Abstract
The electrodeposition of cobalt from alternative electrolytes, including ionic liquids and deep eutectic solvents (DES), has become a topic of great interest to the scientific community, with a significant impact in both academic circles and in the development of commercial industrial electrochemical processes. However, very few studies have considered the effects of water on the electrodeposition of metals from deep eutectic solvents. In this work, the electrodeposition of Co from a choline chloride (ChCl) ethylene glycol (EG)-based deep eutectic solvent (DES) containing 10%, 20% and 30% water has been studied, and for the first time a uniform and bright Co deposit has been obtained when the deposition was achieved from an electrolyte containing 20% water. The speciation of Co in a mixed 1:2 ChCl:EG-based liquid (Ethaline 200) has been studied in both the absence and presence of water. The conductivities of the Co electrolyte were increased with increasing amounts of water. The electrochemical properties of the Co electrolytes have been studied using cyclic voltammetry, where it was found that the redox peak current gets larger and shifts in a positive direction when water was included in the Co solution. The resultant surface morphologies, topography, and roughness of the Co deposits were revealed by scanning electron microscopy (SEM) and atomic force microscopy (AFM), which demonstrated that a highly uniform and smooth cobalt coating had been produced when the deposition occurred in Ethaline 200 containing 20% water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cobalt is well known as a hard magnetic metal whose alloys have the excellent magnetic properties required for use in high-performance magnetic applications (Cui et al. 1990; Kelly et al. 1989; Pattanaik et al. 2007). Usually, electrodeposition of Co is achieved from aqueous solution. However, the disadvantages of aqueous solutions limit the deposition of metals generally; aqueous electrolytes suffer, in particular, from issues such as their electrochemical stability and limited potential windows (resulting in gas evolution leading to hydrogen embrittlement) (Abbott et al. 2013; Endres et al. 2017; Zhang et al. 2016). Whilst aqueous baths, for example acid baths, cyanide baths, and alkaline non-cyanide baths, can be used for the electrodeposition of metals (Abbott et al. 2013; Zhang et al. 2016), dealing with such baths requires particular caution due to the toxicity of the electrolytes. Therefore, ionic liquids (ILs) have been employed as alternatives for metal plating from aqueous solutions (Abbott et al. 2013; Abbott and McKenzie 2006; Celik et al. 2016; Schaltin et al. 2007; Yang et al. 2008).
A number of different room temperature ILs have been used as new electroplating systems as they have many advantages over aqueous solvents such as wide electrochemical potential windows, negligibly low vapour pressures, and high ionic conductivities (Abbott et al. 2004b; Abbott and McKenzie 2006; Endres 2002). A number of studies into the electrodeposition of Co have been published on ionic liquids (Carlin et al. 1998; Mitchell et al. 1996; Chen and Sun 2001; Koura et al. 1996; Schaltin et al. 2007; Zell and Freyland 2003). Carlin et al. (1996) noted that the electrodeposition of cobalt could be achieved from a chloroaluminate ionic liquid. Their studies concluded that Co deposition proceeds via a three-dimensional progressive nucleation with diffusion-controlled growth.
Hanke and Lynden-Bell (2003) studied the effects of water content on 1,3-dimethylimidazolium chloride and 1,3-dimethylimidazolium hexafluorophosphate ILs. They suggested that at low concentrations, the water is either isolated or exists in the solution as small independent clusters. The effects of water on 1-butyl-3-methylimidazolium [C4C1im][BF4], 1-octyl-3-methylimidazolium [C8C1im][BF4], and [C8C1im][Cl] were investigated by Feng and Voth, who found strong interactions between the anions and water molecules (Feng and Voth 2010). Some ionic liquids have issues such as high cost, toxicity, and lack of availability, and ILs such as 1-ethyl-3-methylimidazolium and 1-butyl-3-methylimidazolium are relatively sensitive to water. Such issues will clearly reduce their industrial utilities.
Solvents are of particular importance in the field of green chemistry, where new types of ILs have been produced by forming complexes between quaternary ammonium salts R1R2R3R4N+X− and hydrogen bond donors such as acids, amides and alcohols. These liquids are called deep eutectic solvents (DESs) (Abbott et al. 2003). Compared to other types of ILs, DESs have significant physiochemical advantages such as availability, non-toxicity, biodegradability, recyclability, non-flammability, and low cost (Ismail 2017; Smith et al. 2014; Ismail et al. 2019a). As DESs are relatively cheap and environmentally clean compared to the fabrication and use of ILs generally, they represent a much more attractive alternative to ILs from various perspectives. DESs have been used on an industrial scale in electropolishing (Abbott et al. 2006b, c), electroplating (Al-Esary 2017; Bernasconi et al. 2015), metal oxide processing (Abbott et al. 2006a) and polymer synthesis (Alesary et al. 2018; Hillman et al. 2017; Alesary et al. 2019b; Ismail et al. 2019a, b). The reduction processes of a range of metals have been investigated in DES systems including Zn (Abbott et al. 2011), Cr (Saravanan and Mohan 2011), Cu (Abbott et al. 2009; Xing et al. 2014), Ni (Abbott et al. 2008), Co (Cojocaru et al. 2015; Gómez et al. 2011), Al Sn (Pereira et al. 2012), and Ag (Sebastián et al. 2013). Electrodeposition of cobalt via several ILs has been investigated (Ali et al. 2005; Katayama et al. 2007; Schaltin et al. 2007; Zein El Abedin and Endres 2006), but despite this very little information is available on the electrodeposition and reduction mechanisms of pure cobalt in choline chloride–ethylene glycol DESs; similarly, there are only a few studies that have considered the effects of water on the metal’s electrodeposition from choline chloride-based ILs. Koen and Deun have studied the speciation of copper (II) complexes in a choline chloride-based IL and in choline chloride/water mixtures, finding that water can change the copper species present in the IL (De Vreese et al. 2012). Furthermore, they found that the redox potential of Cu has changed as a result of adding specific ratios of water to their Cu electrolyte. The addition of different amounts of water to a choline chloride-urea-based DES (Reline) was studied by Hammond et al. (2017). They found that water leads to weaker interactions between all the components, i.e., Ch–Cl, Ch–urea, urea–Cl and urea–urea, where the authors did not comment in this regard why this happened. Recently, Al-Murshedi investigated DES–water mixtures in the deposition of copper for different water contents (Al-Murshedi 2018). Their study showed that water increased the brightness of the copper deposition on a nickel substrate due to changes in the crystallite size. In addition, water has the effect of changing the physicochemical properties of DESs by increasing mass transport.
