Abstract
Background/Objective
Although promising, data regarding the renal impact and safety of bariatric surgery (BS) are insufficient. We aimed at investigating the benefits and harms of BS for weight loss on kidney function.
Methods
A systematic review and meta-analysis of observational studies reporting data about the impact of BS (any techniques) on serum/plasma creatinine, creatinine clearance, glomerular filtration rate (GFR), proteinuria, nephrolithiasis, and need for renal replacement therapy (RRT)) was performed. Obese adults (non-chronic kidney disease (CKD), CKD or transplanted patients) that underwent BS for weight loss were included. After searching MEDLINE (inception to August 2017), the Cochrane Library (Issue 10–12, October 2017), and the websiteclinicaltrials.gov (August 2017), data were extracted and summarized using a random-effects model.
Results
The final analysis included 23 cohort studies, comprising 3015 participants. Compared with renal function before treatment, BS significantly decreased serum creatinine level (mean difference (MD), − 0.08 mg dl−1; 95% confidence interval (CI), − 0.10 to − 0.06); p < 0.001) and proteinuria (MD, − 0.04 g 24 h−1; 95% CI, − 0.06 to − 0.02; p < 0.001) in the overall group. GFR significantly improved 6 months or more after BS both in the hyperfiltration and CKD subgroups. Renal function also tended to improve in renal transplant patients. Data on nephrolithiasis and the need for RRT were scarce or not reported.
Conclusions
BS apparently has positive effects on kidney function and tends to normalize GFR across different categories of renal impairment (hyperfiltration and CKD patients).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and chronic kidney disease (CKD) are two of the greatest epidemics of the twenty-first century. In 2014, 10.8% of men and 14.9% of women worldwide had a body mass index (BMI) ≥ 30 kg m−2 [1]; also, 13.4% of the global population has CKD [2]. High body fat increases the risk of developing CKD indirectly—not only via diabetes mellitus and hypertension [1, 2] but also through direct renal functional and structural modifications. This is due to an increased renal sodium tubular reabsorption, secondary to kidney compression [3] that triggers the vasodilation of the afferent arteriole (via tubuloglomerular feedback) leading to hyperperfusion, hyperfiltration, and increased glomerular capillary pressure with subsequent albuminuria/proteinuria [3, 4]. As obesity-associated kidney damage progresses, hyperfiltration (increased glomerular filtration rate (GFR)) is replaced by a declining GFR, with progression toward end-stage renal disease (ESRD) [3, 4].
If obesity is responsible for kidney damage, is it possible to reverse the damage through weight loss therapies? Many studies have addressed this question and most of them reported beneficial effects of different weight loss interventions for improving obesity-induced kidney damage [5,6,7]. For each 1 kg reduction in weight, there is a 4% decrease in proteinuria and albuminuria, independently of blood pressure decline [6].
Bariatric surgery (BS) is the most efficient intervention for obtaining and maintaining substantial weight loss and the only curative method that significantly ameliorates obesity-related comorbidities [8]. BS also seems to have a positive impact on renal function [6]; however, it is associated with hyperoxaluria, nephrolithiasis, and oxalate nephropathy [9]. Most importantly, data regarding the impact and safety of BS in patients with kidney impairment are insufficient. This systematic review particularly addresses the effects of BS on kidney function outcomes in non-CKD and CKD patients.
Materials and Methods
Protocol and Registration
The protocol has been registered with the PROSPERO database of prospectively registered systematic reviews in health and social care, registration number CRD42017057916.
Purpose
This review aims to evaluate the benefits and harms of BS for weight loss on kidney function.
Data Sources/Search Strategy
We searched MEDLINE (inception to August 2017), the Cochrane Library (Issue 10–12, October 2017), and the website clinicaltrials.gov (August 2017) without language restriction. Hand search for relevant articles was done on reference lists from textbooks, articles, and scientific proceedings.
