam. A rate of 7.5 L/min/m2 (0.18 gpm/ft2) was subsequently established for fluoroprotein foam. These application rate values are still used by FAA, NFPA, and ICAO to establish minimum agent supplies at airports.
Tests of AFFF alone were conducted by Geyer [32]. These agents, selected from the U.S. Qualified Products List (MIL-F-24385 requirements), were tested on JP-4, JP-5, and aviation gasoline (avgas) fires. Air-aspirating nozzles were used with different AFFF agents. Example results are shown in Fig. 47.8. Similar data were collected by holding the JP-4 fuel fire size constant at 743 m2 (8000 ft2) and varying the flow rates to develop application rate comparisons. These data are shown in Fig. 47.9.
Fire control and extinguishing times as functions of the foam solution application rate using AFFF at 250 gpm (946 L/min), 400 gpm (1514 L/min), and 800 gpm (3028 L/min) on JP-4, JP-5, and avgas fires [32]
Fire control and extinguishing times as a function of solution application rate using AFFF at 250, 400, and 800 gpm on 743-m2 (8000-ft2) JP-4 fuel fires [32]
Additional tests were conducted by Geyer et al. to verify the continuation of the reduction of water when AFFF agents were substituted for protein foam in aviation situations [1]. In 25-, 31-, and 44-m- (82.4-, 101-, and 143-ft-) diameter Jet A pool fires, AFFF, fluoroprotein, and protein foams were discharged with air-aspirating and non-air-aspirating nozzles. The data, summarized in Fig. 47.10, validated the continued allowance of a 30 % reduction in water requirement at certified U.S. airports when AFFF is substituted for protein foam.
Fire control time as a function of solution application rate for AFFF, fluoroprotein, and protein foams for Jet A pool fires [1]
Although some test criteria in standardized methods do not necessarily correlate directly with actual fire and burnback performance, small-scale test data for AFFF formulated to the U.S. military specification (MIL-F-24385) has been shown to correlate with large-scale fire test results. This is based on a comprehensive review of small- and large-scale test data [25]. In these data, a key variable was controlled; that is, all AFFF agents were formulated to meet MIL-F-24385. Ninety percent fire control times were used as the most accurate measure of fire knockdown performance, which were reported in all tests. The use of 90 % control times eliminates the variability of total extinguishment, which might be dependent on test-bed-edge effects or running fuel fire scenarios. Data for tests using air-aspirated or non-air-aspirated nozzles were combined. Low-flashpoint (less than 0°C [32°F]) fuels were evaluated. The evaluation included only tests where manual application was used, eliminating the variable of fixed versus manual application.
The effects of application rate on control and extinguishment times, as demonstrated in Figs. 47.7 through 47.10, were reconfirmed as shown in Fig. 47.11. Control time increases exponentially as application rate decreases, particularly below 4.1 L/min/m2 (0.10 gpm/ft2). Variability of the data is shown by the first standard deviation.
AFFF control time as a function of application rate [25]
The scaling of small fires with large fires is shown in Figs. 47.12 and 47.13, which relate the time needed to control the burning fuel surface as a function of fire size. The time needed to control a unit of burning area (s/ft2 [s/m2]), designated as the specific control time, is plotted as a function of fire size. For low (1.2 to 2.5 L/min/m2 [0.03 to 0.06 gpm/ft2]) and intermediate (2.8 to 4.1 L/min/m2 [0.07 to 0.10 gpm/ft2]) application rates, the specific control times decrease linearly as a function of fire area. These data are in agreement with data from Fiala, which also indicate decreasing specific extinguishment control times as a function of burning area for increasing application rates of AFFF. [30] Also, Fiala showed that, for a constant application rate, AFFFs have lower specific extinguishment times as a function of burning area than those of protein and fluoroprotein foams. Obviously, this linear relationship must change at very large areas; otherwise, the specific control/extinguishment time would go to zero. This is evidenced in Fig. 47.12, where the curve flattens at the high-area end of the plot.
Specific control times for AFFF at low application rates [25]
Specific control times for AFFF at intermediate application rates [25]
Figures 47.12 and 47.13 show that higher specific control times are required for MIL-F-24385 test fires (2.6 and 4.7 m2 [50 and 20 ft2]) compared to large fires. This is readily apparent as actual/control extinguishment times for the small fires are on the same order as results from large fires. FAA and NFPA criteria for minimum quantities of agent are also shown in Figs. 47.12 and 47.13. These criteria are expressed in terms of specific control time as a function of area by using the required control time of 60 s and the practical critical fire areas for airports serving different sizes of aircraft. The data indicate that specific control times with MIL-F-24385 agents are roughly equivalent or less than the specific control times established by NFPA and FAA requirements for large fire areas. This relationship is true even with the AFFF discharged at rates 25–75 % below the minimum NFPA/FAA discharge rate of 5.5 L/min/m2 (0.13 gpm/ft2). From these data, it can be concluded that a scaling relationship exists between MIL-F-24385 small-scale tests and actual large-scale crash rescue and fire-fighting applications. The MIL-F-24385 tests are more challenging than the larger tests in terms of specific control time, but this challenging test produces an agent that can meet NFPA and FAA requirements at less than the design application rate. This factor of safety accounts for variables in actual aviation crash situations, for example, running fuel fires, debris that may shield fires, and crosswinds that may limit foam stream reach.
Aviation Fire Protection Considerations
Historical Basis for Foam Requirements
The underlying principle in aviation fire protection is to temporarily maintain the integrity of an aircraft fuselage after a mishap to allow passenger escape or rescue. When an aircraft is involved in a fuel spill fire, the aluminum skin will burn through in about 1 min. If the fuselage is intact, the sidewall insulation will maintain a survivable temperature inside the cabin until the windows melt out in approximately 3 min. At that time, the cabin temperature rapidly increases beyond survivable levels.
Aircraft rescue and fire-fighting (ARFF) vehicles are designed to reach an incident scene on the airport property in 2–3 min, depending on the standard enforced by the authority having jurisdiction (AHJ). Having reached the scene in this time frame, the agent must be applied to control a fire in 1 min or less. The 1 min critical time for fire control is recognized by FAA, NFPA, and ICAO.
Minimum agent requirements on ARFF vehicles are established using the 1-min critical control time plus the anticipated spill area for the largest aircraft using the airport. A “theoretical critical fire area” has been developed, based on tests, and is defined as the area adjacent to the fuselage, extending in all directions to the point beyond which a large fuel fire would not melt an aluminum fuselage regardless of the duration of the exposure. A function of the size of an aircraft, the theoretical critical fire area was amended to a “practical critical fire area” after evaluation of actual aircraft fire incidents. The practical critical area, two-thirds the size of the theoretical critical area, is widely recognized by the aviation fire safety community, including FAA, NFPA, and ICAO. Vehicles must be equipped with sufficient agent and discharge devices to control a fire in the practical critical area within 1 min. Vehicles must also be equipped with a secondary agent (dry chemical or Halon 1211) for use in combating three-dimensional fuel fires.
The FAA has recently reviewed the basis of airport foam requirements. They considered new large aircraft containing significantly greater jet fuel loads (e.g., Airbus A380), and aircraft containing significantly greater fuselage combustible composite materials (in place of aluminum) [33, 34]. An fire hazard approach which assumed an unlimited size aircraft spill fire was considered, along with loss history. The “critical area” concept was found to be an acceptable and appropriate approach for establishing agent quantities. Research is continuing of the impact of composite materials.
Agent Quantities and Standards
The previous text on critical application rates described the rationale used to develop design application rates used in aviation fire protection. These rates are 5.5 L/min/m2 (0.13 gpm/ft2) for AFFF, 7.5 L/min/m2 (0.18 gpm/ft2) for fluoroprotein foam, and 8.2 L/min/m2 (0.28 gpm/ft2) for protein foam. Using these rates, the practical critical fire area and the 60-s control time criteria, minimum agent quantities are established for airports serving different size aircraft. These criteria are contained in NFPA 403, Standard for Aircraft Rescue and Fire-Fighting Services at Airports, and the FAA Advisory Circular 150/5210-6C, “Aircraft Fire and Rescue Facilities and Extinguishing Agents.” ICAO uses similar criteria. NFPA 403 has adopted the 4.6 m2 (50 ft2) fire extinguishment and burnback criteria from MIL-F-24385 for AFFF agents. UL test criteria are acceptable for protein and fluoroprotein foams. All certified airports in the United States must now use MIL SPEC AFFF when purchasing foam concentrate. Recognizing the limitations of its test methods for aviation applications, UL has deleted references to crash rescue fire fighting from the scope of UL 162, Standard for Foam Equipment and Liquid Concentrates. NFPA 403 recognizes that the standards for foam that it references are widely recognized throughout North America, but may not be recognized in other areas of the world. In particular, the ICAO test method has significantly different test parameters, including test fuel, application rate, and extinguishment density. The NFPA notes that it is incumbent on the national authority having jurisdiction to determine that alternative test methods meet the level of performance established by NFPA 403 test criteria.
NFPA 412, Standard for Evaluating Aircraft Rescue and Fire-Fighting Foam Equipment, provides field test methods to determine the adequacy of foam equipment on crash rescue vehicles. It includes criteria for foam expansion and drainage, and methods to determine foam solution concentration.
Expansion and Drainage
Foam expansion and drainage requirements of the current version of NFPA 412, Standard for Evaluating Aircraft Rescue and Fire-Fighting Foam Equipment, are shown in Table 47.11.
NFPA 412 references a 1600 mL foam sample collector, which was originally adopted by ICAO and ISO/EN. This single method is used to obtain expansion and drainage measurements for all types of foams in hope that similar success could be obtained in using a single fire test method for all foams. The multiple categories of foam test classification in Table 47.8 for the ISO/EN method show how difficult this has been to achieve. Given the different methods of foam flow over a fuel surface, it may not be practical to use a common fire test method predicated on the current means of testing. Further development of fundamental foam-extinguishing principles is recommended.
The 1600 mL expansion and drainage test method replaced two other methods where a 1000 mL cylinder or 1400 mL pan was used as the collection device. MIL-F-24385 still uses the 1000 mL collection method. This situation, plus other different test methods, makes direct comparison of expansion and drainage data difficult. Tests performed by Underwriters Laboratories (UL) identified differences among the three test methods based on expansion and drainage results [35]. UL found that expansion ratios remained the same but that drainage was quicker using the 1600 mL method compared to the 1000 mL method for film-forming foams. Drainage time increased (i.e., doubled) for the protein foams when the 1600 mL method was used compared to the 1400 mL pan method.
No direct correlations have been established between expansion, drainage, and fire-extinguishing performance. There is a relationship between foam drainage and burnback. Longer drainage times generally result in longer burnback times. Refer back to the “Fire Extinguishment and Spreading Theory” section for quantitative relationships.
The expansion and drainage data in Table 47.11 indicate the inherent differences between air-aspirated and non-air-aspirated film-forming foams. The data in Fig. 47.10 showed that non-air-aspirated AFFF was more effective at critical application rates than was air-aspirated AFFF. This conclusion was verified by Jablonski in tests with U.S. Air Force crash trucks, as shown in Fig. 47.14 [36]. Even so, there continues to be debate over air-aspirated and non-air-aspirated foam for manual applications involving aviation fuel spills.
Effects of AFFF aspiration on JP-4 pool fire control times [36]
Under certain conditions, non-air-aspirated AFFF is not as effective as air-aspirated AFFF. The results of the foam tests in the United Kingdom [37, 38] and the results from DiMaio et al. [39] described situations where air-aspirated AFFF resulted in better fire extinguishment performance than non-air-aspirated foam.
Given that one-to-one correlation between expansion, drainage, and fire-extinguishing performance is difficult to identify, there appears to be a lower limit where non-air-aspirated AFFF becomes ineffective. This has not been quantified, but it is speculated that poor performance occurs when the AFFF expansion ratio is less than 2.5:3.0, and drainage is difficult to measure, that is, nearly instantaneous. This is based in part on unpublished data from the Naval Research Laboratory on shipboard bilge AFFF sprinklers [40] and the results of the U.K. tests [37, 38]. The importance of this lower limit of foam aspiration is recognized in NFPA 412 criteria.
Foam Concentration Determination
The most common method of determining foam concentration in the field is by use of a handheld refractometer. The refractive index, n, is defined as
where
-
sin i = Angle of incidence
-
sin r = Angle of refraction
This is depicted graphically in Fig. 47.15.
Manufacturers report that the glycols in AFFF formulations create the necessary refractive characteristics to determine concentration. However, they also report that glycol has a potential detrimental impact on overall agent performance. Elimination of this compound might improve (slightly) the performance of AFFF, but the glycol is also needed as a fundamental component of agent mixing.
The refractive index of water at 20°C (68°F) is 1.333 (air has a refractive index of 1.0002926). Because the refractive index of a solution is proportional to the inverse of the solution density, and density is proportional to temperature, then
where T is the temperature. This relationship is illustrated in Fig. 47.16. Any refractive index measurements must be made considering temperature. Some handheld measurement devices are temperature compensated. It is good procedure to conduct concentration measurements at a constant temperature.
