Abstract
This paper demonstrates the capability of the cobalt electrodeposition from a eutectic mixture of choline chloride and urea at room temperature. Thermodynamic and kinetic aspects of cobalt electronucleation and growth mechanisms onto glassy carbon electrode were investigated. The cobalt electrodeposition, at 303 K, is dominated by progressive nucleation. The behavior of current density transients of the Co electrodeposition can be described by a model comprising the contribution due to three-dimensional nucleation and diffusion-controlled growth from metallic nuclei combined with the effect of the induction – time. The diffusion coefficient of cobalt ions in deep eutectic solvent was determined by cyclic voltammetry and best-fit parameters obtained using the proposed model. Both values were in agreement with the results published in the literature. SEM and EDS verified the presence and the nucleation type of cobalt onto glassy carbon electrode.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Cobalt has been attracted by the scientists because of its strong magnetic and catalytic properties, which are applied in numerous fields such as magnetic sensors, chemical sensors, and catalysts [1, 2]. The cobalt electrodeposition is extensively studied in the aqueous media [2]. It is known that the use of aqueous media causes many problems (i.e. liberation of hydrogen, evaporation) and technological concerns (toxic solutions), which reduces the cobalt electrodeposition process efficiency [2]. Dealing with these problems, the deep eutectic solvent (DES) based on choline chloride [3] is a prospective candidate. Various metals and their alloys have been synthesized by electrochemical method from DESs such as Ni [4], Zn [5], Co [6, 7], etc. They verified that the early stage of cobalt nuclei formation onto glassy carbon electrode (GCE) plays an important role in the cobalt electrodeposition from DES [6, 7]. However, since the viscosity of the DES is high, these works suggested to perform the experiment at relatively high temperature (above 343 K) to reduce the effect of DES’s viscosity. This causes the following problems: i) it requires more accessories for heating the DES and ii) it decreases the volume of DES due to the thermal decomposition [8], which results more difficult in controlling the metal ions concentration during the electrodeposition process and decreasing the efficiency. Therefore, it is important to study the capability of the Co electrodeposition at room temperature and its thermodynamic and kinetic aspects.
This paper focuses on studying mechanisms and kinetics of cobalt nucleation and growth onto glassy carbon from choline chloride-based DES at 303 K by means of cyclic voltammetry (CV) and chronoamperometry (CA) techniques. Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) were used to characterize the morphology and chemical composition of surface electrodeposit, respectively.
2 Materials and Methods
2.1 Electrolyte Preparation
Choline Chloride and Urea are used to prepare DES in a 1:2 molar ratio at 363 K. This mixture was magnet-stirred. The electrolyte solution was obtained by adding cobalt (II) chloride hexahydrate salt CoCl2.6H2O 50 mM to the DES kept stirring for 12 h at 343 K.
2.2 Electrochemical Experiments and Surface Characterization
The cobalt nucleation and growth mechanisms onto a glassy carbon electrode from DES were studied through CV and CA at room temperature. These tests were performed in a cell comprising three electrodes, using a VersaSTAT Potentiostat/Galvanostat, coupled to the VersaStudio software running on a PC to facilitate experimental control and data collection. The electrochemical cell was composed of a polished GCE with a surface area of 0.0707 cm2 as the working electrode, a platinum wire as counter, and a silver wire as quasi reference electrode. The surface morphology of the Co deposit onto GCE from DES was characterized by a field emission scanning electron microscope (SEM JEOL 7000). The elementary analysis of the surface deposit was determined by EDS.
3 Results and Discussion
3.1 Potentiodynamic Study
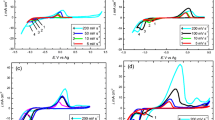
Figure 1 shows the CVs recorded on the GCE of DES and 50 mM Co(II) in DES at 303 K. No peak formation can be observed in black curve (in the absence of Co(II)) in both scan directions, while in the system GCE/50 mM Co(II) in DES, typical reduction and oxidation peaks are clearly formed. The quality of these peaks also verified the capability of DES to electrodeposit Co at room temperature.