Study Selection
We conducted a systematic review and meta-analysis on observational cohort studies in adults with obesity that were treated with BS for weight loss and have reported data about the impact of BS (any techniques) on kidney function endpoints (serum creatinine, creatinine clearance, GFR, proteinuria, nephrolithiasis, and need for renal replacement therapy (RRT)). Patients (non-CKD, CKD or transplanted) were included in this analysis if their biochemical renal function values were reported before and after BS. The surgery itself could be sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), adjustable gastric banding (AGB), and biliopancreatic diversion (BPD) done either by open or laparoscopic surgery. Patients were acting as their own control group, since renal endpoints were compared before (perioperative) and after the surgery. Patients undergoing re-operative intervention for obesity were excluded.
Data Extraction and Synthesis
Data extraction was done independently by two authors (AN and SB) using standard data extraction forms. When more than one publication of a study was found, reports were grouped together and only the publication with the most complete data was included. Data extracted included identifying information, study outcomes, details of the study protocol, and demographic data. We extracted characteristics of each study including baseline renal function values, baseline clinical characteristics of the study population, CKD status, known comorbidities, type of study design, types of surgery, and total duration of follow-up. Any unclear or missing information was requested from the authors by written correspondence, and any relevant information obtained was included in the review. Disagreements were resolved by consultation between all authors.
Risk of Bias
Two reviewers (AN and IN) independently evaluated the quality of the selected studies using the Newcastle-Ottawa scale (NOS) [10]; according to NOS, three methodological categories were used for assessment: selection (score 0–4), comparability (score 0–2), and outcome (score 0–3). Quality was considered high if is score 7–9, intermediate if 4–6, and low if 0–3. Disagreements were resolved by consensus. Publication bias was assessed using the funnel plot technique [11].
Statistical Analysis
We used a random-effects model for meta-analysis and expressed treatment effects as a risk ratio (RR) with 95% confidence intervals (CI) for dichotomous outcomes (need for RRT) and mean difference (MD) for continuous outcomes with 95% CI (e.g., changes in GFR, creatinine level at the end of intervention, etc.) [12]. We used the I2 statistic to assess for inconsistency across individual studies [12]. An I2 > 50% indicated a large inconsistency across studies (heterogeneity) not explained by chance [13]. All statistical analyses were performed using ReviewManager Version 5.2 (The Cochrane Collaboration 2012).
Additional prespecified subgroup analyses were conducted to explore the potential causes of heterogeneity for treatment effect on renal function. Treatment heterogeneity was analyzed also in relation with equations used to estimate the renal function. Subgroup analyses will be conducted for the following subgroups: CKD stages 3 to 5, kidney transplanted patients, and hyperfiltration patients (GFR > 110 ml min−1).
Results
Study Identification
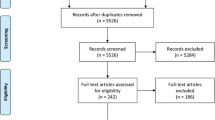
A flow diagram proving the selection process of the included studies is depicted in Fig. 1. The initial search resulted in 1094 potentially relevant articles. A thorough analysis of the abstracts led to the exclusion of 1044 articles due to search overlap, non-relevance, renal function not reported, clinical studies other than observational, case reports, editorials, reviews, or meta-analyses. Fifty articles were studied full text, from which 27 were excluded due to non-relevance or lack of sufficient information. After an in-depth review, 23 observational studies were included in the present systematic review [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] (Fig. 1).
Baseline Study and Patients’ Characteristics
The main characteristics of the 23 studies included in the meta-analysis are presented in Table 1. A total of 3015 patients were included, with a mean BMI ranging from 39.5 ± 9.7 to 57.3 ± 12.6 kg m−2. The mean follow-up period generally varied between 6 and 24 months with only one study reporting a very short follow-up period of 30 days [23] and another study reporting data 3.9 years after the weight loss surgical intervention [28] (Table 1).
Studies reported used various surgical techniques (malabsorptive, restrictive, and hybrid procedures) for achieving weight loss, both laparoscopic or by open surgery (Table 1): the most commonly used technique was Roux-en-Y gastric bypass (RYGB)—performed in more than 50% of cases, followed by sleeve gastrectomy (SG) and adjustable gastric banding (AGB). Other types of gastric bypass (GB) techniques (Fobi Pouch GB, Salmon GB, mini GB) [14, 25, 33] as well as gastroplasty [18] have also been reported. Biliopancreatic diversion (BPD) was performed in two studies [26, 28].
