Abstract
The electrodepositions of Sn(II) and Sb(III) were studied in the [EMIm]BF4 ionic liquid at ambient temperature. Linear sweep voltammetry (LSV) results indicated that the reductions of Sn(II) and Sb(III) on Pt electrode are electrochemically irreversible. The diffusion coefficients of Sn(II) and Sb(III) in the ionic liquid electrolyte were determined in terms of the LSV data. Tin and antimony ions form simpler Sn(II) chlorocomplex species and higher Sb(III) chlorocomplexes, respectively present in the ionic liquid electrolyte. Energy dispersive X-ray spectroscopy (EDX) analysis revealed that tin and antimony alloys can be electroplated in the ionic liquid electrolyte.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Room temperature ionic liquids (RTILs) are a family of liquids only consisting of free moving dissociated ions at or near room temperature. Considerable research efforts have been devoted in the past decade to room temperature ionic liquids due to their attractive properties, such as wide electrochemical window, good thermal and chemical stability, and high ionic conductivity. In particular, RTILs are finding a variety of applications in the field of applied electrochemistry, e.g., rechargeable batteries, capacitors, photoelectrochemical cells, fuel cells, electroplating, electrocatalysis, electrosynthesis, and nuclear waste treatment [1–4]. Many previous reports [5–11] on RTILs in studies of electrochemical and electrodeposition studies have focused on the chloroaluminate-based ionic liquids, which are unfortunately sensitive to moisture. In contrast, the number of reports on air-stable ionic liquids is relatively small.
Tin, antimony, and tin–antimony alloys are popular materials in the semiconductor industry. They are used to produce numerous electronic devices in terms of electrodeposition and/or physical vapor deposition (PVD) techniques. There have been numerous investigations of the electrodeposition of tin and antimony from aqueous solutions or high-temperature molten salts reported in the literature [12–15]. However, there have been few studies on the electrochemistry of tin [5] and antimony [16, 17] in ionic liquids. Hussey and Xe [5] showed that the electrodeposition of Sn on platinum in AlCl3-EMIC is a quasi-reversible process, and the diffusion coefficients of Sn(II) in acidic melt and basic melt were (5.3 ± 0.7) × 10−7 and (5.1 ± 0.6) × 10−7 cm2 s−1, respectively. Osteryoung and Habboush [17] studied the electrochemistry of antimony(III) on glass carbon in AlCl3-BPC at 40 °C. It was revealed that the reduction of Sb(III) to Sb is irreversible while the oxidation of Sb(III) to Sb(V) demonstrates a quasi-reversible behavior. Yang and Sun [16] reported the electrodeposition of antimony(III) in [EMIm]BF4 ionic liquid containing free chloride ions, finding the effect of temperature on the nucleation process and the morphologies of the deposits.
It is apparent that there are considerable limitations to the electrodeposition of tin and/or antimony on active materials like aluminum and lithium, as well as magnesium, in aqueous solutions. On the other hand, the disadvantages of the moisture-sensitive chloroaluminate-based ionic liquid systems exposed to the atmosphere are also obvious in practical applications. Therefore, this pioneering work is intended to explore the possible application of air-stable [EMIm]BF4 ionic liquid systems in the electrodeposition of Sn and Sb as well as their alloys.
2 Experimental
2.1 Apparatus
All the electrochemical experiments were carried out under argon. A CHI 660B electrochemical test system (CH Instruments, Inc.) with a three-electrode electrochemical cell was employed to conduct the electrochemical experiments. A platinum disk (A = 1.96 × 10−3 cm2, Aldrich 99.99%) was used as working and counter electrodes. The reference electrode was a spiral silver electrode immersed in the [EMIm]BF4 liquid saturated with AgBF4, which was separated from the bulk ionic liquid electrolyte through a G4 glass filter. All the electrode potentials are reported relative to this reference electrode. Electrodeposition was conducted on platinum wires (0.5 mm diameter, Aldrich 99.99%). The electroplated wires were immersed in deoxidized acetone for a few minutes, washed with ethanol and deionized water in sequence to remove the ionic liquid residue. A field emission scanning electron microscope (SEM) (FEI QUANTA-200) with an energy dispersive X-ray spectroscopy (EDX) working at 30 kV was used to examine the surface morphology and analyse the elemental compositions of the electrodeposits.
2.2 Chemicals
1-Ethyl-3-methylimidazolium tetrafluoroborate ionic liquid was prepared and purified according to a literature method [18]. The density, absolute viscosity (η), and conductivity of this ionic liquid at 25 °C were determined to be 1.2416 g m−3, 0.357 g m−1 s−1 and 1.37 × 10−2 s cm−1, respectively. Anhydrous tin (II) chloride (Aldrich, 99%), Anhydrous antimony (III) chloride (Aldrich, 99%) were used as received.
3 Results and discussion
3.1 Cyclic voltammogram of tin and antimony
The voltammogram of a Pt electrode in pure [EMIm]BF4 ionic liquid at a linear scan rate of 50 mV s−1 is shown in Fig. 1. It exhibits an electrochemical window of 4.3 V ranging from ca. 2.7 V to −1.6 V versus Ag/Ag(I).