In the forward scan direction of CV, the reduction peak is located in the potential range from – 0.95 V to – 1.20 V. In the opposite direction, the oxidation peak located in the potential range of – 0.4 V to 0.1 V is supposed to the oxidation of Co metallic nuclei into Co (II) ion. The increase of current density at the potential lower than – 1.20 V is attributed to the decomposition of the solvent. To assess the thermodynamic and kinetic behavior of the system GCE/Co (II) in DES, CVs were performed at different scan rates (from 10 to 90 mVs−1). The inset of Fig. 1 indicates that the current density of cathode peak (jcp) displays a linear behavior with square root of the scan rate (v1/2), which demonstrates that the Co nucleation onto GCE from DES follows a diffusion – controlled mechanism described by the Randles – Sevcik equation [7]:

where n is the total number of electrons transferred during the overall electrochemical process, C0 (mol cm−3) is the reduced species bulk concentration, DCo(II) (cm2 s−1) is the diffusion coefficient of Co(II) ions and T (K) is the temperature of medium. Also, the 3D nucleation behavior can be described by the model developed by Scharifker – Mostany (SM) [9]. From Eq. (1), DCo(II) was calculated to be 2.79 × 10−7 cm2 s−1, which is consistent with of the results published by Manh et al. [6].
3.2 Nucleation and Growth Mechanisms of Co Electrodeposition
From CVs, experimental current-time transients (j-t plots) were recorded at – 0.95 V and – 0.96 V at 313 K. The recorded data from experiment was normalized through the maximum of current density for each curve (jm, tm) and then compared with theoretical plots for instantaneous and progressive nucleation [9] to investigate the Co nucleation and growth mechanism.
Figure 2 shows the influence of the induction time by comparing the experimental normalized plot and the theoretical one in both cases (with and without t0). If ignoring the influence of t0, the normalized experimental curve is under the progressive curve (Fig. 2a), out of the validated zone that follows the behavior of the 3D nucleation and diffusion-controlled growth described by the SM model [10].
Therefore, in this situation, the classical form of the SM model cannot depict the nucleation and growth mechanism of Co. For this condition, it is significant to consider the consequence of t0 in the SM model by replacing t = t – t0 [11]. As shown in Fig. 2b, after eliminating the induction-time, the new normalized experimental curve is in the validated zone of SM model, and nearly fit well to the progressive curve. This also evidences that the induction time plays the important part during the early stage of the Co electrodeposition process. Then, the SM model after modifying is below [11]:

where t = t – t0, ρ is the density of the Co deposit and M is its atomic mass, N0 is the number density of active sites on the electrode surface, and A is the nucleation frequency per active site.
Figure 3a shows the comparison between the experimental j – t current density transients and the theoretical plots fitting to the experimental data at 303 K using Eq. (2). It presents a good concordance in the shapes with the experimental j – t plots, which demonstrate that the modified SM model by adding the induction-time correction can depict the nucleation and growth mechanism of Co. Electrodeposition of Co was performed at − 0.96 V at 303 K on copper electrode (Fig. 3b). The grey color able to see in naked eye of copper sheet indicates the formation of Co on the electrode, which is confirmed by the presence of the Cu peak shown in EDS spectrum (Fig. 3d). Furthermore, SEM image in Fig. 3c reveals that the cobalt particles exhibit a hexagonal structure, with a relatively homogeneous distribution, which is consistent with the observation done in [8]. This evidences the ability of Co electrodeposition from DES at room temperature.
(a) Comparison between the j – t plots of experimental CAs obtained in the system GCE/50 mM Co(II) in DES and the theoretical ones (solid lines) fitting to the experimental data after subtracting the induction-time at 303 K (b) The pictures of Co(II) salt solution in DES and Co electrodeposition on copper electrode, (c) SEM images of Co electrodeposited on the copper substrate at −0.96 V at 303 K and (d) EDS analysis of the electrode surface.