With two exceptions [15, 16], all studies reported obesity-related comorbidities—the most prevalent being hypertension (46%), diabetes (36%), and CKD (29.4%), followed by dyslipidemia, metabolic syndrome, obstructive sleep apnea, and cardiovascular/cerebrovascular disease (Table 1).
Study Quality
The quality of the observational studies ranged from 5 to 9, with a mean quality score of 6. This corresponds to a moderate overall risk of bias, mostly due to the absence of a control group (only 4 studies included controls drawn from the same community as the exposed cohort) and lack of control for confounders (only 11 studies performed adequate control for both the most important confounder and additional factors, while 6 studies did not include any confounder adjustment). However, selection bias was low (all studies included representative cohorts with certainty of exposure), outcome assessment was adequately performed in 22 out of 23 studies, follow-up was long enough in most studies (18 out of 23 studies reported outcomes within at least 12 months of follow-up), and there was only one study that lost subjects to follow-up.
Study Outcomes
Results are summarized in Table 2.
Overall Study Analysis
Serum/Plasma Creatinine
Overall, serum/plasma creatinine decreased in 11 [14,15,16, 20, 21, 24, 27,28,29, 32, 36] out of 18 studies [14,15,16,17, 19,20,21,22, 24, 25, 27,28,29,30,31,32, 35, 36] in which this parameter was assessed, irrespective of BS technique. In six of the remaining studies, baseline creatinine values were in the normal range and creatinine did not differ significantly before versus after surgery [17, 19, 22, 25, 31, 35] (Table 2). Creatinine was reported to increase only in the study of Schuster et al. [30] in ten patients with baseline moderate kidney impairment, after more than 24 months of follow-up following BS (see CKD subgroup analysis).
A decrease in serum/plasma creatinine concentrations was also observed in the overall group after BS when a meta-analysis on 17 out of 23 studies was performed (MD, − 0.08 mg dl−1; 95% CI, − 0.10 to − 0.06 mg dl−1) (Fig. 2).
GFR
GFR was assessed in 17 out of 23 studies [14,15,16,17,18,19, 21, 24, 25, 27,28,29, 31,32,33, 35, 36]: Friedman et al. [16] and Chagnac et al. [18] directly measured GFR through plasma iohexol clearance and inulin clearance while the other authors reported estimated GFR (eGFR) by determining: 24-h creatinine clearance [14, 15, 17, 27, 33], Modification of Diet in Renal Disease (MDRD) equation [16, 21, 24, 25, 27,28,29], Cockcroft-Gault and lean body weight-adjusted Cockcroft-Gault (CG-LBW) formulae [19, 27, 31, 32], and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, respectively [16, 31, 35] (Table 3).
eGFR significantly improved in all studies, 6 months or more after BS, irrespective of the surgical method performed and irrespective of the baseline values (hyperfiltration or impaired eGFR), with three exceptions where eGFR either did not change significantly [19] or it decreased [32, 36] (Table 2). Otherwise, GFR was significantly reduced in hyperfiltration patients [14,15,16,17,18, 25, 27, 31,32,33] and at the same time significantly increased in patients with eGFR < 90 ml min−1 ([21, 24, 25, 28, 29, 31, 35] (Table 2) (see CKD subgroup analysis below).
When overall meta-analysis was performed on 13 out of 23 studies, we noticed a lack of significant differences for eGFR changes after surgery in the overall group (MD, − 3.07 ml min; 95% CI, − 13.89 to + 7.74 ml min−1) (Fig. 3). However, for the hyperfiltration group, a 31.87-ml min−1 reduction was observed (95% CI, 38.15 to 25.59 ml min−1) (Fig. 3).
Proteinuria/Albuminuria
Albuminuria and/or proteinuria were reported by 13 out of 23 studies [14, 15, 17, 18, 20, 22,23,24, 26, 32,33,34,35]; it significantly improved in all but one exception [17], where average baseline albuminuria was normal. Reductions in albuminuria and/or proteinuria were seen after various surgical techniques, including RYGB [14, 15, 20, 22, 23, 32,33,34], SG [24, 32, 34, 36], BPD [26], or gastroplasty [18] (Table 2). An overall reduction in albuminuria and/or proteinuria was seen when meta-analysis on 2 out of 23 studies was performed (Fig. 4).