In our previous work (the effect of additives on the electrodeposition of Zn), Zn deposition was achieved from a DES-based IL (Alesary et al. 2019a, b). To the best of our knowledge, there is no previous work in the literature that considers the effect of adding different amounts of water on the electrodeposition of Co from a DES. The aims of this work are to deposit Co from Ethaline 200 in a bright, dense, thick and adherent coating and also to study the effect of water on speciation, conductivity and electrochemical properties of Co in DES. Moreover, to see how water effect on the deposition of cobalt in terms of the deposit morphology and roughness. In the present study, Co species and their conductivities in 1:2 mix of choline chloride and ethylene glycol (Ethaline 200) have been studied with and without the addition of water. Cyclic voltammetry has been used to examine the reduction mechanism of cobalt from the aqueous–Ethaline 200 mixtures. In addition, the effects of adding different concentrations of water on morphologies and roughness of Co deposits were investigated via SEM and AFM.
Experimental
Choline chloride and ethylene glycol were obtained from (Aldrich + 99%). The deep eutectic mixture (Ethaline 200) was prepared by continuous stirring of the two components in a 1:2 ratio of choline chloride:ethylene glycol at 60 °C until a homogeneous, colourless liquid was formed. The cobalt salt CoCl2·6H2O (Aldrich ≥ 98%) was added to form a final concentration in all liquids of 0.3 M. The conductivities of the Co electrolytes, in both the absence and presence of water, were measured as function of temperature between 25 and 85 °C using a Jenway 4510 conductivity meter. A Shimadzu model UV-1601 UV–visible spectrophotometer was used to compare the coordination sphere of the cobalt ion in the Ethaline 200 with varying molar ratios of water. The cell path length was equal to 10 mm, i.e., a standard cuvette was used. Values for λmax were determined using the spectrophotometer’s built-in peak-pick feature, using the uv-probe software.
Cyclic voltammetry was performed using a potentiostat/galvanostat/ZRA with optional FRA/EIS controlled with the GPES2 software using three electrodes: a 0.5 mm platinum disc as the working electrode, a platinum sheet as the counter electrode and an Ag/AgCl reference electrode. Voltammograms of Co were obtained using a potential window of +0.5 V to − 0.7 V. In all experiments, the working electrode was polished using 0.05 alumina paste and cleaned by rinsing with deionized water followed by acetone prior to each experiment.
Bulk electrolysis (Fig. 1.) was carried out using cathodic plates (copper, 50 mm × 42 mm × 1 mm) which were etched with aqueous 0.87 M ammonium persulfate solution, (NH4)2S2O8 (Aldrich) and 0.2 M H2SO4 (Fisher) solution, and then wished with water, then acetone and then dried. An iridium oxide-coated titanium mesh electrode was used as the anode. The electrodepositions were achieved in Ethaline 200 made up to 0.3 M CoCl2·6H2O with and without different concentrations of water (10%, 20% and 30%). The bulk electrodepositions were achieved at a current density of 2.5 mA cm−2 for 1 h and at 70 °C.
The surface morphologies and compositions of the cobalt deposits were characterized using scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) using a Phillips XL30 FEG with an accelerating voltage of 20 kV, giving an average beam current of ca. 120 μA. Both contact and tapping (resonant) modes in the digital instruments nanoscope IV Dimension 300 (Veeco) atomic force microscope with 100 mm scanning head were used to examine the nucleation and roughness of the Co films. Images were acquired in air using the Nanoscope version 6.13 control software.
Results and discussion
Speciation
The coordination environment of the metal species dissolved in the IL to a certain extent determines its electrochemical behaviour. Adding water to the Ethaline 200/CoCl2·6H2O solution can change the chemical species formed with some of the Co ions present. Solution colour is indicative of Co speciation, and indeed various metal salts can be identified because they show significant thermochromism, as has been reported for both conventional ILs and DES solutions (Linert et al. 2001; Wei et al. 2008). Here, UV–Vis spectroscopy was used to study the thermochromic behaviour of CoCl2·2H2O in pure Ethaline 200 in the presence of different water contents, i.e., 10 wt%, 20 wt% and 30 wt%, as shown in Fig. 2. It has been established that the major CoCl2·6H2O species in pure Ethaline 200 is [CoCl4]2− (Hartley et al. 2014). However, the effects of water on the Co species that is subsequently formed in Ethaline 200 need to be investigated thoroughly. There was no difference in the speciation before and after the addition of different concentrations of water, so this indicates that these additives do not tend to change the coordination environment of Co2+. It has been stated that the bivalent cobalt species is dissolved by forming [Co(TFSA)3]− in the EMITFSA IL. Thus, the bivalent cobalt species is considered to be [Co(TFSA)3]− in BMPTFSA (Fujii et al. 2008).