Effects of temperature on refractive index [41]
Other scales may be used. For example, the Brix scale is used as a measure of sucrose weight percent concentration. Units with this scale, commonly found in the food product industry, can be used to measure foam concentration. A typical range of a bench or handheld refractometer is 1.3000 to 1.7000.
NFPA 412, Standard for Evaluating Aircraft Rescue and Fire-Fighting Foam Equipment, describes a method to determine foam concentration using the refractive method. In NFPA 412, the preparation of three standard solutions is recommended: one at the nominal concentration, one at one-third more than the nominal concentration, and one at one-third less than the nominal concentration. A plot of the refractive scale reading against the known foam concentration is made on graph paper. This plot establishes a “calibration” curve against which foam samples from a vehicle or system can be judged. Because refractive index is linear, a calibration curve can be created by
This method is used by the U.S. Navy for checking proportioning system accuracy on board ships.
The limitations of the refractive index technique are described by Timms and Haggar [41]. The accuracy of the refractometer can become poor due to the focusing and setting of the refracted light junction on the crosshairs of the viewing window, and the reading of the graduated scale to four decimal places (where the scale is graduated to only three places). This effect is illustrated in Fig. 47.17, where a calibration curve for a 1 % AFFF concentrate was established using a straight line through the 50 % concentration point and the “water” reading by one of the experimenters. Note that the error between readings by the two experimenters at 1 % concentration exceeds 25 %. In this example, differences in the baseline water reading will create substantial error in the calibration curve. These differences are exaggerated with 1 % concentrates. At 3 % or 6 %, the experimental error in reading the refractometer, for field testing, is generally accepted as adequate.
Alternative methods for measuring AFFF concentration include total fluorine content, optical absorption methods, and electrical conductivity. Because neither the total fluorine content method nor the optical absorption method is suited to field use, the conductivity method has been proposed. Because foams contain electrolytes, their conductance, G, can be measured and described as
where R is resistance (ohms). Conductivity, σ, is conductance per unit length:
Because conductivity is directly proportional to temperature, conductivity increases with temperature (Fig. 47.18). Temperature compensation is appropriate when using this method.
Effects of temperature on the conductance of AFFF solutions [41]
Timms and Haggar showed the influence of the substrate water on both refractive index and conductivity [41] (Figs. 47.19 and 47.20). It is important to note the difference of the characteristic curve for a salt solution. AFFF actually reduces the conductivity of this highly conductive water. Note also that, although conductance may exhibit straight-line characteristics in the area of interest (0 to 10 %), the overall curves from 0 to 100 % are nonlinear.
Effect of substrate water on refractive index of AFFF solutions [41]
Effect of substrate water on conductivity of AFFF solutions [41]
The “sensitivity” of the two methods (i.e., refractive index and conductivity) was shown by these researchers by comparing the difference between readings for solutions of 3 % and 6 % divided by the reading at 6 %. The sensitivities for tap water show that the conductivity method is more sensitive than the refractive index measure (Table 47.12). In repeated readings of refractive index and conductivity, the foam concentration accuracy using conductivity was ±0.1 %, where the accuracy of the refractive index method was ±0.8 % (Table 47.13).
An evaluation was conducted by the U.S. FAA to comparatively test various conductivity meters and retractometers used in testing airport rescue and fire fighting (ARFF) vehicle foam-proportioning systems. [42] During the annual certification inspection of an airport fire department, refractometer and conductivity meter tests are conducted to test the foam concentrate and foam-proportioning systems of the ARFF apparatus. Historically, the refractive index method has been used to determine the proportioning of foam-generating systems. Because of the limited accuracy of the refractometer, particularly for assessing systems using 1 % and 3 % concentrates, the conductivity method is gaining more widespread use. Five conductivity meters were evaluated against a standard refractometer. A range of representative 3 % and 6 % foam concentrates were evaluated. Measured standard solutions prepared from the five conductivity meter tests show very close readings to one another. Typically, the units read within 0.2 mS. More importantly, all five conductivity meters exhibited the same trends in the readings from one foam product to the other. The refractometer data were not as consistent. A significant factor in the use of the refractometer is the fact that the readings can be interpreted differently by several evaluators during the same test. The digital readings from the conductivity meters removed the interpretive errors and proved to have very good repeatability between tests.
When evaluating the various conductivity meters for usability and accuracy, it was determined in the FAA study that all five units were considered better tools for inspecting the foam-proportioning systems than were refractometers. There were some aspects of the various conductivity meters that made some meters slightly better than others. The accuracies of the conductivity meters can be greatly affected by variations between the temperature of the solution and conductivity probe; care should be taken that conductivity measurements are made when the solution and conductivity probe are at the same temperature. One of the units automatically compensated for temperature.
The electrical conductivity method is now recognized in NFPA standards including NFPA 11 and NFPA 412. NFPA 412 cautions against the use of this method for seawater applications. The electrical conductivity method, used for process control in the chemical industry, has recently been adapted for use as a proportioning controller for AFFF systems.
New Airfield Protection Approaches
The U.S. Air Force Research Laboratory (AFRL) has done extensive research and development of novel ARFF-related fire fighting techniques in an attempt to develop smaller, lightweight, air-transportable ARFF vehicles that can be easily carried on cargo aircraft, such as the USAF C-130.
AFRL research has focused on the following technologies:
-
Ultra High Pressure System (UHPS)—This system utilizes high-pressure positive displacement plunger pumps to deliver AFFF at a nominal pump discharge pressure of 1500 psi. Turret residual pressures are in the 1100–1200 psi range, which in effect causes AFFF to be delivered as a foam “mist”. The applied foam has the characteristics of conventional AFFF delivery. It creates a foam blanket and aqueous film formation on the fuel surface, and may have the added fire suppression feature of small droplet mist, namely cooling, flame stripping, and oxygen displacement via water vapor formation.
-
Compressed Air Foam System (CAFS)—In a CAFS system air is injected under pressure into AFFF solution between the pump and the nozzle, so that expanded foam discharges from the nozzle. This allows greater control over the resultant foam expansion ratio and provides a uniform, more expanded, foam delivery to the fuel surface.
-
Combined Agent Fire Fighting System (CAFFS)—Recent testing has focused on the patented “Hydrochem” technology where dry chemical agent, typically PKP, and AFFF are discharged through a concentric nozzle design. PKP is discharged through a central orifice while AFFF, or CAFS, is discharged through the annular opening around the central dry chemical orifice. When flowing simultaneously, the AFFF/CAFS discharge carries the PKP in the center core of the discharge stream providing greater dry chemical discharge range then if discharged separately.
-
Tri/Quad Agent Systems—As a refinement of the twin agent concept widely used for flammable liquid fire fighting for over 30 years, recent delivery systems have been developed to discharge three or four agents (water, AFFF, dry chemical, gaseous/Halogenated agents) either simultaneous or consecutively, often through a single nozzle. This provides the nozzle man the option of easily selecting the desired agent for the particular fire scenario.
Two recent AFRL reports [43, 44] document testing at Tyndall Air Force Base of UHPS, CAFS, and CAFFS. Testing was conducted on fuel fires on a water substrate, fuel on gravel, fuel on soil/sod, and fuel on a hard surface. Since most tests over the years have been conducted with fuel on water (previously cited Geyer and NRL testing, for example), the fuel-on-water test results are described below.
Agent extinguishment tests were conducted against three different size JP-8 fires: 880, 3500 and 5100 ft2. Comparative data was generated against the performance of the primary USAF crash truck, the P-19. Agent flow rates were as follows:
• P-19 | 250/500 gpm AFFF |
• UHPS | 70–100 gpm AFFF |
• CAFs | 250–560 gpm AFFF |
• CAFFS | 125 gpm AFFF/3 pps dry chem. |
220 gpm AFFF/7.5 pps dry chem. |
A total of 114 fuel-on-water fire tests were conducted, with the results as shown in Table 47.14:
The UHPS delivery method produced a mean application density based on pool fire extinguishment of 0.014 gals/ft2, compared to a mean application density with the conventional P-19 of 0.044. The UHPS provided a lower application density. The USAF, after applying an appropriate safety factor to the discharge density, is deploying this technology.
Aircraft Hangar Protection
The two objectives of aircraft hangar protection are (1) protect aircraft and (2) prevent collapse of the hangar roof structure, which is usually unprotected steel. The protection of the aircraft is the principal concern, because its value is generally many times that of the structure. This concern is particularly true for advanced military aircraft. Historically, these protection systems have been deluge-type sprinkler systems with open-head nozzles. They are activated by rapid-response detection systems. Before the development of foam, water-deluge systems were used. The original foam-water sprinkler systems used protein foam. With the development of AFFF, research was performed to determine appropriate application rates and types of discharge devices. The research work, performed primarily by Factory Mutual Research Corporation (FMRC), provides the basis not only for current aircraft hangar protection criteria but also for other sprinkler suppression system criteria.
Overhead Sprinkler Protection
Before the advent of foam, hangars were protected by conventional spray sprinklers using water. Water-deluge systems having discharge rates on the order of 10.4 L/min/m2 (0.25 gpm/ft2) were used in conjunction with sloped floors and drains to protect aircraft. Even with these systems, activated by detection systems, burnthrough protection of aircraft fuselages (e.g., 1 min) could not be ensured. Ceiling temperatures in an 18.3-m-(60-ft-) high space on the order of 427 to 816°C (800 to 1500°F) have been recorded for fuel spill fires where this protection was provided. For a 121 m2 (1300 ft2) JP-4 fuel fire, 927°C (1700°F) ceiling temperatures have been recorded within 30 s of ignition prior to deluge system discharge.
Protein foam systems, discharging at a rate of 8.2 L/min/m2 (0.20 gpm/ft2), were an improvement on the water systems. Air-aspirating sprinklers were required to make effective protein foam. Because of the high centerline velocities of a pool fire plume, the foam flow from the perimeter toward the center of the fire was thought to be the dominant suppression mechanism [41].
With the development of AFFF, FMRC conducted a series of tests for the U.S. military to establish appropriate design parameters. In a series of baseline comparison tests, FMRC compared AFFF with protein foam. The tests consisted of 83.6 m2 (900 ft2) JP-4 pool fires in an 18.3-m (60-ft) high space. Air-aspirating, standard upright, and old-style upright sprinklers were evaluated at application rates of 4.1 to 8.2 L/min/m2 (0.10 to 0.20 gpm/ft2). In one test, a low-level turret nozzle discharging AFFF was used in conjunction with sprinklers discharging water. Table 47.15 summarizes the results of the AFFF tests. A comparison of Tests 4 and 5 with Test 3 indicates improved results from the use of standard sprinklers compared to foam-water sprinklers. At application rates of 6.6 L/min/m2 (0.16 gpm/ft2), the standard sprinklers were 1.3 to 1.6 times as effective in achieving extinguishment compared to air-aspirating foam-water sprinklers. At an application rate of 8.2 L/min/m2 (0.20 gpm/ft2), the extinguishment times with AFFF from foam-water sprinklers were comparable to results from protein foam tests. Rapid suppression with the turret nozzle (at 8.3 L/min/m2 [0.22 gpm/ft2]) combined with an overhead water system was demonstrated in Test 7. No adverse effects were evident from the water discharged from the overhead sprinklers after the foam ran out.
The superior performance of the standard sprinklers was attributed to more effective plume penetration by higher density foam particles. The maximum centerline velocities measured were 23.2 m/s (76 ft/s), with 15.2 m/s (50 ft/s) at the centerline of the fire. The fire plumes tended to bend due to air currents within the test building. Because the terminal velocity of the foam agents was estimated to be on the order of 9.1 m/s (30 ft/s) maximum, the droplets near the centerline never reached the fire. This result supports the theory that extinguishment occurs from the outside perimeter inward. Because foam droplets from standard sprinklers are about twice as dense as air-aspirated particles, the terminal velocities are greater. Greater velocities allow greater penetration of the fire plume. The same mechanisms explain why air-aspirated AFFF provides similar performance to protein foam. When the AFFF is air aspirated, there is no longer any advantage of increased droplet terminal velocity.
Additional work by FMRC established estimates for the terminal velocity of foam, as shown in Table 47.16 [46, 47]. Plume theory was used to estimate roughly that velocity on the order of 18.3 m/s (60 ft/s) could be expected in an 18.3-m (60-ft) high space with an 83.6 m2 (900 ft2) JP-4 fire. This estimate was in good agreement with the experimental results. Based on an average foam particle diameter of 6.3 mm (0.25 in.), a maximum terminal velocity of 7.3 m/s (24 ft/s) could be expected. For a JP-4 pool fire, this translates into a 0.7-m2 (8-ft2) maximum fire size before plume penetration is not possible.
The practical significance of AFFF discharged through non-air-aspirating sprinklers was demonstrated by Breen et al. [46] Air-aspirating sprinklers require 207 kPa (30 psi) nozzle pressure to be effective. Standard sprinklers can discharge effective AFFF solution at pressures as low as 69 kPa (10 psi). This capability had important retrofit considerations where foam-proportioning system losses could be made up through reduced sprinkler pressures.