4 Conclusions
The capability of Co electrodeposition, at room temperature, onto glassy carbon from DES based on choline chloride was studied through the CV and CA analyses. It was found that the Co nucleation onto the GCE from the DES occurred by a diffusion – controlled mechanism, which can be described by adding the contribution of the induction-time to the classic SM model. The diffusion coefficient of Co(II) ions was determined to be 2.79 × 10−7 cm2 s−1. Based on the results, it was obvious to conclude that the early stage of the Co electrodeposition is dominant by progressive nucleation mechanism at 303 K. Finally, the ability of cobalt electrodeposition in DES at low temperature can expand the potential applications of DES in different engineering fields.
References
Leslie-Pelecky, D.L., Rieke, R.D.: Magnetic properties of nanostructured materials. Chem. Mater. 8, 1770–1783 (1996)
Rios-Reyes, C.H., Granados-Neri, M., Mendoza-Huizar, L.H.: Kinetic study of the cobalt electrodeposition onto glassy carbon electrode from ammonium sulfate solutions. Quim. Nova 32(9), 2382–2386 (2009)
Abbott, A.P., Capper, G., Davies, D.L., Rasheed, R.K., Tambyrajah, V.: Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. (Camb), (1) 70–1 (2003)
Huang, P., Zhang, Y.: Electrodeposition of nickel coating in choline chloride-urea deep eutectic solvent. Int. J. Electrochem. Sci. 13, 10798–10808 (2018)
Vieira, L., Whitehead, A.H., Gollas, B.: Mechanistic studies of zinc electrodeposition from deep eutectic electrolytes. J. Electrochem. Soc. 161(1), D7–D13 (2014)
Manh, T.L., Arce-Estrada, E.M., Mejía-Caballero, M.I., Aldana-González, J., Romero-Roma, M., Palomar-Pardavé, M.: Electrochemical synthesis of cobalt with different crystal structures from a deep eutectic solvent. J. Electrochem. Soc. 165, 285–290 (2018)
Cao, X., Xu, L., Shi, Y., Wang, Y., Xue, X.: Electrochemical behavior and electrodeposition of cobalt from choline chloride- urea deep eutectic solvent. Electrochim. Acta 295, 550–557 (2019)
Yizhak, M.: Properties of Deep Eutectic Solvents, pp. 45–110. Springer International Publishing (2019)
Scharifker, B., Hills, G.: Theoretical and experimental studies of multiple nucleation. Electrochim. Acta 28(7), 879–889 (1983)
Scharifker, B.R., Mostany, J.: Three-dimensional nucleation with diffusion-controlled growth. J. Electroanal. Chem. 177, 13–23 (1984)
Manh, T.L., Arce-Estrada, E.M., Mejıa-Caballero, I., Rodrıguez-Clemente, E., Sanchez, W., Aldana-Gonzalez, J., Lartundo-Rojas, L., Romero-Romo, M., Palomar-Pardave, M.: Iron electrodeposition from Fe(II) ions dissolved in a choline chloride: urea eutectic mixture. J. Electrochem. Soc. 165(16), D808–D812 (2018)
Acknowledgement
This research is funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 103.02-2019.28.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Phuong, T.D.V., Thuy, H.T.T., Tam, P.D., Le Manh, T. (2021). On Room-Temperature Electrodeposition of Cobalt from a Deep Eutectic Solvent: A Study of Electronucleation and Growth Mechanisms. In: Sattler, KU., Nguyen, D.C., Vu, N.P., Long, B.T., Puta, H. (eds) Advances in Engineering Research and Application. ICERA 2020. Lecture Notes in Networks and Systems, vol 178. Springer, Cham. https://doi.org/10.1007/978-3-030-64719-3_67
Download citation
DOI: https://doi.org/10.1007/978-3-030-64719-3_67
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64718-6
Online ISBN: 978-3-030-64719-3
eBook Packages: EngineeringEngineering (R0)