It has also been reported that changing the geometry of the cobalt (II) from octahedral or dodecahedral to tetrahedral does not occur in ILs during the heating or cooling process (Banić et al. 2014). Quantitatively, it should be noted that the octahedral complexes are typically pale red or purple, while many common tetrahedral complexes are an intense blue (e.g., in Ethaline 200). In each case, the visible spectrum is influenced by the highest energy \(^{4} A_{2 } \to T_{1} \left( P \right)\) transition for tetrahedral and the \(^{4} T_{{1{\text{g}}}} \left( F \right) \to^{4} T_{{1{\text{g}}}} \left( P \right)\) transition for octahedral complexes, where in octahedral systems the 4A2g level is typically closer to the 4T1g (P) level and the transitions to these two levels can frequently overlap.(Cotton et al. 1988) Because the 4A2g state is derived from T 32g E4 electron configuration, while the 4T1g(F) ground state is derived mainly from a T 52g E 2g configuration, the \(^{4} T_{{1{\text{g}}}} \left( F \right) \to^{4} A_{{2{\text{g}}}}\) transition is essentially a two-photon process and thus weaker by about a factor of 10−2 than other transitions. In the tetrahedral system, the visible transition is stronger and shifted to lower energies, consistent with the colour observed, as mentioned above. For the octahedral complex, there is one more spin allowed transition 4T1g(F) → 4T2g, which occurs in the near infrared region (Cotton et al. 1988). For tetrahedral complexes, there is also a transition in close to the infrared region [4A2 → 4T1(F)], in addition to one of quite low energy (4A2 → 4T2), which is rarely detected because it is in an obscure region of the spectrum (1–2 µm) and, more importantly, because it is formally forbidden.
In Fig. 2, in the case of the cobalt solution that does not contain water, a broad spectrum was obtained that contained three distinct peaks at 632.12 nm, 667.01 nm and 694.54 nm. This spectrum corresponded to the [CoCl4]2 complex (Hartley et al. 2014). There was no difference between the speciation before and after addition of water; however, the intensity of the peaks decreased with increasing water content, which could purely have been due to the decreasing concentration of [CoCl4]2− in the solution as a result of producing new species such as [Co(H2O)6]2+ or [CoCl(H2O)5]+, or could have been due to a change in the geometry of Co complexes from tetrahedral to octahedral, where the colour of the Co solution changed gradually from blue to purple with the increasing quantity of water added. The visible transition in the tetrahedral example has complex envelopes because several transitions to the doublet excited state can be observed in the same region and these obtain some intensity by means of spin–orbit coupling.
Conductivities of Co electrolytes
In the majority of ionic liquid electrodeposition studies to date, little attention has been paid to the effect that the addition of water has upon the resultant conductivity. In general, most ILs have low conductivities compared to the aqueous electrolyte at room temperature. Generally, the formation of the large number of hydrogen bonds leads to an increase in the viscosity of ionic liquids, thus reducing their conductivities. This could be due to a lattice disruption effect of the liquid that alters the coordination of the Cl− interaction. In electrochemical applications, a high conductivity is required to reduce cell resistance and Ohmic heating during bulk electrolysis (Garcia et al. 2015; Zhang et al. 2012). In this work, the effects of adding water to the 1ChCl:2EG liquid containing 0.3 M CoCl2·6H2O at 25 °C are demonstrated in Fig. 3a. It can be seen that with increasing amounts of water, the conductivities of the Co solutions actually increased. Figure 3a shows a significant change in the conductivities of the CoCl2·6H2O electrolytes with increasing water content. The highest conductivity, 29.2 mS cm−1, for the CoCl2·6H2O solution was recorded for a solution containing 30 wt% water.
In addition, the influence of temperature on the conductivities of CoCl2·2H2O in pure Ethaline 200 and in the presence of 10 wt%, 20 wt% and 30 wt% water was studied, as shown (Fig. 3b). It is clear from this figure that the conductivities of Co solution increased with increasing temperature due to the associated increase in ionic mobility, which is consistent with previous results in the literature (Abbott et al. 2004a; Al-Murshedi 2018; Cao et al. 2015; Popescu et al. 2014).
In this section, the key point is to provide a model for this increase in conductivity increasing upon the addition of water. Clearly, it can be predicted that the conductivity of the Co electrolyte will increase as the proportion of water increases due to the magnitude of the interaction between water and the ions of the IL being smaller than ion–ion interactions (Vila et al. 2006). According to Eq. (1)(Vila et al. 2006)
where D is the diffusion coefficient of the holes (ions), γ is the surface tension of the liquid, kB the Boltzmann constant, T is the absolute temperature of the liquid and m is the mass of a particle. This effect can be simulated via whole theory by modification of the surface tension of the liquid when adding water. Consequently, a structural model for the IL + water system is required to determine the effects of the water on ionic transport. Figure 3a shows that there is no huge change in the conductivity of the Co solution that contained a relatively small amount of water (10%). Thus, it can be assumed that the addition of small amounts of water to the IL does not lead to a significant change in the structural picture of hole theory; that is, the conduction still relies on the existence of cavities in the interior of the liquid being occupied by ions in the course of their motion. It is clear that the validity of this hypothesis is restricted to very small amounts of water in the IL solutions because, in limited concentrations of water, a water droplet network will form, as will isolated water molecules and water groups (Hanke and Lynden-Bell 2003). In ionic mixtures, more so than water molecules, the latter tend to be isolated from one another (Cammarata et al. 2001; Hanke and Lynden-Bell 2003; Vila et al. 2006) and strongly linked to any anions present (Hanke et al. 2002; Vila et al. 2006). Therefore, it can be suggested that absorbed molecules are the anion–water complexes produced when water is added to pure IL. This results in a reduction in the viscosity of the liquid and a concomitant increase in conductivity. The increase in conductivity found for the Co electrolytes that contained 20% and 30% water was 21.1 mS cm−1 and 29.2 mS cm−1, respectively. We can conclude that the change in conductivity of Co bath as a result of adding water can be clarified within the framework of conventional hole theory resulting in the modification of the surface tension of the liquid. Therefore, the size of the hole increases, leading to an increase in ion mobility in the IL–water mixture, thus increasing the conductivity.