Additional tests were conducted with closed-head sprinklers in an 18.3-m (60-ft) high hangar [48]. Potential cost benefits would have resulted from reduced hardware costs and unwanted discharges from deluge systems. These tests demonstrated that this concept was not feasible for the hangar scenario because of the large number of sprinklers that opened during the 83.6 m2 (900 ft2) fire tests.
The superior performance of standard sprinklers compared to air-aspirating sprinklers is reflected in the criteria of NFPA 409, Standard on Aircraft Hangars. If standard sprinklers are used with AFFF, the design application rate for overhead deluge systems may be reduced to 6.6 L/min/m2 (0.16 gpm/ft2) from 8.2 L/min/m2 (0.20 gpm/ft2) required for air-aspirated sprinklers. This decrease represents a 20 % reduction in foam required when standard sprinklers are used.
It should be noted that AFFF discharging from sprinklers cannot be assumed to control a three-dimensional Class B fire. In tests conducted by the U.S. Navy, AFFF sprinklers were discharged on a simulated ruptured fuel tank fire [49]. Deluge, air-aspirating sprinklers were discharged at 6.5 L/min/m2 (0.16 gpm/ft2) from a 8.5-m (28-ft) high simulated hangar. The three-dimensional fire consisted of 28 L/min (7.5 gpm) of marine diesel (simulating JP-5) flowing down over a shielded cascade assembly. The assembly simulated a ruptured fuel tank below a damaged aircraft wing, which prevented direct AFFF application to the running fuel fire. Although the contiguous pool fire was suppressed, the running fire was not. It was also found that delaying AFFF discharge time for this scenario had an adverse effect on suppression.
Care must be taken in designing AFFF sprinklers for both pool fire suppression and extinguishment of other hazards. In fire tests for shipboard vehicle storage areas, a fire scenario involving a Class B pool fire (marine diesel, simulating vehicle diesel fuel or JP-5) and ordinary combustibles was performed [50]. Tests were conducted in an 8.5-m (28-ft) high space with air-aspirating sprinklers discharging 6.5 L/min/m2 (0.16 gpm/ft2). The Class A fuel load was simulated by a 1.6 m (6 ft) stack of wood pallets. The Class B pool fire was readily controlled and extinguished, but the Class A pallet fire was not controlled or suppressed. It was concluded that a higher sprinkler application rate was required to control/suppress the Class A fire. This is consistent with NFPA 13, Standard for the Installation of Sprinkler Systems, requirements for a minimum application rate of 8.1 L/min/m2 (0.2 gpm/ft2) or greater for wooden pallets stacked 1.8 m (6 ft) high or more. Fires within actual vehicles will likely require a higher sprinkler application rate to prevent spread to adjacent vehicles [51].
Low-Level Application of AFFF
With the increase in wingspan areas of large aircraft, it was recognized that significant damage could occur before extinguishment of the pool fire underneath the wing. Using overhead sprinklers only, FMRC demonstrated the times required for the foam to spread and extinguish fires (see Table 47.15). The concept of low-level application of foam, using monitors or turret nozzles, was developed to reduce extinguishment time where shielded fires may occur. This concept was later extended to include side-mounted nozzles and discharge outlets, and flush-mounted nozzles installed in a floor or deck.
These systems are effective because AFFF solution droplets do not have to penetrate the fire plume. They also typically deliver, at spot locations, high densities of foam. A high density allows the foam to gain a “bite” or toehold on the fire. Low-level AFFF systems have been used successfully for over two decades on U.S. Navy air-capable ships, protecting flight decks and special hazard areas.
Table 47.17 summarizes fire test data for low-level application of AFFF. As seen, control and extinguishment times are quite rapid. NFPA 409, Standard on Aircraft Hangars, criterion of 4.1 L/min/m2 (0.10 gpm/ft2) for low-level applications is based on a fire control time of 30 s and extinguishment in 60 s. Data indicate that a JP-5 pool fire can be 90 % controlled in 60–90 s and 99 % extinguished in 2 min when an application rate of 2.4 L/min/m2 (0.06 gpm/ft2) is used. The system can be effective at rates as low as 1.6 L/min/m2 (0.04 gpm/ft2). For low-flashpoint fuels (e.g., avgas), control time increases. Control and extinguishment times can be reduced by increasing the application rates on JP-5 fuel fires. Based on these results, the U.S. Navy adopted an AFFF application rate of 2.4 L/min/m2 (0.06 gpm/ft2) for protecting aircraft carrier flight decks [56].
Although they may help control a three-dimensional (spill) fire, low-level application systems cannot be assumed to suppress totally a running fuel fire. Running fuel fires at a spill rate of 189 L/min (50 gpm) are typically used in U.S. Navy flight-deck suppression tests using the flush-deck system. The running fuel fire, shielded by simulated aircraft debris, requires aggressive handline attack for extinguishment [57].
Obstructions, such as parked vehicles, may block low-level nozzles. Testing for a flight-deck weapons staging area showed that a side-mounted low-level system could be effective even when nozzles were obstructed [55]. In these tests, 5 of the 12 deck-edge nozzles were obstructed to simulate vehicle tires blocking edge-mounted nozzles. Even with 40 % reduction, the fire was controlled and extinguished in less than 1 min (compared to 15–30 s when unobstructed). As with overhead sprinklers, low-level AFFF nozzles should not be relied on to extinguish three-dimensional Class B fires.
Cost of installation, maintainability, and reliability are factors when considering a low-level application system. Reliability issues with turrets/monitors have been identified by both FMRC and the U.S. Navy. The flush-deck system adopted by the U.S. Navy took considerable effort before a high degree of reliability and maintainability could be achieved. This open deluge nozzle, originally installed as a water washdown nozzle, incorporates a ball-check feature in the nozzle orifice to prevent debris from clogging the nozzle. Clean-out traps are installed in system piping for maintenance. Pop-up nozzles have been proposed as an alternative to flush-deck nozzles. These nozzles have their own reliability and maintainability issues. Unless there are very high costs associated with the loss of an aircraft, in-floor or flush-deck nozzles are generally cost-prohibitive for commercial aviation facilities. For high-risk/cost applications, in-floor nozzles may be justified. This may be the case for advanced military aircraft; for example, research has been performed on an inverted deluge system that not only can suppress a pool fire but also can cool exterior combustible components of the airframe. Initial installations have suffered from design and installation problems. [58] Lack of experience with these types of systems was the significant single cause of problems with these systems. Acceptance testing and maintenance were found to be lacking.
Side-mounted nozzles are the most reliable systems, consisting of open-pipe or -spray nozzles. The spreading rate of foam from an aspirated open-pipe system increases control and suppression time. Open-spray nozzles can be very effective, but their reach is limited.
New Hangar Fire Protection Design Concepts
Issues related to asset protection, reliability of fixed systems, and environmental impact led the U.S. Navy to reevaluate its approach to hangar fire protection systems. A goal was established to install reliable and easily maintained fire protection systems that prevent damage to the hangar structure and to aircraft not directly involved in an initial spill fire ignition. This goal resulted in a multidiscipline study to address all associated technical issues.
All military service branches in North America have been plagued with false activation involving foam-water-deluge sprinkler systems over aircraft with open cockpits. These false activations have been caused by numerous sources including lightning strikes that introduced transient voltage spikes into the fire alarm system; water hammers in aging underground water distribution systems; accidental releases by maintenance personnel; deliberate acts of vandalism; accidental activation of manual pull stations; failure of pressure relief valves at pumping stations; roof-water leakage into overhead heat detection systems; and false activation of fire detection systems. This situation prompted the pursuit of alternative fire protection designs that would provide the desired level of protection.
Alternative designs included the use of closed-head AFFF overhead sprinkler systems and greater reliance on low-level monitor nozzle AFFF systems as the primary extinguishing component as described in the previous section. Low-level systems were originally designed to provide supplementary protection for the area shadowed from the overhead system by large wing areas. In pursuing these alternative designs, technical and operational issues and limitations of both existing and proposed new systems were identified:
-
Thermally activated systems may result in unacceptably high damage to assets prior to fire control/extinguishment, particularly in very high bay hangar ceilings (see Gott et al. [59]).
-
Although it is readily accepted that conventional hangar fire protection systems were not designed to extinguish a three-dimensional fire, some fire protection engineers believed that AFFF extinguishing systems could be designed to control a spill fire and limit the area of the fire to only those aircraft intimate with the initial ignition source.
-
Different aviation fuels are now commonly being used, for example, JP-5 and JP-8 are now the predominant fuels, compared to the lower flashpoint JP-4 previously used.
-
Low-level AFFF monitor nozzle systems are
-
Relatively inefficient in terms of pattern distribution
-
Unreliable
-
Susceptible to blockage by equipment
-
Commonly found out of service in the field
-
-
Any new AFFF low-level nozzle should be designed for minimal overspray and should not be significantly impacted by water discharge from any water-only protection system.
-
Optical fire detectors are
-
Prone to false alarms
-
Currently tested, listed, and approved using fuels that are not typical in aviation
-
Subjected to few if any sources of false alarms in currently recognized approval standards
A concept was developed by the U.S. Navy to meet the desired performance goals. This concept included the following:
-
-
Use of low-level AFFF deluge nozzles, having minimal overspray, to control/extinguish liquid fuel pool spill fires
-
Operation of the low-level AFFF system using improved optical detectors designed to
-
Be highly immune to false alarms
-
Rapidly detect JP-5 fuel spill fires
-
-
Installation of a quick-responding, closed-head, wet-pipe sprinkler system in the hangar ceiling
-
Implementation of lessons learned from all military hangar design experiences in a comprehensive, new, improved design
Most of the research and development associated with the process has been completed and is described in Gott et al. [59], Szepezi et al. [60], Back et al. [61], Gottuk et al. [62], Scheffey et al. [63], and Parker [64]. Two aspects of U.S. Navy research and development are germane to the performance of AFFF. The first involves the performance of AFFF when subjected to water spray from sprinklers. The second is a developmental effort initiated to design a reliable, low-profile AFFF nozzle that could be installed in the floors of hangars.
Twenty-three full-scale fire tests were conducted to evaluate the effects of overhead water sprinklers on AFFF foam blankets [61]. One AFFF application rate (4.0 L/min/m2 [0.1 gpm/ft2]) and two sprinkler application rates (6.5 and 10.2 L/min/m2 [0.16 and 0.25 gpm/ft2]) were included in this evaluation. The tests were conducted on a range of spill fires. The spill fires were produced using either JP-5 or JP-8 aviation fuels and were evaluated on a concrete pad with similar drainage characteristics typical of navy hangars. The spill fires continued to burn (i.e., were shielded) during water/foam application. The heating effect on the burnback resistance of foam, with and without sprinkler water application, was evaluated.
The results show that the use of water sprinklers in conjunction with a low-level AFFF fire suppression system (with an application rate of 4.0 L/min/m2 [0.1 gpm/ft2]) had minimal effects on the ability of the system to suppress the fire and resist burnback. In all tests, the low-level AFFF system was capable of quickly extinguishing the test fire (control ~30 s and extinguishment ~1 min) independent of the sprinkler application rate. The time required for the fire to burnback across the fuel surface was apparently a function of the drainage characteristics of the hangar and was only slightly affected by the application of water through the overhead sprinklers. The tests also show that the flashpoint of the fuel has an effect on the control, extinguishment, and burnback resistance capabilities of the system. Although the burnback times for the lower flashpoint fuels were faster than the higher flashpoint fuels, the duration of protection was not significantly altered. These tests show that overhead water sprinklers have minimal effect on AFFF foam blankets, independent of the test fuel, particular fire, and sprinkler application rate. A combined low-level AFFF extinguishing system operating in conjunction with an overhead water sprinkler system provided adequate burnback protection during AFFF discharge, but this protection may be lost shortly (a few minutes) after the end of AFFF discharge.
The new low-level fire-extinguishing system was designed to discharge AFFF adequately across a hangar floor, to be less likely to be affected by obstructions, and to reduce the likelihood of damage to exposed aircraft electronic equipment [63]. To achieve these objectives, the nozzle was designed to
-
Produce a nominal AFFF application rate of 4.0 L/min/m2 (0.1 gpm/ft2)
-
Operate at a nominal pressure of 2.8 bar (40 psi)
-
Provide coverage to a distance of 7.6 m (25 ft) from a hangar floor drainage trench (centerline of two parallel trenches spaced 15.2 m [50 ft] apart)
-
Spray AFFF so that the pattern height does exceed 0.3 m (1 ft) above the deck
The nominal AFFF application rate of 4.0 L/min/m2 (0.1 gpm/ft2) was selected based on current design practices as described in the previous two sections of the chapter. The nozzle operating pressure was selected based on standard, commercially available pump performance curves and preliminary estimates of friction loss for the system.