After adjustment for confounders, improvement in albuminuria/proteinuria was both weight- and blood pressure-independent in most studies [20, 22, 23, 32]. Only Amor et al. [34] reported normalization of albumin excretion as being associated with a larger decrease in waist circumference and BMI in type 2 DM patients. Predictors of albuminuria reduction in the studies included in this review are baseline albuminuria, insulin sensitivity/change in HbA1c levels, and adiponectin [20, 22, 23, 34].
Nephrolithiasis
The occurrence of nephrolithiasis was assessed only by Palomar et al. [26] which found a decrease in calcium, phosphate, uric acid, and citrate urinary excretion and a tendency toward an increase in oxalate urinary excretion but no increase in renal stone production [26] (Table 4).
Need for RRT
The need for RRT was not reported in the included studies with the exception of Palomar et al. [26] that did not find any cases of kidney failure after BPD (Table 4).
CKD Subgroup Analysis
Eight studies (six retrospective [21, 25, 28, 30, 31, 35] and two prospective [19, 24]) included 877 patients with CKD (kidney transplant patients are discussed separately) from which 766 had CKD stage 3 or higher (Schuster et al. [30] did not report eGFR, but only creatinine values, therefore CKD stage could not be assessed in this case).
In five out of eight studies, eGFR and/or creatinine levels improved regardless of the type of BS performed [21, 24, 25, 31, 35]. Kidney function (eGFR and/or creatinine) was also stated to improve in other two studies [19, 28], but complete data were not available. Only in one study the creatinine values had the tendency to rather increase 24 months after BS in patients with baseline moderate renal impairment (creatinine > 1.6 mg dl−1) [30].
When the CKD subgroup meta-analysis was performed, only the eGFR (MD, 10.04 ml min−1; 95% CI, 6.07 to 14.02 ml min−1; p < 0,001) and not creatinine (MD, − 0.12 mg dl−1; 95% CI, − 0.27 to 0.03 mg dl−1; p = 0.12) was significantly ameliorated (Figs. 2 and 3).
A greater improvement in eGFR was seen in patients with overt CKD (CKD stage 3 or greater) compared with patients with eGFR > 60 ml min−1 [28]. eGFR improvement was independent of changes in blood pressure [19, 30, 31], BMI [19, 31], presence of diabetes [30, 31], or restrictive versus malabsorptive BS procedures in CKD patients [25].
Albuminuria/proteinuria, the occurrence of nephrolithiasis and the need for RRT, respectively, were not assessed/reported in CKD patients in any of the studies.
Transplantation Subgroup Analysis
Only the study of Golomb et al. [36] included kidney transplant patients (ten patients with normal eGFR after transplantation). Creatinine values and proteinuria significantly decreased 12 months after BS in the ten patients included, while in five out of ten subjects, their median creatinine clearance had the tendency to decrease. The incidence of nephrolithiasis and the need for RRT were not assessed/reported.
Discussion
Summary of Findings
The present systematic review shows an improvement in renal parameters after BS: (1) although creatinine did not change in some studies after surgery, it significantly decreased in most of them, (2) eGFR profile improved in almost all studies (decreased in patients with hyperfiltration and increased in patients with reduced eGFR), and (3) proteinuria/albuminuria decreased significantly in all studies with one exception [17].
Obese patients initially develop kidney hyperfiltration with increased eGFR. As kidney structural damage occurs, eGFR progressively declines to CKD values. BS tends to stabilize eGFR across different categories of kidney function in bariatric patients, with reduction toward the normal range in hyperfiltration and increase toward the normal range in CKD, respectively. The apparent deterioration of renal function in some studies [32, 36] was accompanied by improvement of the other renal parameters (decrease in creatinine levels and albuminuria) and rather reflects a weight loss-induced reduction of ultrafiltration and not a real kidney injury (possible confounders include ethnic minorities, cohorts composed mainly of females, and unknown differences in duration of comorbidities) [32, 36]. Also, the apparent increase in creatinine values in moderate CKD patients in the study of Schuster et al. [30] rather reflects the natural course of the disease in patients with a more severe baseline kidney disease stage, especially as creatinine tended to decrease in mild CKD patients in the same study [30]. With regard to these discrepancies, one must take into account that creatinine is only a crude indicator of eGFR, due to its variable tubular secretion and reabsorption especially in kidney disease [37]; also, it is difficult to accurately estimate GFR using formulae in obese patients, due to body size confounders. Unfortunately, equations that properly account for obesity have not yet been established. MDRD significantly underestimates, while Cockcroft-Gault highly overestimates GFR when compared with 24-h creatinine clearance [27]. However, 24-h creatinine clearance determination is burdensome, may be hampered by 24-h urinary output collection and also exceeds true GFR due to tubular secretion [27]. Also, measured GFR does not seem to significantly correlate to body surface area or weight in obese individuals [16]. According to Friedman et al. [16], the best predictor in obese patients is the CKD-EPI-derived equation that uses both serum creatinine and cystatin C, which estimates GFR within 30% of its value more than 80% of the time [16].