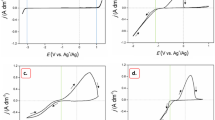
Sn(II) and Sb(III) were introduced into the [EMIm]BF4 along with SnCl2 and SbCl3. A previous report [19] indicated that Sn(II) exists as [SnCl3]− and [SnCl4]−, of which the former is the most common species, while Sb(III) [17] exists as [SbCl4]− complex anions in chloroaluminate ionic liquids. A typical cyclic voltammogram of the Pt electrode in the [EMIm]BF4 ionic liquid containing 25 mM Sb(III) at 25 °C is shown in Fig. 2a. The electroreduction of Sb(III) occurs at the cathodic wave peak c1 and the oxidation of the electrodeposited Sb occurs at the wave peak a1. A typical cyclic voltammogram of 25 mM Sn(II) on the Pt electrode in the [EMIm]BF4 at 25 °C is presented in Fig. 2b. This cyclic votammogram reveals that the electrodeposition of Sn (peak c2) occurs at a potential more negative than the deposition of the Sb. On the anodic scan, Fig. 2b shows an electrochemical oxidation peak a2, corresponding to stripping of the bulk Sn deposit. Figure 2c shows the cyclic voltammogram of a solution containing 25 mM Sb(III) and 25 mM Sn(II) in [EMIm]BF4 at 25 °C. The anodic wave peak a2 accounting for the stripping of bulk Sn deposit and anodic wave peak a1 attributed to the stripping of bulk Sb deposit reduce significantly while new peaks a ′1 and a ′2 become apparent. These features in the strongly suggest that the co-deposition of Sb–Sn takes place in the electrochemical reduction process.
3.2 Reduction of Sn(II) and Sb(III)
In the linear sweep voltammetric (LSV) experiments, the cathodic peak potentials (E c p ) of Sn(II) shifted negatively as the potential scan rate increased. Since the current is of the order of a few microamperes, the effect of ohmic drop in the electrolyte should be negligible [20]. This indicates that the reduction of Sn(II) is electrochemically irreversible on Pt in the ionic liquid electrolyte. The typical LSV data (i c p , E c p , and E p/2 represent cathodic peak current, cathodic peak potential, and half-peak potential, respectively) of 25 mM Sn(II) in [EMIm]BF4 ionic liquid at various potential scan rates, v (mV s−1), are given in Table 1.
The transfer coefficient, α, for reduction of Sn(II) to Sn is estimated to be 0.537 by plotting E c p , the cathodic peak potentials of the different scan rates, against the logarithm of the corresponding scan rate, v (V s−1), as shown in Fig. 3, and using the following equation[21]
where k 0, E 0′, D O , R, T, and F are the standard heterogeneous rate constant, formal potential in V, diffusion coefficient of substance O, gas constant in J mol−1 K−1, absolute temperature in K and Faraday constant in C mol−1, respectively.
The diffusion coefficient of Sn(II), D Sn(II), in [EMIm]BF4 is estimated to be 6.07 × 10−7 cm2 s−1 from the plot of peak current density (j p ) against square root of scan rate, v (V s−1), as shown in Fig. 4, using Eq. 2 [21]:
where α, n a , C *Sn(II) , i c p , and A stand for transfer coefficient, the number of electrons involved in the electrochemical reduction, bulk concentration of Sn(II) in mmol dm−3, cathodic peak current, and the surface area of the working electrode, respectively.
The diffusion coefficient is related to the viscosity of the solution, η, and the solvodynamic radius of a diffusing species, r solv, according to the Stokes–Einstein equation
where k is the Boltzman constant. The Stokes–Einstein product, ηD Sn(II) /T, is inversely proportional to the solvodynamic radius. The ηD Sn(II) /T value of Sn(II) in the [EMIm]BF4 system is 7.27 × 10−10 g cm s−2 K−1, which is larger than that of Sn(II) in AlCl3-[EMIm]Cl ionic liquid [5], as listed in Table 2. Comparing this result with aprevious one, the larger ηD Sn(II) /T value of Sn(II) may be attributed to the simpler Sn(II) chlorocomplex species in the [EMIm]BF4 than that in the AlCl3-[EMIm]Cl.
The typical cyclic voltammogram of Sb(III) in the [EMIm]BF4 ionic liquid at Pt is shown in Fig. 2a. The large peak potential separation, \( E_p^{c_1 } - E_p^{a_1 } \), indicates that the electrochemical reduction of Sb(III) is a mixed diffusion and kinetic controlled process. The cathodic peak potential, E c p , for the reduction of Sb(III) shifts negatively with increase in potential scan rate, as shown in Table 3. As a result, the reduction of Sb(III) is electrochemically irreversible. In other words, the reduction reaction rate of Sb(III) is controlled by mass transport rather than charge transfer.
The transfer coefficient, α, for the reduction of Sb(III) to Sb was calculated from the slope of the plot of E c p against the logarithm of scan rate, as shown in Fig. 5, and the diffusion coefficient of Sb(III), D Sb(III), in [EMIm]BF4 was calculated from the slope of the plot of peak current density against square root of scan rate, as shown in Fig. 6, using Eqs. 1 and 2. The results are summarized in Table 4.