Cyclic voltammetric studies
Cyclic voltammetry was used to investigate the electrochemical behaviour of cobalt in the 1ChCl:2EG-based liquid containing 0.3 M CoCl2·6H2O at different temperatures (25, 50, 70 °C) as shown in Fig. 4. The CVs were obtained using a Pt working electrode in the potential range 0.5 V to − 0.7 V (vs. Ag/AgCl) at a scan rate of 10 mV s−1. There is a clear change in the cyclic voltammograms of Co when the cyclic voltammetry was performed at different temperatures. The Co reduction peak was shifted anodically by about 150 mV when the experiment was run at 70 °C, as illustrated in Fig. 4, due to the decrease in viscosity and IR ohmic drop with increasing temperature, causing the Co2+ to deposit at a lower potential. The temperature mainly influences the viscosity and conductivity of the plating liquid, which affects the electrochemical processes. With increasing temperature, the free void volume in the ILs will increase, enhancing the movement of the cations and anions in the liquid, and accelerating the stripping and reduction processes through increased mass transport towards the electrode surfaces. In this case, the high temperatures increased the oxidation and reduction current peak of Co due to the decrease in viscosity and increase in conductivity of the Co solution, as per the explanation given in the previous section (see Fig. 3b).
In addition, the electrodeposition of the Co could be inhibited by the specific adsorption of free chloride ions at the electrode surface. High temperatures can work to reduce the incorporation of chloride ions into the electrochemical double layer, where at the high temperature of 70 °C the concentration of [CoCl4]−2 species at the electrode surface will be increased due to deceased activity and mobility of the chloride ions. Thus, less energy is required to reduce the Co ions, and the oxidation and reduction currents peaks of the Co species will increase with increasing temperature, as illustrated in Fig. 4. This means that the rate of Co deposition increased as a result of applying the potential at 70 °C.
No stripping peak can be seen cyclic voltammogram of Co when the experiments were conducted at 25 °C and 50 °C. However, a clear stripping peak can be observed at about − 0.1 V when the process is performed at 70 °C. This indicates that the Co species have been deposited on the Pt electrode surface at 70 °C and that the film was dissolved when the scan was reversed to its starting potential. The above was identical to our previous work, where it was found that Zn deposits could be successfully produced from a bath at higher temperature (70–80 °C) (Alesary et al. 2019a, b). Therefore, it was suggested that bulk Co deposition should be conducted at higher temperatures (for example, 70 °C or 80 °C). A Co film was produced on the copper substrate when the deposition was conducted at 70 °C which was examined via SEM and EDX, as illustrated in Fig. 5.
In addition, cyclic voltammetry of 0.3 M CoCl2·6H2O in Ethaline 200 at different scan rates was studied as demonstrated in (Fig. 6a). Here, these experiments were also recorded using a polished Pt disk as the working electrode, and using a Pt flag counter electrode and an Ag/AgCl reference electrode. These voltammograms were initially scanned from +0.5 to − 0.7 V and then reversed to the initial point a constant temperature of 70 °C. The cathodic peak potential shows no change with increasing scan rate, while there is an obvious increase in the stripping/reduction peaks with increased scan rate, as expected (Abbott et al. 2007; Al-Esary 2017; Alesary et al. 2019a, b). The theoretical value of the difference between the oxidation and reduction potentials (ΔEp) for a one-electron Nernstian process and for a reversible system of metals redox is 59 mV at T = 298 K (Monk 2008). However, here, in the voltammograms recorded for Co in Ethaline 200 (Fig. 6a), the difference between the cathodic and anodic peak potentials is around 180 mV. This indicates that the electrode reaction of Co2+ to Co is more likely to be irreversible and proceeds via a one-step, two-electron transfer process.
a Cyclic voltammograms of Ethaline 200 containing 0.3 M CoCl2·6H2O recorded with a Pt working electrode, Pt flag counter electrode and an Ag/AgCl as a reference electrode at different scan rates and at 70 °C. b Reduction current peaks for Co in Ethaline 200 as a function of the square root of the scan rate
Figure 6b shows that a good linearity was obtained in plots of ipc against the square root of scan rate (v1/2), and there is no obvious shift in the reduction potential of Co (Fig. 6a) with increasing scan rate. Therefore, it can be suggested that the electrodeposition of Co from Ethaline 200 is not significantly affected by mass diffusion and can be controlled by electron transfer or surface chemical reaction steps.
Cyclic voltammetry in the presence of water
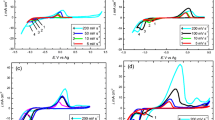
The aim of this work is to examine the electrodeposition of Co from Ethaline 200 containing water; thus, it is more important to study the electrochemical behaviour of Co species in this type of liquid in the absence and presence of different concentrations of water. Figure 7 shows the cyclic voltammetry of 0.3 M CoCl2·6H2O in Ethaline 200 on a Pt electrode as a function of different amounts of water. The experiments were recorded at 70 °C, with a scan rate of 10 mV s−1 and where the potential window was between 0.5 and − 0.7 V. It was concluded from the conductivity data (Fig. 3a) that the conductivities of the Co solutions increased with increasing amounts of water, which corresponded to an increase in ion mobility in the IL–water due to its decreased viscosity and increased void volume.