eGFR improvement did not correlate with BMI reduction/weight loss per se [16, 19, 32] but was rather a consequence of lower blood pressures values [15, 18] and improved metabolic parameters (e.g., glycemia) [18]. On the contrary, improvement in albuminuria was weight independent and blood pressure independent in most studies in which adjustment for BMI and blood pressure as possible confounders was performed [20, 22, 23, 32]. Reduction of albuminuria seems to be influenced by baseline albuminuria, insulin sensitivity/change in HbA1c levels, and adiponectin [20, 22, 23, 34].
Of particular interest are the beneficial effects of BS in patients with overt CKD. The resolution of comorbidities such as hypertension, metabolic dysfunctions, and sleep apnea as a result of BS is the main contributor to renal function enhancement [8, 38]. Nonetheless, BS also attenuates renal inflammation and fibrosis via weight loss: the serum and urinary levels of macrophage migration inhibitory factor (MIF), monocyte chemoattractant protein-1 (MCP-1), and chemokine-ligand 18 (CCL-18)—proinflammatory and profibrotic major mediators of renal damage- significantly decrease after BS procedures [24]. Renal tissue expression of transforming growth factor beta (TGF-β) is also attenuated in animal models of diabetic nephropathy after Roux-en-Y esophagojejunostomy [39].
Weight gain is a very common problem in kidney transplant recipients that increases the risk for kidney dysfunction, graft loss, and complications [40]. Bariatric surgery is a much more efficient weight loss procedure compared with medical treatment in CKD patients [41], but there is currently little knowledge about it in renal transplant recipients. Golomb et al. [36] showed a favorable effect of LSG on renal outcomes up to 14 months after BS in transplanted patients. However, the creatinine clearance reduction in five patients in this study urges for assessment of long-term outcomes.
The results of BS in CKD patients are encouraging and give rise to the following question: is BS appropriate and safe for all stages of CKD? Turgeon et al. [42] have demonstrated a positive trend between CKD severity and the incidence of complications after BS, even after controlling for diabetes and hypertension. However, the 30-day overall mortality of 0.12% and the absolute incidence of complications of less than 10% in both open surgery (associated with a higher risk) and laparoscopic procedures combined [42] is comparable with that of the general population (0.3 and 4.1%, respectively) [43]. Potential renal pitfalls include an increased risk for oxalate nephropathy [9] and for acute kidney injury in the CKD population [44].
On the long term, BS is associated with nephrolithiasis, with incidence rates as high as 3% and a rate of recurrence of more than 30%. The major cause is hyperoxaluria, which is maintained 2 years or more after GB [9]. Although the only study that assessed incident nephrolithiasis after BS [26] did not report any modifications in oxalate excretion after BS (BPD) and no increase in the incidence of lithiasis, oxalate nephropathy may accelerate CKD progression in patients with pre-existing CKD, leading even to initiation of dialysis (data mainly from case reports or case series, therefore incidence could not be quantified) [45]. The need for RRT was not reported in the described studies (only Palomar et al. [26] reported no incident kidney failure after BPD) especially as, with few exceptions [19, 21, 24, 25, 28, 30, 31, 35, 36], CKD was an exclusion criteria or not mentioned at all.
Our findings regarding the positive impact of BS upon renal function are in concordance with the meta-analysis of Navaneethan et al. [5] and the systematic reviews of Afshinnia et al. [6] and Bolignano and Zoccali [7] which globally investigated the renal effects of various weight loss interventions (surgical and non-surgical).