Over 50 nozzles were evaluated for this application [64]. Testing of these nozzles indicated that, although a limited number of commercially available nozzles could meet the design requirements, manufacturing, installation, and operation of these nozzles under normal hangar conditions were not feasible. Existing pop-up nozzles were not designed for the high flow rates or spray characteristics required of this application. As a result of these deficiencies, a prototype nozzle was developed. The prototype concept was subsequently developed into a commercially available nozzle. Foam pattern, distribution, and flow tests were conducted by Underwriters Laboratories Inc. on a nozzle with a flow coefficient of 22.6 k (gpm/psi1/2).
There is no universal agreement on the proper approach to military hangar fire protection in North America. For example, the U.S. Air Force recognizes the use of high-expansion foam. Many of these systems have recently been installed.
The Canadian Ministry of Defense (MoD) is using compressed-air foam, or CAF. The primary advantage of CAF systems is for situations where there is an extremely limited water supply. Unlike traditional foam systems where air aspiration occurs at or near the discharge device, CAF systems inject air prior to the discharge device [65, 66]. Foam is generated by injecting air under pressure into a foam solution stream. As the solution moves through the piping system, foam is produced by the combined momentum of the foam solution and the injected-air stream in the hose or pipe. In the hangar system, AFFF is used as the foam concentrate, proportioned at 2 % compared to 3 % for traditional foam sprinklers. Also, the effective application rate (foam solution, L/min/m2 or gpm/ft2) is lower than similarly listed low-expansion foam sprinkler systems. NFPA 11, Standard for Low-, Medium-, and High-Expansion Foam, recognizes this system and provides design and installation requirements.
Additionally, the U.S. Army has evaluated the use of early suppression fast-response (ESFR) closed-head water sprinkler protection for helicopter hangars [67]. FMRC concluded that both 93 °C (200 °F) temperature-rated ESFR sprinklers discharging at 345 kPa (50 psig) (41 L/min/m2 [1.0 gpm/ft2]) and 517 kPa (75 psig) (49 L/min/m2 [1.2 gpm/ft2]) and temperature-rated, k = 5.6 (gpm/psi½) quick-response sprinklers at 345 kPa (50 psig) (16 L/min/m2 [0.40 gpm/ft2]) can provide adequate fire protection for the hangar against a 61-m2 (200-ft2), 473 L (125 gal) JP-4 aviation fuel-pan fire. For some tests, fuel depletion was necessary for the fire to be controlled.
New fire detection technology, potentially applicable to hangar fire protection, has become available. Video image flame detection (VIFD) is a software-based method of flame detection that can be implemented by a range of video image analysis techniques. VIFD systems can analyze images for changes in features such as brightness, contrast, edge content, loss of detail, and motion. The detection equipment can consist of cameras producing digital or analog (converted to digital) video signals and processing unit(s) that maintain the software and interface to the fire alarm control unit. The technology potentially speeds detection, while improving immunity to false alarms. NFPA 72®, National Fire Alarm Code®, now recognizes this technology.
Foam-Water Sprinkler Systems
This chapter has dealt with foam characteristics, foam concentrate, test standards, and manual application techniques. In particular, applications in the aviation industry were described. The text on aircraft hangar protection addressed the concept of fixed foam protection systems. Much of the foam-water sprinkler system test data was originally developed for aircraft hangars. Herein, foam-water sprinkler system design criteria for other applications are described. Again, emphasis is placed on AFFF systems because they are more effective for extinguishment than protein or fluoroprotein systems.
Codes, Standards, and Regulations
Overhead foam-water sprinkler systems, as specified in the NFPA standards, are generally designed to serve dual purposes: (1) to control and/or suppress a fuel spill fire and (2) when the foam runs out, to cool materials with water. Because the systems are designed to provide protection for flammable/combustible liquid hazards and ordinary combustibles, the specified application rates reflect this dual-protection approach. Table 47.4 shows the fundamental application rates used by Underwriters Laboratories on hydrocarbon fuel fires to evaluate sprinklers discharging foam-water. The fire must be extinguished within 5 min for AFFF discharged at 4.1 L/min/m2 (0.10 gpm/ft2) for standard sprinklers and 6.6 L/min/m2 (0.16 gpm/ft2) for agents discharged from foam-water sprinklers (air aspirating). However, because most deluge and closed-head sprinkler systems are installed in industrial occupancies for property protection concerns, they must usually meet highly protected risk (HPR) insurance requirements. As a result, the NFPA standard for deluge and closed-head AFFF systems (NFPA 16, Standard for the Installation of Foam-Water Sprinkler and Foam-Water Spray Systems) requires 6.6-L/min/m2 (0.16-gpm/ft2) minimum water application. This water application rate also provides a safety factor over the 4.1 L/min/m2 (0.10 gpm/ft2) rate at which AFFF discharged from sprinklers is effective on pool fires. The safety factor is reflected in Table 47.4 under the column heading Minimum Application Rate.
Table 47.18 summarizes current requirements from NFPA standards. NFPA 11, Standard for Low-, Medium-, and High-Expansion Foam, is geared toward petroleum and chemical industry protection. Previous requirements from NFPA 11 allowed 4.1 L/min/m2 (0.10 gpm/ft2) for loading racks, for example, tank truck loading facilities. The latest requirements for NFPA 11 eliminate this design criterion and reference NFPA 16 requirements, which require 6.6 L/min/m2 (0.16 gpm/ft2). In special situations, 4.1 L/min/m2 (0.10 gpm/ft2) is permitted by NFPA 11, but only where there is low-level or manual application for a hydrocarbon fuel spill. NFPA 16 is consistent in requiring 6.6 L/min/m2 (0.16 gpm/ft2); it references other NFPA standards for special exceptions, for example, NFPA 409, Standard on Aircraft Hangars, and NFPA 30, Flammable and Combustible Liquids Code. NFPA 409 requirements were previously discussed. Chapter 48 provides an example for calculating foam quantities based on design application rates and areas to be protected.
With the publication of the 1998 edition of NFPA 11, marine foam application was specifically addressed. Foam application rates are required to be not less than the greatest of that required for deck spills, the largest tank, or the largest monitor solution flow rate as shown in Table 47.19 for hydrocarbon fuels and Table 47.20 for polar solvents. For polar solvents, standardized fire tests are used to determine the minimum foam design application rate for the most difficult extinguishment case. Foam concentrates for hydrocarbon fuels must be approved using a 9.29-m2 (100-ft2) fire test similar to UL 162. The fixed-nozzle gasoline fire test has an extinguishment application density of 12.2 l/m2 (0.30 gal/ft2).
Model building and fire codes in the United States have adopted AFFF protection criteria for the storage of flammable and combustible liquids. Criteria of insurance companies are usually similar to the NFPA requirements, but this needs to be verified. Insurance authority guidelines should be referenced for specific projects, because there can be differences in protection criteria.
Protection of Stored Flammable and Combustible Liquids
Flammable and combustible liquids are stored in containers ranging in size from less than one quart to several hundred gallons. These liquids may be stored for display in a retail outlet or “superstore,” stored for distribution in a general-purpose warehouse housing many different combustibles, or stored in “liquid” warehouses containing large quantities of the liquid. NFPA 30, Flammable and Combustible Liquids Code, addresses these situations. This code includes requirements for tank storage, piping systems, containers, and operations. Criteria for suppression system protection is addressed in the sections dealing with container storage.
The protection of flammable and combustible liquids is a function of many factors, including the liquid properties, the ignition (which can be a factor of the storage occupancy), the packaging system (e.g., stored in cardboard cartons), the container design and material (e.g., steel, plastic, glass, fiberboard), and the arrangement of storage (e.g., rack versus pallet, storage height, aisle width, and mixture of other combustibles in the array). Based on these factors, a suppression system is provided to control or suppress the anticipated fire and protect the structure. The system may be designed to (1) control a fire so that the fire department can ultimately extinguish or suppress the burning material or (2) suppress the fire. Variables in suppression system design include application rate, fire fighting agent, and sprinkler orifice size, spacing, response time index (RTI), temperature rating, and use of in-rack sprinkler protection.
The basis of protection criteria in NFPA 30 is now well documented. Fire test references and associated citations in technical literature are now included with all protection criteria [68]. The basis of the protection criteria can now be directly linked to test data or engineering extrapolations of the data. Material in Appendix E of NFPA 30 provides guidance and an example test protocol for evaluating protection of liquids stored in the containers. This includes consideration of the source of the fire, which may be a “point” ignition (i.e., small ignition) or a large spill/three-dimensional fire. Depending on other variables, such as container type and packaging material, one of these scenarios may be more difficult to protect. Annex E of NFPA 30 provides detailed guidance on this subject.
Stored liquids may be protected using water sprinklers, foam, or other approved methods. Figure 47.21 shows a conceptual grouping of water and AFFF protection methods as a function of container type and storage method for water protection of liquids. The reader should consult Nugent [68]. The basis of AFFF protection is described in the following sections.
Protection of Drum and Tank Storage
Some of the earliest work using AFFF sprinklers involved the protection of 208 L (55 gal) drums. In work conducted at Factory Mutual Research Corporation, sponsored by Allendale Insurance, Factory Insurance Association (FIA), and the 3 M Company, the effectiveness of standard sprinklers supplied with AFFF for controlling drum fires was determined [69]. Five fire tests were conducted in simulated flammable liquid-drum storage using two types of storage arrangements. Three tests were conducted with two-, three-, and four-high palletized drum storage, respectively. Two tests were conducted with five-tier high-rack storage of palletized drums.
In all tests, a heptane fuel supply simulated leakage from the upper level of storage. Except for one rack-storage test that used a 57 L/min (15 gpm) spill rate, fuel spillage was 7.6 L/min (2 gpm). Ceiling protection employed high-temperature sprinklers at discharge rates of either 12.3 or 24.6 L/min/m2 (0.30 or 0.60 gpm/ft2). In-rack supplemental protection for the rack-storage tests was provided at three levels, with ordinary temperature sprinklers each discharging 113 L/min (30 gpm). The success of each test was based on storage stability, that is, no pile collapse, and limitation of drum pressure to 104 kPa (15 psig).
AFFF was effective in controlling spill fires on the floor. The exception was in areas not reached by the discharge from operating sprinklers, where the flow of foam was blocked by pallets. Protection was not effective on the three-dimensional spill fires. Fire exposure and resultant pressure development within drums was more severe with increased clearances between storage and sprinklers due to greater delays in sprinkler operation.
Generally, results were considered good in the rack-storage tests, where in-rack sprinklers were provided in each tier. For palletized storage, the AFFF protection controlled the floor fire although pallets hindered the spread of foam. Ceiling sprinklers alone did not adequately protect palletized storage where an elevated spill resulted in a three-dimensional fire within the pile.
The results of these tests were used, along with engineering judgment, to develop AFFF protection criteria in NFPA 30, Flammable and Combustible Liquids Code. AFFF protection of 12.3 L/min/m2 (0.30 gpm/ft2) at the ceiling for rack protection of metal drum/tank storage up to 7.6 m (25 ft) high is specified. In-rack protection (e.g., sprinklers in alternating tiers or every tier) is a function of the liquid (flashpoint) container style (relieving vs. nonrelieving), and capacity of the container.
The results of the original Factory Mutual (FM) drum tests were extended in a series of tests conducted by Southwest Research Institute [70]. The objective was to test the effectiveness of relieving-style steel drums and varying degrees of overhead sprinkler protection to mitigate fire hazards associated with the storage of flammable liquids. Nylon plugs inserted in the 5.1 cm (2.0 in.) pour hole and 1.3 cm (0.5 in.) vent hole were designed to melt under fire conditions, allowing the drum to vent any built-up pressure. Heptane, a Class IB flammable liquid, was used as the stored commodity.
Tests were designed to model credible, worst-case loss scenarios involving the 208 L (55 gal) storage of the commodity. The fire modeled the accidental puncture of a full drum, and either an immediate or a delayed ignition source. Sprinkler suppression of the fire was monitored for the duration of the spill, and until flames were either under control or completely extinguished. Commodity was stacked in a 3 × 3 palletized array, to varying heights (2, 3, or 4 high), and protected with varying sprinkler types and densities.
The relieving-style closures were successful at mitigating the hazards associated with overpressurizing drums during a fire. The installed suppression systems were capable of either extinguishing or controlling the fire for the duration of the spill. A summary of the successful protection configurations for the commodity tested is provided in Table 47.21.
The fuel spill rate (7.6 vs. 56.8 L/min) was found to have a substantial impact on the fire exposure of the drums. When taken in conjunction with the effect of the ignition scenario, the fuel spill rate had a strong influence on the number of initial heads operating and on the duration of the fire exposure. The ignition of the fuel source also played a role in the number of heads actuated during a test. The immediate ignition of fuel (simulating a spill onto an existing ignition source) resulted in a slower growing fire, actuating fewer sprinkler heads. Alternately, an ignition scenario where a 7.6 L spill was allowed to develop prior to ignition resulted in the actuation of four heads within the first minute of fire exposure. A comparable test with the immediate ignition scenario resulted in only two heads operating in a time in excess of 2 min and 30 s. The involvement of fewer sprinkler heads and the prolonged fire exposure implied that the immediate ignition provided a more challenging scenario.