Figure 7 shows a clear change in the CVs of Co as a result of adding water to the Co electrolyte. In the system without water, Co species start to deposit at − 0.4 V. However, the potential of the deposition shifted positively by about 50 mV when the process was conducted in the system containing 10% water. Moreover, an enhancement in the stripping/deposition current peaks was obtained as a result of adding water to the plating bath. The first reason for this change in the CVs is the associated decrease in viscosity and increase in conductivity of the Co solution. The second reason could be due to the reduced adsorption of the choline cations and chloride ions on the Pt electrode surface due to the addition of water.
Water molecules can be adsorbed or link to the IL anions and then allow to Co species to deposit more easily. This leads to an increased rate of Co deposition and then an increase in the dissolution of the Co deposit. In this work, it can be seen that the stripping current peaks (Fig. 7) increased as a result of the increasing amount of Co that has been deposited. From the UV–Vis data (Fig. 2), we expected that a change in the geometry of the Co spices had occurred (tetrahedral to octahedral) and that the new species could be deposited more easily, thus modifying the CV of Co. In our previous work, the choline cation was believed to have adsorbed on the cathode electrode surface and cooperated in the formation of the double layer (Alesary et al. 2019a, b; Alesary 2019). Water can reduce the effect of these cations through the attraction between them (Vila et al. 2006). Thus, adsorption of anions/cations will result in their reduction on the cathode electrode surface, enhancing the growth of the Co.
A nucleation loop can be observed in the cyclic voltammograms of electrolytes that contained high concentrations of water (for example, 20% and 30%). This indicates that the nucleation/growth rate for the Co deposition was high compared to the nucleation rate for Co deposition from the system without water, and indeed that with only 10% water. Figure 7 also shows the change in the stripping potential of Co deposited from electrolytes in the presence of water, where the Co deposit began to be removed from the electrode surface at − 0.21 V, and where this potential was shifted negatively by about 50 mV when the coating was produced from systems that contained water. This was either due to the increasing amount of Co being deposited or the formation of different Co phases, for example on the microscale where the latter requires less energy to oxidize.
Cobalt deposit morphology
The morphology of the Co deposits was studied via SEM and AFM. Figure 8 displays the photographs, morphologies and AFM images of the Co films that were deposited from Ethaline 200 containing 0.3 M CoCl2·6H2O in (a) the absence of water, and (b) in the presence of 10% H2O, (c) 20% H2O, and (d) 30% H2O. The deposition was achieved at 70 °C for 1 h on a copper substrate using a current density of 2.5 mA cm−2. A black Co deposit was produced when the deposition occurred from the Co bath in the absence of water, as can be seen in (Fig. 8a). No significant change was observed in the morphology of the Co film when the deposition was achieved from a bath containing 10% water, although a non-homogeneous, dark grey Co film was formed, as presented in (Fig. 8b). However, a quite remarkable modification was noted in the morphology of the Co films deposited from a bath containing 20% water, where a homogeneous and bright Co deposit was formed as per Fig. 8c. The morphology of the Co deposit obtained from the Co electrolyte containing 30% water was somewhat improved compared to that obtained from the bath containing 10% of water, where it can be seen that the grain size of Co deposit was somewhat smaller (Fig. 8d), although the coating was not as homogenous as that obtained from the bath containing 20% water. The viscosity and conductivity of the plating liquid are key parameters affecting the electrochemical processes, and these are mainly influenced through the use of water. Figure 8 also shows the nucleation and the roughness of the Co deposits examined using AFM, where the roughness of the Co deposited in pure Ethaline was 327 nm but which decreased significantly to 30 nm when the deposition was performed in the electrolyte with 20% water. The roughness of the Co deposit formed from the system with 30% water was around 123 nm, which is less than the roughness of the Co coating in the system containing 10% water, though it is still greater than that formed from the bath with a 20% water content.
Photographic images, SEM images and AFM topographies of Co deposits from Ethaline 200 containing 0.3 M CoCl2·6H2O in a the absence of water, and b in the presence of 10% H2O, c 20% H2O and d 30% H2O (All depositions were achieved at 70 °C for 1 h on a Cu substrate at an applied current density of 2.5 mA cm−2)
It was mentioned earlier in this work that water molecules can be linked to ionic liquid ions and reduce their activities such as adsorption on the electrode surface and reducing the viscosity of the solution, which leads to the increased movement of the cations and anions in the liquid and, thus, an increased rate of Co deposition. Electrodeposition of Co could be inhibited in Ethaline 200 by the incorporation of chloride ions in the electrochemical double layer. Adding water may work to reduce the specific adsorption of free chloride ions at the electrode surface and promoting Co reduction as the activity and mobility of the chloride ions decreased (Abbott et al. 2011). It can be concluded that the SEM revealed that the morphologies of the Co deposition were extremely dependent on the proportion of water in the plating bath.
Adding water to the plating bath did not affect the current efficiency of electroplating of Co; consequently, this means that low hydrogen evolution can be formed from the cathode surface. Whereas the current efficiency of the Co electrodeposition formed from Ethaline 200 in the presence of 20% H2O was calculated, its efficiency was found to be ~ 97%. According to Faraday’s Law (Q = nFN), the charge measured is directly proportional to the number of species deposited in moles (N); the thickness of cobalt coating produced from the bath containing 20% was about 6.16 where this is considered to be the efficiency of the current close to 100%. Thus, it can be said that there is no significant effect for hydrogen evolution on electrodeposition of Co from Ethaline 200 in the presence of water.