The small value of Stokes–Einstein products, ηD Sb(III) /T, indicates that some unidentified higher chlorocomplexes of Sb(III) form in the [EMIm]BF4 ionic liquid. It is common for the higher chlorocomplexes to form in high temperature molten salts [19].
3.3 Electrodeposition of tin and antimony
The electrodeposition experiments were carried out in the [EMIm]BF4 ionic liquid containing 25 mM Sn(II), 25 mM Sb(III) and 25 mM Sn(II) + 25 mM Sb(III) at room temperature, respectively. The metal deposits were plated on 0.5 mm diameter platinum wires under argon. The potential of electrodeposition was controlled at −1.1 V versus Ag/Ag(I). The metal plated wires were then immersed in pure acetone solvent for a few minutes and rinsed with ethanol and deionized water in sequence to remove the melt residue [15].
The surface morphologies were examined by SEM and are presented in Fig. 7a, b, and c, respectively. EDX analysis of the electrodeposits revealed that they were pure metals without other elemental contaminants, such as Cl, B, and F, indicating that no ionic liquid residue was left on the deposit surfaces. As shown in Fig. 7a, the tin deposit produced at −1.1 V versus Ag/Ag(I) was just needle-type islands, not a continuous plating film on Pt surface. In contrast, the antimony electrodeposit formed a dense, compact layer of plating film on the Pt surface, as shown in Fig. 7b. It appeared that the Sn–Sb alloy deposit was a dense film with additional tin deposit needles on the surface as shown in Fig. 7c. The crystal size of the Sn–Sb alloy deposit is estimated ca. 300∼500 nm according to the higher magnification SEM micrograph in Fig. 7d. The EDX analysis result of the alloy deposit is listed in Table 5. The composition of the crystal is tin and antimony with a one-to-one ratio of atom.
4 Summary and conclusions
SnCl2 and SbCl3 can be dissolved in the [EMIm]BF4 ionic liquid at room temperature. The electrochemical reductions of Sn(II) and Sb(III) were studied in terms of linear sweep voltammetry (LSV). The Sn(II) and Sb(III) reductions on Pt electrode exhibit electrochemically irreversible behavior. The diffusion coefficients and Stoke–Einstein products calculated from LSV data for Sn(II) and Sb(III) indicate the formation of simpler Sn(II) chlorocomplex species and higher Sb(III) chlorocomplexes, respectively, in the [EMIm]BF4 ionic liquid. The electrodepositions of Sn and Sb were investigated on Pt. Scanning electron microscope (SEM) analysis showed that the deposits of tin are isolated needle crystals, but antimony forms a uniform plating film on the platinum electrode. EDX analysis revealed that tin and antimony can be co-electrodeposited from the [EMIm]BF4 ionic liquid.
References
Fang YS, Zhou RQ (1999) J Power Sources 81:891
Neo EL, Kare L, McEwen AB (1999) J Electrochem Soc 146:1687
Lewandowski A, Galinski MJ (2004) Phys Chem Solids 65:281
Gen M, Kentaro T, Takaya S (2004) Electrochim Acta 49:3603
Hussey CL, Xu XH (1993) J Electrochem Soc 140:618
Robinson J, Osteryoung RA (1980) J Electrochem Soc 127:122
Moffat TP (1994) J Electrochem Soc 141:L115
Pitner WR, Hussey CL, Stafford GR (1996) J Electrochem Soc 143:130
Carlin RT, Trulove PC, De Long HC (1996) J Electrochem Soc 143:2747
Mitchell JA, Pitner WR, Hussey CL (1996) J Electrochem Soc 143:3448
Gau W-J, Sun I-W (1996) J Electrochem Soc 143:914
Verdieck RG, Yntema LF (1944) J Phys Chem 48:286
de Fremont RM, Rosset R, Leroy M (1964) Bull Soc Chim Fr 16: 706
Texier P (1968) Bull Soc Chim Fr 3:4716
Fung KW, Begun GM, Mamantov G (1973) Inorg Chem 12:53
Yang MH, Sun I-W (2003) J Appl Electrochem 33:1077
Osteryoung RA, Habboush DA (1984) Inorg Chem 23(4):1726
Chen P-Y, Sun I-W (1999) Electrochim Acta 45:441
Cotton FA, Wilkinson G (1988) Advanced inorganic chemistry 5th edn. Wiley, New York, p 288
Katayama Y, Dan S, Miura T, Kishi T (2001) J Electrochem Soc 148:C102
Bard AJ, Faulkner LR (1980) Electrochemical methods: fundamentals and applications. Wiley, New York, chapter 6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, W., Cang, H., Tang, Y. et al. Electrodeposition of tin and antimony in 1-ethyl- 3-methylimidazolium tetrafluoroborate ionic liquid. J Appl Electrochem 38, 537–542 (2008). https://doi.org/10.1007/s10800-007-9470-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-007-9470-6