The AFFF system used in the test program was successful in generating a good blanket of foam within 1 to 2 min of actuation (depending on the number of initial heads actuated). The foam quality was such that it was free to flow over drum heads, providing cooling to the tops and sides of drums, and forming a blanket at the floor to suppress pool fires. The foam system (in Tests 6 through 8) was also effective at limiting the fire at the fuel introduction point, periodically extinguishing the source. In general, by the time fuel flow to the array was complete, the foam system had suppressed all pool fires, leaving only small pallet fires for manual suppression.
An initial survey of closure obstruction versus venting phenomenon indicated that there was little or no effect on the obstruction of a plug and its ability to vent. This is indicated by the low number of drums that exhibited bulging during tests. The bulging of a drum indicates an unusual buildup of pressure. This phenomenon was not consistent, even in drums where both closures were obstructed. It was also noted that even partial venting of either opening was sufficient in reducing the pressure within the drum.
Drum deformation was recorded on a subjective basis. Typical deformation involved bulging of the head of the drum by 1.2 to 2.5 cm (0.5 to 1.0 in.). In some cases, deformations were seen on the order of 7.6 to 10.2 cm (3 to 4 in.) with some unfurling of the head chime.
It is difficult to attribute the level of deformation with a corresponding internal pressure. Several drums were deformed to a degree consistent with hydrostatic pressures of 207 to 241 kPa (30 to 35 psi); however, no pressures of this magnitude were recorded. A possible reason for higher levels of deformation at lower pressures may lie in the exposed temperatures of the drums. Several drums were subjected to uneven heating. The uneven heating phenomenon is present where a drum is located directly above a pallet containing venting drums. This scenario sets the subject drum over an isolated flame source, heating it from below.
The results of these tests have been included in the NFPA 30 protection criteria tables for palletized steel drum storage up to four high when protected using AFFF. The use of listed relieving devices is recommended; the exact details of this listing procedure are being developed.
Liquid Spill and Container Storage
Table 47.22 summarizes early closed-head AFFF sprinkler testing on a flammable liquid spill [71]. In a 9.1-m (30-ft) high ceiling room, n-heptane was discharged in a simulated spill to create a three-dimensional spill and a two-dimensional pool fire. Fuel spill rate was varied up to 113 L/min (30 gpm). AFFF application rates were 4.5 to 12.3 L/min/m2 (0.11 to 0.30 gpm/ft2). The primary variables were the temperature rating of the sprinkler and the application rate. Non-air-aspirating sprinklers were used. The data show that high-temperature-rated sprinklers, activated at about the same time as ordinary temperature sprinklers, controlled the fire in comparable times (roughly 2 min control time), and resulted in significantly fewer sprinklers operating (7 versus 32). An increase in application rate when the high-temperature sprinklers were used resulted in fewer heads operating, but did not decrease overall control and extinguishment time. Fires were controlled, but not totally extinguished as a result of the three-dimensional spill fire. These tests showed the advantage of using high-temperature-rated sprinklers in AFFF closed-head suppression systems.
In response to the concerns related to flammable liquid warehouse protection, the National Fire Protection Research Foundation (NFPRF) initiated the International Foam-Water Sprinkler Research Project. The objectives were to document the performance of foam-water sprinkler systems designed for real-world storage and ignition scenarios and provide a design basis and minimum design parameters for foam-water sprinkler systems. Five tasks were performed, including a literature search, range-finding tests, and large-scale tests involving palletized and rack storage of liquids.
The literature search identified over 1100 sources of information related to flammable liquid fires and foam protection, but a dearth of data related to water and foam-water sprinkler suppression of liquid storage fires [72]. The range-finding tests indicated that the Class IB flammable liquids (heptane) provided a greater challenge than water-miscible fuels (e.g., isopropanol) [73]. Breach of steel containers exposed to a flammable liquid pool fire without sprinkler protection occurred over a range of times between 2 and 7.5 min, depending on the particular type of container. Plastic containers were quickly breached and discharged their contents to the exposing pool fire.
Large-scale tests were conducted under an 8.2-m (27-ft) high ceiling at the Underwriters Laboratories fire test facility in Northbrook, Illinois [74]. A series of 14 fire tests involving the protection of 3.8 and 18.9 L (1 and 5 gal) metal and 18.9 L (5 gal) plastic containers filled with heptane (Class IB flammable liquid) were conducted. The use of closed-head foam-water sprinkler systems for the protection of these fuel packages was investigated. Quantities of fuel used in the fire tests varied from 605 to 7260 L (1601920 gal); fuel storage densities ranged from 160 to 1907 L/m2 (3.9–46.5 gal/ft2); and storage heights ranged from 4.3 to 42.7 m (1.3–13 ft). Each fire test was initiated using a 37.8 L (10 gal) flammable liquid (heptane) spill, recognizing the larger spill ignition scenarios observed in large-loss fires.
Fire tests involving palletized storage of 3.8 L (1 gal) metal F-style containers of heptane, packaged four containers in a corrugated cardboard carton, were conducted. The results indicated that the 37.8 L (10 gal) flammable liquid spill fire could be suppressed by a closed-head foam-water sprinkler system at a 16.4 L/min/m2 (0.40 gpm/ft2) design application rate for storage heights up to 3.3 m (10.7 ft) under the 8.2 m (27 ft) ceiling prior to any container breach or fuel loss. Fires involving 18.9 L (5 gal) metal containers of heptane could be suppressed by a closed-head foam-water sprinkler system application rate of 12.3 L/min/m2 (0.30 gpm/ft2) for a palletized storage height of up to 3.6 m (12 ft). Plastic pour spouts in the 18.9 L (5 gal) tight-head metal containers safely vented and prevented container breaching.
Fires involving 18.9 L (5 gal) plastic containers of heptane could not be suppressed by a preprimed, closed-head foam-water sprinkler system with an application rate of 12.3 L/min/m2 (0.30 gpm/ft2), where containers were stacked one high (483 mm [19 in.]), due to container breaching and flammable liquid spillage prior to foam-water discharge.
Rack-storage tests also conducted in the NFPRF International Foam-Water Sprinkler Research Project did not lead to conclusive results [75].
Based on the results of the NFPRF foam-water sprinkler testing, the FMRC original AFFF drum testing, and engineering judgment/extrapolation, the NFPA 30 Technical Committee adopted protection criteria for palletized and rack storage of liquids in metal containers when protected by AFFF. Variables that affect the specific level of protection include container size, class of liquid stored, inclusion of exterior packaging material, and storage height. Ceiling application rates are on the order of 12.3–16.4 L/min/m2 (0.30–0.40 gpm/ft2). Protection criteria shown in Table 47.23 are recommended for palletized storage of small containers that are nonrelieving style (i.e., do not readily vent when exposed to fire). Additional criteria are included in NFPA 30 for foam protection of palletized relieving-style containers based on extrapolation of the NFPRF data and engineering judgment. Where the hazard involves a water-miscible fuel, an alcohol-type foam should be used. The application rate should be at least as great as the rate established by foam listing requirements. AFFF solution should be discharged when four sprinklers are operating.
AFFF protection of flammable and combustible liquids should be used where large spills of low flashpoint fuels are a realistic scenario. Other protection options are available and have recently been adopted or are currently being considered by NFPA 30 and the model building/fire prevention codes. Designers of warehouse protection should have a thorough knowledge of these criteria and the available test data (including water-only protection) when considering design options for the protection of stored combustible and flammable liquids. Nugent [68] and NFPA 30 provide detailed data and guidance for water-only protection. Additional guidance for warehouse protection is available from the Center for Chemical Process Safety [76].
Foam Environmental Considerations
There has been increasing concern about the consequences of the discharge of foam in the environment. This concern affects the users of foam, the manufacturers of foam agents, the fire safety authority having jurisdiction, and environmental authorities. The issue is not a new or unique development but has received increased notice as a result of increased attention to environmental impact of fire-fighting agents.
Factors related to the impact of fire-fighting foam on the environment include
-
1.
Discharge of foam solutions and fuel-contaminated foam solutions to waterways and the potential toxicity to aquatic life
-
2.
Effects on water treatment facilities
-
3.
Persistence and biodegradability of chemicals in foam concentrates and solutions
-
4.
Combustion products of fuel/foam solutions
In the United States, all fire-fighting foams are regulated at some point during their life cycle, and all have the potential to impact the environment. Fire-fighting foams have several intrinsic properties that cause environmental impacts, including foaming, oxygen demand, aquatic toxicity, biodegradability, and oil emulsification. Because of these properties, fire-fighting foams will impact surface water and groundwater if released into the environment. If sent to a wastewater treatment plant, they can cause disruption of the plant, preventing sewage from being treated and forcing the plant to discharge raw sewage.
All fire-fighting foams have these properties because they consist of ingredients that exhibit these properties. The main ingredients in fire-fighting foams are water, surfactants, solvents, and other ingredients used to make the foam work in a particular system or under specified conditions. Some of these ingredients are specifically listed in U.S. environmental laws because of their environmental impacts (e.g., butyl carbitol, dipropylene glycol methyl ether, ethylene glycol, etc.). Although it is easier to highlight these as being regulated because they are specifically named, almost all of the ingredients in fire-fighting foams are regulated due to their properties.
The properties and ingredients of fire-fighting foams make them subject to U.S. federal environmental laws that regulate their manufacture, storage, use, release, cleanup, remediation, and disposal. These laws include the Clean Water Act (CWA); Clean Air Act (CAA/CAAA-90); Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA or Superfund); the Superfund Amendments and Reauthorization Act (SARA); the Emergency Planning and Community Right-to-Know Act (EPCRA); the Resource Conservation and Recovery Act (RCRA); the Hazardous and Solid Waste Amendments of 1984 (HSWA); the Safe Drinking Water Act (SDWA); the Toxic Substances Control Act (TSCA); and the Uniform National Discharge Standard (UNDS). These laws cover the entire life cycle of fire-fighting foams from manufacture to final disposition.
Whether foams are used in fixed facility systems or on crash fire-rescue vehicles, mitigating the environmental impacts is best accomplished through careful planning and management. This forethought may include engineered systems for capture and containment, temporary containment equipment, improved standard operating procedures, and other measures. Simple substitution of one “environmentally friendly” foam for another will not eliminate environmental impacts. In order to make the final decision to use any type of system for fire protection, it is essential to fully review the fire and environmental risks and benefits of using fire suppression chemicals and their associated systems. The review should involve both fire protection and environmental professionals to ensure a balanced approach that guarantees maximum fire protection and environmental protection at the same time.
Fluorinated Surfactants
AFFF fire fighting agents contain fluorinated surfactants (fluorosurfactants). They are key ingredients that provide AFFF with the required low surface tension and positive spreading coefficient that enables film formation on top of hydrocarbon fuels. This provides for superior fire extinguishment capability as described earlier.
Environmental regulators have investigated AFFF chemicals for their persistence, tendency to bioaccumulate, and toxicity (PTB). As part of this assessment process, the AFFF manufacturing industry has formed a coalition to represent the fire fighting foam industry’s interests on all issues related to the environmental acceptability of AFFF agents. The Fire Fighting Foam Coalition (FFFC) provides periodic updates on the status and use of chemicals used to create AFFF [77]. They note that the chemicals used to produce fluorosurfactants can be manufactured by different processes and have different chemical structures. The fluorosurfactants used in AFFF have historically been produced from fluorochemicals manufactured by two methods: electrochemical fluorination and telomerization.
In 2002, the US manufacturer of fluorosurfactants using the electrochemical fluorination process voluntarily stopped production of a number of products including AFFF agents because they contain and degrade into perfluorooctane sulfonate (PFOS). PFOS is considered by environmental authorities to be PBT. Regulations in the United States, Canada, European Union, Australia, and Japan act as a ban on new production of PFOS-based products including foams. These regulations do not currently restrict the use of existing stocks of PFOS-based foam in the US, Australia, or Japan. In the EU and Canada, existing stocks of PFOS-based foam must be removed from service over a set time period. A general overview of this regulatory restriction on PFOS in the US is available in the general fire service literature [78].
Other AFFF agents contain telomer-based fluorosurfactants. Telomer-based AFFF agents do not contain or break down into PFOS. Telomer-based AFFF agents are not made with any chemicals that are currently considered by environmental authorities to be PBT. The US Environmental Protection Agency (EPA) has indicated that some telomer-based fluorochemicals can break down in the environment into perfluorooctanoic acid (PFOA) or other perfluorocarboxylic acids (PFCAs). EPA’s concern is focused on long-chain perfluorinated chemicals (LCPFCs) containing eight carbons or more (C8, C10, C12). Existing data shows that shorter-chain compounds (C6 and below) have a lower potential for toxicity and bioaccumulation. Under an EPA stewardship program, fluorochemical manufacturers have voluntarily agreed to reduce both plant emissions and product content of PFOA, PFOA precursors, and related chemicals. This will result in the reformulation of many existing telomer-based AFFF agents.