Conclusions
No previous work has previously been undertaken on Co electrodeposition from choline chloride–ethylene glycol deep eutectic solvents containing water. In this study, the electrodeposition of Co from 1ChCl:2EG (Ethaline 200) containing 10%, 20% and 30% water on a Cu substrate at 70 °C was investigated. The properties of the Co electrodeposit have been optimized compared to the corresponding system without water, where a bright cobalt deposit was obtained when the coating was performed using a system containing 20% water. Clear differences in the cyclic voltammograms of Co were observed when water was added to the Co electrolytes, which corresponded to the increase in conductivity of the plating liquids and possibly to a change in the Co species present.
No remarkable change in the morphology of the Co deposits from the system containing 10% water was observed; however, the coating morphology was found to be significantly improved when the proportion of water in the plating bath was increased to 20%. A reduced roughness in the Co deposit (30 nm) was achieved when the deposition occurred in the electrolyte containing 20% water, while the roughness of Co films from the baths containing 10% and 30% water was 327 nm and 120 nm, respectively. The morphology of the Co deposit formed form the electrolyte with the 30% water content was of lower quality than those from the Co bath with 20% water. This was due to the increased hydrogen evolution and the formation of a passivation layer. It can be concluded that a uniform, bright Co deposit was formed when the deposition occurred in Ethaline 200 as a DES that further contained 20 wt% water.
References
Abbott AP, McKenzie KJ (2006) Application of ionic liquids to the electrodeposition of metals. Phys Chem Chem Phys 8:4265–4279. https://doi.org/10.1039/B607329H
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V (2003) Novel solvent properties of choline chloride/urea mixtures. Chem Commun 1:70–71. https://doi.org/10.1039/B210714G
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004a) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126:9142–9147. https://doi.org/10.1021/ja048266j
Abbott AP, Capper G, Davies DL, Rasheed R (2004b) Ionic liquids based upon metal halide/substituted quaternary ammonium salt mixtures. Inorg Chem 43:3447–3452. https://doi.org/10.1149/1.2760185
Abbott AP, Capper G, McKenzie KJ, Ryder KS (2006a) Voltammetric and impedance studies of the electropolishing of type 316 stainless steel in a choline chloride based ionic liquid. Electrochim Acta 51:4420–4425. https://doi.org/10.1016/j.electacta.2005.12.030
Abbott AP, Capper G, Davies DL, McKenzie KJ, Obi SU (2006b) Solubility of metal oxides in deep eutectic solvents based on choline chloride. J Chem Eng Data 51:1280–1282. https://doi.org/10.1021/je060038c
Abbott AP, Capper G, McKenzie KJ, Glidleb A, Ryder KS (2006c) Electropolishing of stainless steels in a choline chloride based ionic liquid: an electrochemical study with surface characterisation using SEM and atomic force microscopy. Phys Chem Chem Phys 8:4214–4221. https://doi.org/10.1039/B607763N
Abbott AP, Barron JC, Ryder KS, Wilson D (2007) Eutectic-based ionic liquids with metal-containing anions and cations. Chem Eur J 13:6495–6501. https://doi.org/10.1002/chem.200601738
Abbott AP, El Ttaib K, Ryder KS, Smith EL (2008) Electrodeposition of nickel using eutectic based ionic liquids. Trans Inst Met Finish 86:234–240. https://doi.org/10.1179/174591908X327581
Abbott AP, El Ttaib K, Frisch G, McKenziea KJ, Ryder KS (2009) Electrodeposition of copper composites from deep eutectic solvents based on choline chloride. Phys Chem Chem Phys 11:4269–4277. https://doi.org/10.1039/B817881J
Abbott AP, Barrona JC, Frischa G, Ryder KS, Silva AF (2011) The effect of additives on zinc electrodeposition from deep eutectic solvents. Electrochim Acta 56:5272–5279. https://doi.org/10.1016/j.electacta.2011.02.095
Abbott AP, Frisch G, Ryder KS (2013) Electroplating using ionic liquids. Annu Rev Mater Res 43:335–358. https://doi.org/10.1146/annurev-matsci-071312-121640
Al-Esary HFN (2017) Influence of additives on electrodeposition of metals from deep eutectic solvents. PhD Thesis, Department of Chemistry, University of Leicester. http://hdl.handle.net/2381/40869
Alesary HF, Ismail HK, Khudhair AF, Mohammed MQ (2018) Effects of dopant ions on the properties of polyaniline conducting polymer. Orient J Chem 34:2525. https://doi.org/10.13005/ojc/340539
Alesary HF, Cihangira S, Ballantynea AD, Harrisa RC, Westonc DP, Abbotta AP, Ryder KS (2019a) Influence of additives on the electrodeposition of zinc from a deep eutectic solvent. Electrochim Acta 304:118–130. https://doi.org/10.1016/j.electacta.2019.02.090
Alesary HF, Khudhair AF, Rfaish SY, Ismail HK (2019b) Effect of sodium bromide on the electrodeposition of Sn, Cu, Ag and Ni from a deep eutectic solvent-based ionic liquid. Int J Electrochem Sci 14:7116–7132. https://doi.org/10.20964/2019.08.80
Ali M, Nishikata A, Tsuru T (2005) Electrodeposition of cobalt from cobalt chloride-N-(n-butyl) pyridinium chloride molten salt. Indian J Chem Technol 12:648–653
Al-Murshedi AYM (2018) Deep eutectic solvent–water mixtures. Department of Chemistry, University of Leicester. http://hdl.handle.net/2381/42799
Banić N, Vraneš M, Abramović B, Csanádia J, Gadžurić S (2014) Thermochromism, stability and thermodynamics of cobalt (II) complexes in newly synthesized nitrate based ionic liquid and its photostability. Dalton Trans 43:15515–15525. https://doi.org/10.1039/C4DT01836B
Bernasconi R, Zebarjadi M, Magagnin L (2015) Copper electrodeposition from a chloride free deep eutectic solvent. J Electroanal Chem 758:163–169. https://doi.org/10.1016/j.jelechem.2015.10.024
Cammarata L, Kazarian SG, Salterb PA, Welton T (2001) Molecular states of water in room temperature ionic liquids. Phys Chem Chem Phys 3:5192–5200. https://doi.org/10.1039/B106900D
Cao Q, Lu X, Wu X, Guo Y, Xu L, Fang W (2015) Density, viscosity, and conductivity of binary mixtures of the ionic liquid N-(2-hydroxyethyl) piperazinium propionate with water, methanol, or ethanol. J Chem Eng Data 60:455–463. https://doi.org/10.1021/je500380x
Carlin RT, Trulove PC, De Long HC (1996) Electrodeposition of cobalt-aluminum alloys from room temperature chloroaluminate molten salt. J Electrochem Soc 143:2747–2758. https://doi.org/10.1149/1.1837102
Carlin RT, De Long HC, Fuller J, Trulove PC (1998) Microelectrode evaluation of transition metal-aluminum alloy electrodepositions in chloroaluminate ionic liquids. J Electrochem Soc 145:1598–1607. https://doi.org/10.1149/1.1838524
Celik YC, Pulletikurthi G, Endres F (2016) Electrodeposition of Al, Zn, and Pt on silver-coated textile fibres from ionic liquids. J Solid State Electrochem 20:2781–2790. https://doi.org/10.1007/s10008-016-3276-6
Chen PY, Sun IW (2001) Electrodeposition of cobalt and zinc-cobalt alloys from a lewis acidic zinc chloride-1-ethyl-3-methylimidazolium chloride molten salt. Electrochim Acta 46:1169–1177. https://doi.org/10.1016/S0013-4686(00)00703-9
Cojocaru A, Mares ML, Prioteasa P, Liana A, Visan T (2015) Study of electrode processes and deposition of cobalt thin films from ionic liquid analogues based on choline chloride. J Solid State Electrochem 19:1001–1014. https://doi.org/10.1007/s10008-014-2711-9
Cotton FA et al (1988) Advanced inorganic chemistry. University of London, London
Cui CQ, Jiang SP, Tseung ACC (1990) Electrodeposition of cobalt from aqueous chloride solutions. J Electrochem Soc 137:3418–3423
De Vreese P, Brooks NR, Hecke KV, Meervelt LV, Matthijs E, Binnemans K, Deun RV (2012) Speciation of copper (II) complexes in an ionic liquid based on choline chloride and in choline chloride/water mixtures. Inorg Chem 51:4972–4981. https://doi.org/10.1021/ic202341m
El Abedin SZ, Endres F (2006) Electrodeposition of metals and semiconductors in air-and water-stable ionic liquids. ChemPhysChem 7:58–61. https://doi.org/10.1002/cphc.200500288
Endres F (2002) Ionic liquids: solvents for the electrodeposition of metals and semiconductors. ChemPhysChem 3:144–154. https://doi.org/10.1002/1439-7641(20020215)3:2%3c144:AID-CPHC144%3e3.0.CO;2-%23
Endres F, Abbott A, MacFarlane D (2017) Electrodeposition from ionic liquids. Wiley, Berlin. https://doi.org/10.1002/9783527682706
Feng S, Voth GA (2010) Molecular dynamics simulations of imidazolium-based ionic liquid/water mixtures: alkyl side chain length and anion effects. Fluid Phase Equilib 294:148–156. https://doi.org/10.1016/j.fluid.2010.02.034
Fujii K, Nonaka T, Akimoto Y, Umebayashi Y, Ishiguro S (2008) Solvation structures of some transition metal (II) ions in a room-temperature ionic liquid, 1-ethyl-3-methylimidazolium bis (trifluoromethanesulfonyl) amide. Anal Sci 24:1377–1380. https://doi.org/10.2116/analsci.24.1377
Garcia G, Aparicio S, Ullah R, Atilhan M (2015) Deep eutectic solvents: physicochemical properties and gas separation applications. Energy Fuels 29:2616–2644. https://doi.org/10.1021/ef5028873
Gómez E, Cojocarub P, Magagninb L, Vallesa E (2011) Electrodeposition of Co, Sm and SmCo from a deep eutectic solvent. J Electroanal Chem 658:18–24. https://doi.org/10.1016/j.jelechem.2011.04.015
Hammond OS, Bowron DT, Edler KJ (2017) The effect of water upon deep eutectic solvent nanostructure: an unusual transition from ionic mixture to aqueous solution. Angew Chem Int Ed 56:9782–9785. https://doi.org/10.1002/anie.201702486
Hanke C, Lynden-Bell R (2003) A simulation study of water-dialkylimidazolium ionic liquid mixtures. J Phys Chem B 107:10873–10878. https://doi.org/10.1021/jp034221d
Hanke CG, Atamas NA, Lynden-Bella RM (2002) Solvation of small molecules in imidazolium ionic liquids: a simulation study. Green Chem 4:107–111. https://doi.org/10.1039/B109179B
Hartley JM, Ip CM, Forrest GCH, Singh K, Gurman SJ, Ryder KS, Abbott AP, Frisch G (2014) EXAFS study into the speciation of metal salts dissolved in ionic liquids and deep eutectic solvents. Inorg Chem 53:6280–6288. https://doi.org/10.1021/ic500824r