The restrictions on the manufacture and use of AFFF has resulted in industry creating a new class of foams, “fluorine-free” foam. Preliminary test data show that these non-film forming foams take longer to extinguish fires compared to AFFF [79], but have better fire extinguishing performance than protein or fluoroprotein foams. This is not surprising since the fluorosurfactants used to create the “film” in AFFF have been eliminated. Users may be faced with trade-offs in selecting foams for fire performance and environmental impact. If reduced fire extinguishing performance must be accepted for lower environmental impact, priorities for protecting hazards may have to be established. An example methodology has been published by the U.S. Navy [80].
Perspective on the Use of Foam Agents
In order to assess the impact of foam on the environment, the likely scenarios under which AFFF may be discharged should be considered. Based on these scenarios, the overall impact can be assessed and, where appropriate, potential mitigation strategies can then be developed. Likely scenarios include uncontrolled fires, potential hazardous situations, fire-fighting training evolutions, and fixed or mobile vehicle suppression system discharge testing.
Uncontrolled Fires
There are many fires for which foam may be used, including flammable liquid storage, process industry protection, aviation protection, and marine applications. For most fires, the elimination of foam as a suppression agent results in the potential for dramatically increased environmental impact. This impact results from the potential increase in hydrocarbon fuel effluent to the environment (due to smoke from uncontrolled burning and fuel/fire-fighting water effluent). Consider the example shown in Fig. 47.22. A 929 m2 (10,000 ft2) section of a warehouse containing combustible and flammable liquids may be protected using traditional water sprinklers discharging at a rate of 12.3 L/min/m2 (0.30 gpm/ft2). If these sprinklers fail to control a large spill fire, the fire may develop and spread past the design area of the sprinklers. The example assumes the fire is contained within the fire wall; this may not always be the case for high-challenge fires. If the fire department aggressively combats the fire, a rough estimate of fire-fighting water that may be used is 15 to 50 times the minimum anticipated agent required for suppression [6, 81, 82]. A rough estimate of the potential fuel-contaminated effluent (neglecting the actual quantities of hydrocarbon liquid) is shown in Fig. 47.22. In the alternative situation, a properly specified foam-water sprinkler system designed for a high degree of reliability can control or suppress the fire. Using application rates and discharge times based on recent tests and building code requirements, the anticipated fuel/foam-water effluent for this scenario can be estimated (see Fig. 47.22). The use of the foam-water system reduces the potential effluent by a factor of nearly 500 compared to the “unsuccessful” water sprinkler scenario where handlines are used. This reduction neglects the impact of smoke discharged to the atmosphere during the uncontrolled burning in the water-only scenario.
In some cases, it may be possible to collect the effluent from an uncontrolled fire. In other situations, it may not be possible. Any foam solution that has been used in fire suppression is likely to be contaminated with fuel and diluted with water.
Potential Hazardous Situations
Potential hazardous situations may result from a fuel spill where there is a likely ignition source. In this situation, foam may be applied for ignition prevention. The potential impact of ignition and resulting uncontrolled fire must be assessed against the potential additional environmental impact by discharging foam for ignition prevention. The potential environmental effects from an uncontrolled fire should be considered as described in the previous text. Another consideration is the assessment of any additional impact of foam when applied to a fuel spill. For example, would the resulting fuel with foam have any greater impact on the environment than the fuel alone? If so, how is this impact quantitatively determined?
Training Evolutions
Fire-fighting training is usually conducted under conditions conducive to collection of fuel, water, and foam. A separation process might be used to recover fuel. Water/foam solution may then be treated or reused. Alternatively, simulated hydrocarbon fuel spill scenarios might be used, with a simulated foam agent. Propane-fired burners are typically used. The disadvantage of these systems is the potential loss of realism of the simulated fire/agent interaction. These techniques may potentially reduce training effectiveness. Quantitative comparisons have not been performed to assess these differences.
System Discharge Testing
Facilities protected by foam systems may have containment systems that can hold effluent. Requirements for these containment systems are becoming more widespread in model building and fire codes. An alternative to discharge testing with foam is the use of a simulant that can be measured using concentration determination methods. For example, salt solutions can be used as the “concentrate” to test AFFF systems, with the simulant concentration measured using the conductivity method. Simulators may be more difficult to use for protein-based systems, where viscosity factors influence proportioning system accuracy.
Because of their persistent nature, NFPA 11 recommends that emissions of fluorochemical surfactants to the environment be minimized whenever possible using the following techniques:
-
1.
Use training foams that do not contain fluorochemical surfactants.
-
2.
Provide for containment, treatment, and proper disposal of foam discharges.
-
3.
Follow applicable industry standards on the design, installation, and maintenance of foam systems and extinguishers.
-
4.
Minimize false discharges from fixed foam systems by using approved detection, actuation, and control systems as required by industry standards.
-
5.
When appropriate consider treating collected wastewater with granular activated carbon (GAC) or a membrane process such as reverse osmosis to remove the fluorochemical surfactants prior to disposal.
Methods of Assessment
Biodegradability
The primary component of AFFF solution is water. Examples of other components are nonfluorinated surfactants, glycol ethers, and fluorinated surfactants. Freeze-resistant concentrate may contain ethylene or propylene glycol. Alcohol-type foams contain xanthan or similar gums. The fluorinated surfactants are particularly resistant to biodegradation. Further, the less-effective protein-based foams were largely assumed to be nonpolluting because of their “natural” organic base. An early review of the available literature by Factory Mutual Research Corporation indicated that both types of agents, that is, AFFF and protein-based, present inherent environmental issues and that effluents containing either should be processed in some form of sewage treatment facility or diluted prior to discharge into a stream [47].
A conventional method used to determine the biodegradability of a material is comparison of the chemical oxygen demand (COD) of the material with its biological oxygen demand (BOD). This method is particularly important for waste treatment facilities where the stability of the treatment process may be upset. The method typically used is specified in Standard Methods for the Examination of Water and Wastewater [83]. BOD measures the amount of oxygen consumed by microorganisms in breaking down a hydrocarbon. COD measures the maximum amount of oxygen that could theoretically be consumed by microorganisms. Therefore, a BOD/COD ratio is representative of the ability of microorganisms to biodegrade the components in a foam. The higher the BOD/COD ratio, the more biodegradable the foam. Results reported for BOD/COD of AFFF range from 0.60 to 0.99. MIL-F-24385 requires a maximum COD of 500,000 mg/l and a minimum 20-day BOD/COD ratio of 0.65 for 6 % concentrate. AFFF agents have been reported to have higher BOD and COD values than protein foams. [47] AFFF solutions are high-BOD materials compared to the normal influent to treatment plants. Large quantities can “shock load” wastewater treatment facilities.
The fluorochemical-based surfactants in AFFF have a carbon–fluorine chain that apparently does not break down in either the BOD or the COD test. The AFFF might then appear to be completely “biodegradable,” even though the carbon-fluorine chain remains.
If nonbiodegradability concerns are based on the persistence of the fluorochemical surfactants, then the environmental impact tests currently used to assess foams do not address this concern. There is speculation that the undegradable material is biologically inert, but no published data confirm this. Because the fluorinated surfactants are required to create surface-tension reduction of the solution, replacement with less persistent chemicals is problematic. There is a need for a more thorough understanding and testing related to the environmental impact of fluorosurfactants and possible alternatives.
The persistence of fluorosurfactants in soil has been quantified in a study of fire-training facilities [84]. In a study of training sites having long-term use, perfluorocarboxylates were detected using gas chromatography/mass spectrometry. These chemicals were detected at sites that were inactive for a period of 7–10 years. The results are consistent with the view that biodegradation of the long chain perfluorocarbon is unlikely. The influence of the perfluorinated compounds on the biotransformation and transport of other cocontaminants (e.g., training fuel) and other site characterization parameters (e.g., dissolved organic carbon and inorganics) is unknown.
Methods for detecting AFFF in aqueous solutions have been investigated [85]. A Fourier Transform Infrared Spectroscopy (FTIR) method and drain-time test were found to be effective in evaluating the level of AFFF contamination in wastewater and soil. The drain-time method was proposed as a simple, easy-to-use field test. Using these methods, procedures were developed to estimate AFFF contamination levels in wastewater and soil. Analysis of wastewater and soil for AFFF contamination was broken into two groups: nonbiodegraded samples and biodegraded samples. Nonbiodegraded samples were screened for AFFF, then analyzed further if deemed necessary. Samples were initially screened using the drain-time test. Samples with no drain time contain less than a 1:240 dilution of AFFF (5 ppm of fluorosurfactant). If the sample had a drain time, it was recommended that the FTIR analysis be performed on the sample. In solutions with fluorosurfactants, FTIR analysis can provide a quantitative level of AFFF in the sample if the fluorosurfactant source solution is available to develop a calibration curve. Otherwise, FTIR provides a qualitative estimate of the AFFF level in the solution.
Biodegraded wastewater samples were difficult to analyze because the hydrocarbon surfactants and a portion of the fluorosurfactant molecule were degraded. With these foam-making constituents degraded, the drain-time test results were found to be unreliable. However, the fluorine-carbon tail of the fluorosurfactant is not biodegraded, making FTIR analysis on biodegraded samples possible. With biodegraded samples, FTIR analysis can provide a qualitative measure of AFFF levels.
Toxicity
In sufficient concentrations, foams may affect aquatic life. A number of fish toxicity studies have been performed. In tests using fathead minnows, the U.S. Air Force found that these fish could live in a simulated effluent stream containing 250 ppm AFFF without fatality for up to 8 days. LC50 values (i.e., the concentration causing deaths of 50 % of the fish exposed) at 96 and 24 h were 398 and 650 ppm, respectively [86]. MIL-F-24385 requires AFFF toxicity testing in accordance with ASTM E729, using dynamic procedures with killifish. LC50 of 1000 mg/l for 6 % concentrate is permitted.
Alone, these values may be considered as having a low degree of fish toxicity using environmental regulation rating scales. Localized concentrations in ponds or streams may exceed the values cited, if there is limited water movement.
Published data do not exist for the phytotoxicity of foam solutions; however, there have been no published reports of plant kills resulting from foam solution discharges.
Manufacturers report that thermal decomposition products from AFFF do not present a health hazard during fire fighting. Again, there are no data published in the literature. Manufacturers’ product environmental data for AFFF include references to a test where a layer of AFFF was burned in a pan of gasoline inside an enclosure. Two measurements of hydrogen fluoride recorded above the sample were 0.23 and 0.16 ppm [87].
Foaming and Emulsification of Fuels
The surfactants in AFFF solutions can cause foaming in treatment aeration ponds. This foaming process may suspend high BOD solids in the foam. If these are carried over to the outfall of the treatment facility, nutrient loading in the outfall waterway may result. Foam aeration may also cause foam bubble backup in sewer lines.
In uncontrolled fires, spills, and live fire-training scenarios, foams may contain suspended fuels. The fuel may become emulsified in the foam-water solution.
A bench-scale study has been conducted to evaluate the potential inhibitory effects of untreated AFFF wastewater on the biological nutrient removal process [88]. In this study, bench-scale reactors simulating the nitrification process were loaded at various AFFF concentrations, and the influence on process performance was evaluated. The results indicated that AFFF in concentrations between 10 and 60 ppm did not show any inhibition to biological nitrification, and effluent did not exhibit any pass-through toxicity. These range-finding tests did indicate that nitrification inhibition did occur above 60 ppm AFFF. Some reductions in percent COD removal were observed as AFFF concentrations were increased.
Mitigation Strategies
Foam discharges are more easily handled where there is an in-place collection capability. This situation may be available at warehouses, tank farms, and fire-fighting training facilities. Where these facilities are not available, temporary diking is an alternative where time and resources permit.
Investigations have been conducted to develop foam-water separators using aeration and agitation techniques. To date, these techniques have not been optimized.
Discharge to water treatment facilities is recommended by many foam vendors when the solution is uncontaminated by fuel. Metering or dilution may be required to prevent levels of foam that will upset treatment facility reactions or cause excessive foaming. The use of defoamers to reduce aeration has been suggested.
Where fuels contaminate foam solutions, fuel-water separators might be used to skim off the hydrocarbon fuel. AFFF solutions have a tendency to form emulsions with fuels, potentially reducing the effectiveness of fuel-water separators. An alternative is to hold the solution in a pond or tank until the emulsion breaks and the separation process can be used. Agitation should be avoided to prevent the emulsion from reforming. In some situations (e.g., training), the fuel and treated water have been reused. Many fire training facilities collect foam solution for ultimate discharge to water treatment facilities.
To ensure that unbalanced conditions do not occur in water treatment facilities, foam discharge should be carefully monitored. Different ranges of discharge rates have been suggested. This is an area requiring further investigation. Manufacturers of the foam solution should be consulted in conjunction with the wastewater treatment operator.