Hillman AR, Ryder KS, Ismail HK, Unala A, Voorhaar A (2017) Fundamental aspects of electrochemically controlled wetting of nanoscale composite materials. Faraday Discuss 199:75–99. https://doi.org/10.1039/C7FD00060J
Ismail HK (2017) Novel battery chemistries using electrically conducting polymers synthesised from deep eutectic solvents and aqueous solutions. PhD Thesis, Department of Chemistry, University of Leicester. http://hdl.handle.net/2381/39875
Ismail HK, Alesary HF, Al-Murshedi AY, Kareem JH (2019a) Ion and solvent transfer of polyaniline films electrodeposited from deep eutectic solvents via EQCM. J Solid State Electrochem 23:3107–3121. https://doi.org/10.1007/s10008-019-04415-1
Ismail HK, Alesary HF, Mohammed MQ (2019b) Synthesis and characterisation of polyaniline and/or MoO2/graphite composites from deep eutectic solvents via chemical polymerisation. J Polym Res 26:65. https://doi.org/10.1007/s10965-019-1732-6
Katayama Y, Fukui R, Miura T (2007) Electrodeposition of cobalt from an imide-type room-temperature ionic liquid. J Electrochem Soc 154:D534–D537. https://doi.org/10.1149/1.2768298
Kelly PE, O’Grady K, Mayo PI, Chantrell RW (1989) Switching mechanisms in cobalt-phosphorus thin films. IEEE Trans Magn 25:3881–3883. https://doi.org/10.1109/20.42466
Koura N, Endo T, Idemoto Y (1996) The electrodeposition of amorphous Co-Zn alloy from ambient temperature molten salt electrolytes. J Non-Crystal Solids 205:650–655. https://doi.org/10.1016/S0022-3093(96)00289-X
Linert W, Fukudab Y, Camard A (2001) Chromotropism of coordination compounds and its applications in solution. Coord Chem Rev 218:113–152. https://doi.org/10.1016/S0010-8545(01)00359-9
Mitchell JA, Pitner WR, Hussey CL, Stafford GR (1996) Electrodeposition of cobalt and cobalt-aluminum alloys from a room temperature chloroaluminate molten salt. J Electrochem Soc 143:3448–3455. https://doi.org/10.1149/1.1837235
Monk PM (2008) Fundamentals of electroanalytical chemistry. Wiley, New York
Pattanaik G, Kirkwood DM, Xu X, Zangar G (2007) Electrodeposition of hard magnetic films and microstructures. Electrochim Acta 52:2755–2764. https://doi.org/10.1016/j.electacta.2006.07.062
Pereira NM, Pereira CM, Silva AF (2012) The effect of complex agents on the electrodeposition of tin from deep eutectic solvents. ECS Electrochem Lett 1:D5–D7. https://doi.org/10.1149/2.003202eel
Popescu AM, Donath C, Constantin V (2014) Density, viscosity and electrical conductivity of three choline chloride based ionic liquids. Bulg Chem Commun 46:452–457
Saravanan G, Mohan S (2011) Electrodeposition of Fe–Ni–Cr alloy from deep eutectic system containing choline chloride and ethylene glycol. Int J Electrochem Sci 6:1468–1478
Schaltin S, Nockemannb P, Thijsb B, Binnemansb K, Fransaera J (2007) Influence of the anion on the electrodeposition of cobalt from imidazolium ionic liquids. Electrochem Solid State Lett 10:D104–D107. https://doi.org/10.1149/1.2760185
Sebastián P, Vallés E, Gómez E (2013) First stages of silver electrodeposition in a deep eutectic solvent. Comparative behavior in aqueous medium. Electrochim Acta 112:149–158. https://doi.org/10.1016/j.electacta.2013.08.144
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082. https://doi.org/10.1021/cr300162p
Vila J, Ginésa P, Riloa E, Cabezaa O, Varela LM (2006) Great increase of the electrical conductivity of ionic liquids in aqueous solutions. Fluid Phase Equilib 247:32–39. https://doi.org/10.1016/j.fluid.2006.05.028
Wei X, Yu L, Wang D, Jina X, Chen GZ (2008) Thermo-solvatochromism of chloro-nickel complexes in 1-hydroxyalkyl-3-methyl-imidazolium cation based ionic liquids. Green Chem 10:296–305. https://doi.org/10.1039/B715763K
Xing S, Zanella C, Deflorian F (2014) Effect of pulse current on the electrodeposition of copper from choline chloride-ethylene glycol. J Solid State Electrochem 18:1657–1663. https://doi.org/10.1007/s10008-014-2400-8
Yang W, Cang H, Tang Y, Wang J, Shi Y (2008) Electrodeposition of tin and antimony in 1-ethyl-3-methylimidazolium tetrafluoroborate ionic liquid. Phys Chem Chem Phys 38:537–542. https://doi.org/10.1007/s10800-007-9470-6
Zell C, Freyland W (2003) In situ STM and STS study of Co and Co-Al alloy electrodeposition from an ionic liquid. Langmuir 19:7445–7450. https://doi.org/10.1021/la030031i
Zhang Q, Vigier KDO, Royera S, Jérôme F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41:7108–7146. https://doi.org/10.1039/C2CS35178A
Zhang Q, Wang Q, Zhang S, Lu X, Zhang X (2016) Electrodeposition in ionic liquids. ChemPhysChem 17:335–351. https://doi.org/10.1002/cphc.201500713
Acknowledgements
The authors wish to acknowledge the University of Kerbala, Kufa and Koya for providing the required materials and instruments for this work. They would also like to thank Dr. Mark Watkins for notable comments and for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Murshedi, A.Y.M., Al-Yasari, A., Alesary, H.F. et al. Electrochemical fabrication of cobalt films in a choline chloride–ethylene glycol deep eutectic solvent containing water. Chem. Pap. 74, 699–709 (2020). https://doi.org/10.1007/s11696-019-01025-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-01025-z