The entire area of environmental aspects of foam discharge requires additional evaluation and development of generally recognized guidance. Until generally recognized guidance is promulgated, users must rely on manufacturers’ data and guidance. In all situations, discussions with the operator of the wastewater treatment facility and the environmental regulatory authorities are appropriate. Work is continuing in an effort to identify appropriate policy and criteria covering foam discharge for facilities having foam suppression systems. These efforts are focusing on identifying applicable codes and standards, analyzing environmental impact, and evaluating containment options.
References
G.B. Geyer, L.M. Neri, and C.H. Urban, “Comparative Evaluation of Fire Fighting Foam Agents,” Report FAA-RD-79-61, Federal Aviation Administration, Washington, DC (1979).
J.H. Aubert, A.M. Kraynik, and P.B. Rand, “Aqueous Foams,” Scientific American, 19, 1, pp. 74–82 (1988).
V.L. Francen, “Fire Extinguishing Composition Comprising a Fluoroaliphatic and a Fluorine-Free Surfactant,” U.S. Patent 3,562,156 (1971).
M.J. Rosen, Surfactants and Interfacial Phenomena, John Wiley and Sons, New York, Chapters 1, 5, 7 (1989).
R.L. Tuve, H.B. Peterson, E.J. Jablonski, and R.R. Neill, “A New Vapor-Securing Agent for Flammable Liquid Fire Extinguishment,” NRL Report 6057, Naval Research Laboratory, Washington, DC (1964).
H.-Z. Yu and J.S. Newman, “Theory of Fire Extinguishment,” Fire Protection Handbook, 20th ed. (A.E. Cote et al., eds.), National Fire Protection Association, Quincy, MA, p. 2–85 (2008).
C.P. Hanauska, J.L. Scheffey, R.J. Roby, and D.T. Gottuk, “Improved Formulations of Firefighting Agents for Hydrocarbon Fuel Fires,” SBIR Phase I Final Report, Hughes Associates, Inc., Baltimore, MD (1994).
B. Persson and M. Dahlberg, “A Simple Model of Foam Spreading on Liquid Surfaces,” SP Report 1994:27, Swedish National Testing and Research Institute, Boras, Sweden (1994).
H. Persson, “Fire Extinguishing Foams—Resistance Against Heat Radiation,” SR Report 1992:54, Swedish National Testing and Research Institute, Boras, Sweden (1992).
B.Y. Lattimer, C.P. Hanauska, J.L. Scheffey, and F.W. Williams, “The Use of Small-Scale Test Data to Characterize Some Aspects of Fire Fighting Foam for Suppression Modeling,” Fire Safety Journal, 38, pp. 117–146 (2003).
S. Ikasson and H. Persson, “Fire Extinguishing Foam—Test Method for Heat Exposure Characterisation,” SP Report 1997:09, Swedish National Testing and Research Institute, Boras, Sweden (1997).
A.M. Kraynik, “Foam Drainage,” Sandia Report SAND-83-0844, Sandia National Laboratories, Albuquerque, NM (1983).
B. Persson, A. Lonnermark, H. Persson, D. Mulligan, A. Lancia, and M. Demichela, “FOAMSPEX Large Scale Foam Application–Modelling of Foam Spread and Extinguishment,” SP Report 2001:13, Boras, Sweden (2001).
A.M. Kraynik, “Foam Flows,” Annual Review of Fluid Mechanisms, 20, pp. 325–357 (1988).
J.A. Fay, “The Spread of Oil Slicks on a Calm Sea,” in Oil on the Sea (D.P. Hoult, ed.), Plenum, New York, pp. 53–64 (1964).
J.A. Fay and D.P. Hoult, “Physical Processes in the Spread of Oil on a Water Surface,” Coast Guard Final Report, Massachusetts Institute of Technology, Cambridge, MA (1971).
P. Cann, H.A. Spikes, and G. Caporico, “Spreading of Perfluorinated Fluids on Metal Surfaces,” in 4th International Colloquium on Synthetic Lubricants and Operation Fluids, Oatfildern, Germany, pp. 631–638 (1984).
H.E. Moran, J.C. Burnett, and J.T. Leonard, “Suppression of Fuel Evaporation by Aqueous Films of Fluorochemical Surfactant Solutions,” NRL Report 7247, Naval Research Laboratory, Washington, DC (1971).
T. Briggs, and B. Abdo, “Emphasis on Spreading Quality of AFFF Could Be Misleading,” FIRE, 80, 94 (1988).
J.L. Scheffey, R.L. Darwin, J.T. Leonard, C.R. Fulper, R.J. Ouellette, and C.W. Siegmann, “A Comparative Analysis of Film-Forming Fluoroprotein Foam (FFFP) and Aqueous Film-Forming Foam (AFFF) for Aircraft Rescue and Fire Fighting Services,” Hughes Associates, Inc. Report 2108-A01-90, Hughes Associates Inc., Baltimore, MD (1990).
G.B. Geyer, “Status Report on Current Foam Fire Fighting Agents,” International Conference on Aviation Fire Protection, Interlaken, Switzerland (1987).
P.J. Chiesa and R.S. Alger, “Severe Laboratory Fire Test for Fire Fighting Foams,” Fire Technology, 16, 1, pp. 12–21 (1980).
Underwriters Laboratories Inc., Standard for Foam Equipment and Liquid Concentrates, 6th ed., Northbrook, IL (1994, with revisions through 2015).
Chevron Foam Concentrate Team, “Chevron Firefighting Foam Test Report,” Report to the American Petroleum Institute, Washington, DC (2001).
J.L. Scheffey, J. Wright, and C. Sarkos, “Analysis of Test Criteria for Specifying Foam Firefighting Agents for Aircraft Rescue and Firefighting,” FAA Technical Report, DOT/FAA/CT-94/04, Atlantic City, NJ (1994).
International Organization for Standardization, “Fire Extinguishing Media—Foam Concentrates—Part 3: Specification for Low-Expansion Foam Concentrates for Surface Application to Water-Immiscible Liquids,” European Standard EN 1568–3, ISO, Geneva, Switzerland (2008).
B.P. Johnson, “A Comparison of Various Foams When Used Against Large-Scale Petroleum Fires,” Home Office Fire Research and Development Group, London, UK (1993).
W.M. Carey and M.R. Suchomel, “Testing of Fire Fighting Foam,” Underwriters Laboratories Report No. CG-M-1-81, Underwriters Laboratories Inc., Northbrook, IL (1980).
M.D. Thomas, “UK Home Office Research into Domestic Fire Fighting,” First International Conference on Fire Suppression Research Proceedings, Stockholm, Sweden, pp. 283–289 (1993).
R. Fiala, “Aircraft Post-Crash Firefighting/Rescue,” from AGARD Aircraft Fire Safety Lecture Series No. 123 (1982).
G.B. Geyer, “Evaluation of Aircraft Ground Firefighting Agents and Techniques,” Technical Report AGFSRS 71–1, Tri-Service System Program Office for Aircraft Ground Fire Suppression and Rescue, Wright-Patterson AFB, OH (1971).
G.B. Geyer, “Firefighting Effectiveness of Aqueous Film-Forming Foam (AFFF) Agents,” FAA Technical Report FAA-NA-72-48, DOD Aircraft Ground Fire Suppression and Rescue Unit (ASD-TR-73-13), Washington, DC (1973).
J.L. Scheffey, R.L. Darwin, and S. Hunt, “A Technical Review of Methodologies for Calculating Firefighting Agent Quantities Needed to Combat Aircraft Crash Fires,” DOT/FAA/AR-11/29, Federal Aviation Administration, Atlantic City Int’l Airport, NJ (2012).
J.L. Scheffey, R.L. Darwin, and S. Hunt, “Analysis of Suppression Effects on Aviation Fuel Fires Around an Aircraft,” DOT/FAA/AR-11/27, Federal Aviation Administration, Washington, DC (2011).
W.M. Carey, Improved Apparatus for Measuring Foam Quality, Underwriters Laboratories Inc., Northbrook, IL (1983).
E.J. Jablonski, “Comparative Nozzle Study for Applying Aqueous Film-Forming Foam on Large-Scale Fires,” U.S. Air Force Report, CEEDO-TR-78-22, Tyndall AFB, FL (1978).
J.A. Foster, “Additions for Hosereel Systems: Trials of Foam on 40 m2 Petrol Fires,” Scientific Research and Development Branch Report 40/87, Home Office, London (1987).
P.L. Parsons, “Trials of Foam on Petrol Fires at the Fire Service Technical College,” Scientific Advisory Branch Report No. 14/75, Home Office, London (1976).
L.R. DiMaio, R.F. Lange, and F.J. Cone, “Aspirating vs. Non-Aspirating Nozzles for Making Fire Fighting Foams—Evaluation of a Non-Aspirating Nozzle,” Fire Technology, 20, 1, pp. 5–10 (1984).
J.L. Scheffey, “Submarine Bilge AFFF Sprinklers,” Naval Research Laboratory Project, 64561N-S1946–2024000, unpublished data (1988).
G. Timms and P. Haggar, “Foam Concentration Measurement Techniques,” Fire Technology, 26, 1, pp. 41–50 (1990).
K. Bagot, “Evaluation of Conductivity Meters for Firefighting Foams,” DOT/FAA/AR-02/115, Final Report, Federal Aviation Administration, Airport and Aircraft Safety Research and Development Division, Atlantic City International Airport, NJ (2002).
M.J. McDonald, D.S. Dierdorf, J.L. Kalberer, and K.D. Barrett, “Fire Extinguishing Effectiveness Tests,” AFRL-ML-TY-TR-2004-4554, Applied Research Associates, Tyndall AFB, FL (2004).
C.P. Menchini, J.L. Kalberer, D. Dierdorf, M.J. McDonald, K.S. Cozart, and A.C. Abildgaard, “The Development and Design of a Prototype Ultra High Pressure P-19 Firefighting Vehicle,” AFRL-ML-TY-TR-2007-4525, Applied Research Associates, Tyndall AFB, FL (2007).
D.E. Breen, “Hangar Fire Protection with Automatic AFFF Systems,” Fire Technology, 9, 2, pp. 119–131 (1973).
D.E. Breen, L.M. Krasner, B.G. Vincent, and P.J. Chicarello, “Evaluation of Aqueous Film Forming Foam for Fire Protection in Aircraft Hangars,” FMRC Technical Report, Serial No. 21032, Factory Mutual Research Corporation, Norwood, MA (1974).
L.M. Krasner, D.E. Breen, and P.M. Fitzgerald, “Fire Protection of Large Air Force Hangars,” AFWL-TR-75-119, Factory Mutual Research Corporation, Norwood, MA (1975).
L.M. Krasner, “Closed-Head AFFF Sprinkler Systems for Aircraft Hangars,” FMRC S. I. 0C6N3.RG, RC 79-T-58, Factory Mutual Research Corporation, Norwood, MA (1979).
J.L. Scheffey, S.M. Bryant, H.V. Pham, M.A. Harrison, J.P. Farley, F.W. Williams, and M.P. Hunstad, “Aircraft Carrier Hangar Bay Test Program–Test Series 3, AFFF System Testing and Manned Firefighting Scoping Tests,” NRL Letter Report, Serial No. 6180/0272, Naval Research Laboratory, Washington, DC (2004).
A.C. Luers, M.A. Harrison, H.V. Pham, J.A. Lynch, J.L. Scheffey, F.W. Williams, J.P. Farley, and M.P. Hunstad, “Test Report for the Well Deck and Vehicle Stowage Area Vulnerability and Fire Model Validation Tests, Series 1—An Evaluation of Aqueous Film Forming Foam (AFFF) Suppression Systems for the Protection of LHA(R) Well Deck and Vehicle Stowage Areas,” NRL Letter Report, Serial No. 6180/0369, Naval Research Laboratory, Washington, DC (2004).
M. Shipp, K. Annable, and C. Williams, “Assessment of the Fire Behaviour of Cargo Loaded on Ro-Ro Vehicle Decks in Relation to the Design Standards for Fire Suppression Systems,” BRE Fire and Security Report 227974, prepared for the Maritime and Coastguard Agency, Garston, UK (2006).
“Pop-Up-Type Floor-Mounted Foam Sprinklers for Aircraft Hangars,” Technical Record TR44/153/14(L), Department of Housing and Construction, Australia.
China Lake CVA Fire Fighting Tests, Phase III, unpublished data (1972).
J.L. Scheffey, “Flow, Pattern, and Fire Performance Characteristics of a Prototype Pop-Up Nozzle for Use on Aircraft Carrier Flight Decks,” Report 2429–17, Hughes Associates, Inc., Columbia, MD (1985).
J.L. Scheffey and J.T. Leonard, “AFFF Protection for Weapons Staging Areas,” Fire Safety Journal, 14, 14, pp. 47–63 (1988).
Department of the Navy, “General Specifications for Ships of the United States Navy, 1991 Edition,” NAVSEA S9AA0-AA-SPN-010/GEN-SPEC, Section 555, Department of the Navy, Washington, DC (1991).
H.W. Carhart, J.T. Leonard, R.L. Darwin, R.E. Burns, J.T. Hughes, and E.J. Jablonski, “Aircraft Carrier Flight Deck Fire Fighting Tactics and Equipment Evaluation Tests,” NRL Memorandum Report 5952, U.S. Naval Research Laboratory, Washington, DC (1987).
Rolf Jensen and Associates, “Comprehensive Aqueous Film Forming Foam Central Fire Suppression System Analysis/Report, B1-B Aircraft Hangars,” Technical Report A6406, U.S. Air Force, Washington, DC (1989).
J.E. Gott, D.L. Lowe, K.A. Notarianni, and W. Davis, “Analysis of High Bay Hangar Facilities for Fire Detector Sensitivity and Placement,” NIST TN1423, National Institute of Standards and Technology, Gaithersburg, MD (1997).
D.B. Szepezi, G.G. Back, J.L. Scheffey, F.W. Williams, and J.E. Gott, “Aircraft Hangar Fire Suppression System Evaluation—Intermediate Scale Studies,” NRL/MR/6180-99-8422, Naval Research Laboratory, Washington, DC (1999).
G.G. Back, F.W. Williams, J.E. Gott, A.J. Parker, and J.L. Scheffey, “Aircraft Hangar Fire Protection System Evaluation Full Scale Study,” NRL Letter Report, Serial No. 6180/0620, Naval Research Laboratory, Washington, DC (1998).
D.T. Gottuk, J.L. Scheffey, F.W. Williams, J.E. Gott, and R.J. Tabet, “Optical Fire Detection (OFD) for Military Aircraft Hangars: Final Report on OFD Performance to Fuel Spill Fires and Optical Stress,” NRL/MR/6180-00-8457, Naval Research Laboratory, Washington, DC (2000).
J.L. Scheffey, A.J. Wakelin, J.E. Gott, R.J. Tabet, and F.W. Williams, “Aircraft Hangar Fire Suppression System Design Study,” NRL/MR 6180-00-8464, Naval Research Laboratory, Washington, DC (2000).
A.J. Parker, G.G. Back, J.L. Scheffey, J.J. Schoenrock, J.P. Ouellette, F.W. Williams, J.E. Gott, and R.J. Tabet, “The Development of a Prototype Low-Level AFFF Nozzle System for U.S. Navy Aircraft Hangars,” NRL Letter Report, Serial No. 6180/0004, Naval Research Laboratory, Washington, DC (2000).
G.P. Crampton, A.K. Kim, and J.K. Richardson, “A New Fire Suppression Technology,” NFPA Journal, July/Aug. (1999).
G. Crampton and A. Kim, “The Comparison of the Fire Suppression Performance of Compressed-Air Foam with Foam Water Sprinklers on Free-Flowing Heptane Spill Fires,” Research Report RR-174, National Research Council Canada, Ottawa, Canada (2004).
B.G. Vincent, H.C. Kung, and P. Stavrianidis, “Fire Protection for U.S. Army Helicopter Hangars,” FMRC J.I.OXON1.RA, Factory Mutual Research Corporation, Norwood, MA (1993).
D.P. Nugent, Directory of Fire Tests Involving Flammable and Combustible Liquids in Small Containers, 3rd ed., Schirmer Engineering, Deerfield, IL (2004).
R.M. Newman, P.M. Fitzgerald, and J.R. Young, “Fire Protection of Drum Storage Using ‘Light Water’ Brand AFFF in a Closed-Head Sprinkler System,” FMRC Technical Report, Serial No. 22464, RC 75-T-16, Factory Mutual Research Corporation, Norwood, MA (1975).
Southwest Research Institute, “Performance Testing of Automatic Sprinkler Systems in the Protection of Palletized, 55-Gal Storage of Heptane in Self-Relieving Style, Steel Drums,” SwRI Project No. 01-2016-001, San Antonio, TX (1999).
J.R. Young and P.M. Fitzgerald, “The Feasibility of Using ‘Light Water’ Brand AFFF in a Closed-Head Sprinkler System for Protection against Flammable Liquid Spill Fires,” FMRC Technical Report RC 75-T-4, Serial No. 22352, Factory Mutual Research Corporation, Norwood, MA (1975).
Schirmer Engineering Corporation, “International Foam-Water Sprinkler Research Project: Task 1—Literature Search,” Technical Report No. 10-90001-04-00, National Fire Protection Research Foundation, Quincy, MA (1992).
J.P. Hill, “International Foam-Water Sprinkler Research Project: Task 3—Range Finding Tests,” Technical Report OTOR6.RR, National Fire Protection Research Foundation, Quincy, MA (1991).
Underwriters Laboratories Inc., “International Foam-Water Sprinkler Research Project: Task 4—Palletized Storage Fire Tests 1 through 13,” Technical Report 91NK14873/NC987, National Fire Protection Association, Quincy, MA (1992).
W.M. Carey, “International Foam-Water Sprinkler Research Project: Task 5—Rack Storage Fire Tests,” Technical Report, National Fire Protection Research Foundation, Quincy, MA (1992).
Center for Chemical Process Safety, Guidelines for Safe Warehousing of Chemicals, American Institute of Chemical Engineers, New York, (1998).
Fire Fighting Foam Coalition, “Fact Sheet on AFFF Fire Fighting Agents,” Arlington, VA, www.fffc.org/AFFF (undated).
A. Riecher, “The Day the Bubble Burst: 3M Abandons Class B AFFF Fire Fighting Foam,” Industrial Fire World (May-June, 2000).
B. Williams, T. Murray, C. Butterworth, Z. Burger, R. Sheinson, J. Fleming, C. Whitehurst, and J. Farley, “Extinguishment and Burnback Tests of Fluorinated and Fluorine-free Firefighting Foams with and without Film Formation,” Suppression, Detection and Signaling Research and Applications – A Technical Working Conference (SUPDET 2011), Orlando, FL (2011).
J.L. Scheffey, R.L. Darwin, W. Leach, S. Fallis, and F.W. Williams, “Performance Analysis of Foam Agents Required to Combat Liquid Fuel Hazards,” NRL/MR/6180-02-8608, Naval Research Laboratory, Washington, DC (2002).
D.J. Rasbash, “The Extinction of Fire with Plain Water: A Review,” Proceedings from the First International Symposium on Fire Safety Science (C.E. Grant and P.J. Pagni, eds.), National Institute of Standards and Technology, Gaithersburg, MD, pp. 1145–1163 (1986).
J.L. Scheffey and F.W. Williams, “The Extinguishment of Fires Using Low-Flow Water Hose Streams—Part II,” Fire Technology, 27, 4, pp. 291–320 (1991).
American Public Health Association, Standard Methods for the Examination of Water and Wastewater, 18th ed., Washington, DC (1992).
C.A. Moody and J.A. Field, “Determination of Perfluorocarboxylates in Groundwater Impacted by Fire-Fighting Activity,” Environmental Science and Technology, 33, 16, pp. 2800–2806 (1999).
Hughes Associates, Inc., “Development of Detection Method for Aqueous Film Forming Foam (AFFF),” Armstrong Laboratories Environics Directorate, Dayton, OH (1997).
National Environmental Health Laboratory, “Biological Treatment of Fire Fighting Foam Waste,” Report No. REHL(K) 67–14, Kelly AFB, Texas (1967).
“Light Water Brand AFFF Waste Disposal Recommendations and Hazard Evaluation,” 3M Product Environmental Data Sheet, 3M Company, St. Paul, MN (1991).
M. Enten-Unal, S. Paranjape, G.C. Schafran, and F.W. Williams, “Evaluation of the Effects of AFFF Inputs to the VIP Biological Nutrient Removal Process and Pass-Through Toxicity,” NRL/MR/6180-98-8141, Naval Research Laboratory, Washington, DC (1998).
Additional Readings
ASTM D1331, Standard Test Methods for Surface and Interfacial Tension of Solutions of Surface-Active Agents, American Society for Testing and Materials, Philadelphia, PA (2014).
ASTM E729, “Standard Practice for Conducting Acute Toxicity Tests with Fish, Macroinvertebrates, and Amphibians,” American Society for Testing and Materials, Philadelphia, PA (2014).
FAA, “Aircraft Fire and Rescue Facilities and Extinguishing Agents,” FAA Advisory Circular 150/5210-6C. Federal Aviation Administration, Washington, DC (1985).
ICAO, “Rescue and Firefighting,” Airport Services Guide, 4th ed., International Civil Aviation Organization, Montréal, Québee (2014).
NFPA 11, Standard for Low-, Medium-, and High-Expansion Foam, National Fire Protection Association, Quincy, MA (2010).
NFPA 13, Standard for the Installation of Sprinkler Systems, National Fire Protection Association, Quincy, MA (2013).
NFPA 16, Standard for the Installation of Foam-Water Sprinkler and Foam-Water Spray Systems, National Fire Protection Association, Quincy, MA (2015).
NFPA 30, Flammable and Combustible Liquids Code, National Fire Protection Association, Quincy, MA (2015).
NFPA 72 ®, National Fire Alarm Code ®, National Fire Protection Association, Quincy, MA (2013).
NFPA 403, Standard for Aircraft Rescue and Fire-Fighting Services at Airports, National Fire Protection Association, Quincy, MA (2014).
NFPA 409, Standard on Aircraft Hangars, National Fire Protection Association, Quincy, MA (2016).
NFPA 412, Standard for Evaluating Aircraft Rescue and Fire-Fighting Foam Equipment, National Fire Protection Association, Quincy, MA (2014).
UL 162, Standard for Foam Equipment and Liquid Concentrates, Underwriters Laboratories Inc., Northbrook, IL (1994, with revisions through 1999).
UL Fire Protection Equipment Directory. Underwriters Laboratories Inc., Northbrook, IL (2008).
U.S. Military Specification, MIL-F-24385F, Department of the Navy, Washington, DC (1992).
U.S. Government, “Federal Specification, Foam Liquid, Fire Extinguishing, Mechanical.” O-F-55B, U.S. Government Protein Foam Specification, Washington, DC (1964).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Nomenclature, Subscripts and Superscripts
- AFFF%sample
-
percentage of AFFF present in the sample
- αfoam
-
absorptivity of foam
- BOD
-
biological oxygen demand (mg/l)
- γ a
-
surface tension of liquid a (dynes/cm)
- γ b
-
surface tension of liquid b (dynes/cm)
- γ l
-
interfacial tension between liquids a and b (dynes/cm)
- COD
-
chemical oxygen demand (mg/l)
- ER
-
expansion ratio
- ΔH v
-
combined latent and sensible heads of vaporization (kJ/kg)
- δ
-
viscous boundary layer thickness (cm)
- G
-
conductance (mhos)
- g
-
acceleration of gravity (cm/s2)
- h
-
foam thickness
- h c
-
critical thickness of the foam layer
- i
-
angle of incidence
- k
-
foam spreading coefficient, dimensionless or nozzle coefficient (L/min/kPa½)
- k d
-
foam drainage coefficient, dimensionless
- k e
-
foam evaporation coefficient, dimensionless
- l
-
length of foam spread
- n
-
refractive index, dimensionless
- μ
-
viscosity (cm2/s)
- ṁ add
-
foam addition rate
- ṁ fuel
-
fuel mass loss rate
- ṁ drain
-
foam mass loss due to drainage
- ṁ drop
-
foam loss rate due to drop-out
- ṁ vap
-
foam mass loss rate due to vaporization
- mS
-
milli siemens
- v
-
kinetic viscosity (cm2/s)
- n water
-
refractive index of water, dimensionless
- n foam
-
refractive index of foam solution, dimensionless
- n concentrate
-
refractive index of foam concentrate, dimensionless
- P v
-
vapor pressure of fuel
- ρfuel
-
fuel density (g/cm3)
- ρfoam
-
foam density (g/cm3)
- \( {\dot{q}}^{{\prime\prime} } \)
-
rate of heat transfer
- \( {\dot{q}}_{\mathrm{rad}} \)
-
rate of heat transfer due to radiation
- q ″rad
-
radiative heat release rate from pool fire
- R
-
resistance (ohms)
- r
-
angle of refraction
- σ
-
spreading coefficient (dynes/cm) or conductivity (mhos)
- S a/b
-
spreading coefficient between liquids a and b (dynes/cm)
- T
-
temperature (°C)
- t
-
time (s)
- T i
-
foam temperature (°C)
- T s
-
fuel temperature (°C)
- V
-
volume (cm3 or l3)
- v s
-
spreading velocity of foam (cm/s)
- add
-
addition of foam
- drain
-
drainage of foam
- drop
-
drop-out of foam
- rad
-
radiation
- vap
-
vaporization
- ⋅
-
rate of change, as in ṁ
- ″
-
per unit area
Rights and permissions
Copyright information
© 2016 Society of Fire Protection Engineers
About this chapter
Cite this chapter
Scheffey, J.L. (2016). Foam Agents and AFFF System Design Considerations. In: Hurley, M.J., et al. SFPE Handbook of Fire Protection Engineering. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-2565-0_47
Download citation
DOI: https://doi.org/10.1007/978-1-4939-2565-0_47
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-2564-3
Online ISBN: 978-1-4939-2565-0
eBook Packages: EngineeringEngineering (R0)