Regarding non-surgical weight-loss methods, diet and medical interventions are not without caveats in CKD: low-carbohydrate diets are usually rich in proteins and therefore have a negative impact upon kidney function [46]; at the same time, no weight-loss medication has been adequately tested in overt CKD [47]. As BS is an emerging option for weight loss in renal patients [41], our review focused only on the impact of BS upon kidney function. Likewise, we only found one meta-analysis that specifically addressed the effects of BS on renal function, recently published in 2016: Li et al. [48] have confirmed the improvement of measured GFR and/or eGFR in both hyperfiltration and CKD stage 2 obese patients and also reported reductions in albuminuria/proteinuria after BS. However, the meta-analysis did not assess comorbidities or confounders and also the occurrence of adverse renal effects such as nephrolithiasis or the need for RRT. Moreover, their study focused on CKD stage 2, while we included all stages of CKD.
More long-term prospective studies that evaluate overall complications and renal complications after different BS procedures are needed. Also, studies that evaluate the effect of BS in ESRD patients on dialysis patients and/or in CKD patients that are kidney transplant recipients or candidates for transplantation are necessary.
Limitations and Strengths
The strengths of the present study include the systematic approach and extensive review of literature, with data extraction and appraisal performed by two independent reviewers. We assessed the overall kidney function by evaluating the effect of BS upon creatinine values, eGFR, and albuminuria/proteinuria and also the possible adverse renal effects associated with BS, such as nephrolithiasis and need for RRT. We also reviewed the results from all-stage CKD patients independently of populations with normal kidney function.
However, most cohorts were very small and the available evidence, mostly observational, is at moderate risk of bias and limited by heterogeneity among studies with regard to the effect of BS upon creatinine levels (although reported results regarding GFR and proteinuria were rather homogenous, thus providing reliable results), indirect comparisons and inconsistency for some outcomes (e.g., proteinuria, nephrolithiasis). Our review could not exclude publication bias of original studies, as probably authors that have not found positive effects of BS or did not find any effect at all are less likely to publish their results.
Conclusion
BS seems to have positive effects on the kidney function, including creatinine values, GFR, and albuminuria/proteinuria. BS tends to normalize GFR across different categories of renal impairment such as hyperfiltration and reduced GFR patients. Finally, future studies specifically addressing CKD subpopulations that also investigate CKD progression and need for RRT would allow for more precise and firm conclusions to be drawn with regard to the effects of BS on kidney disease.
References
NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–96.
Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765.
Hall ME, do Carmo JM, da Silva AA, et al. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renov Dis. 2014;7:75–88.
Garland JS. Elevated body mass index as a risk factor for chronic kidney disease: current perspectives. Diabetes Metab Syndr Obes. 2014;7:347–55.
Navaneethan SD, Yehnert H, Moustarah F, et al. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4(10):1565–74.
Afshinnia F, Wilt TJ, Duval S, et al. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant. 2010;25(4):1173–83.
Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrol Dial Transplant. 2013;28(Suppl 4):iv82–98.
Piche M, Auclair A, Harvey J, et al. How to choose and use bariatric surgery in 2015. Can J Cardiol. 2015;31(2):153–66.
Duffey BG, Alanee S, Pedro RN, et al. Hyperoxaluria is a long-term consequence of Roux-en-Y gastric bypass: a 2-year prospective longitudinal study. J Am Coll Surg. 2010;211(1):8–15.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Higgins JPT, Green S. Handbook for systematic reviews of interventions, version 5.1.0 (updated March 2011) 2011.
DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Navarro-Diaz M, Serra A, Romero R, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol. 2006;17(12 Suppl 3):S213–7.
Serpa-Neto A, Biaco Rossi FM, Dal Moro Amarante R, et al. Effect of weight loss after Roux-en-Y gastric bypass, on renal function and blood pressure in morbidly obese patients. J Nephrol. 2009;22(5):637–46.
Friedman AN, Moe S, Fadel WF, et al. Predicting the glomerular filtration rate in bariatric surgery patients. Am J Nephrol. 2014;39(1):8–15.
Saliba J, Kasim NR, Tamboli RA, et al. Roux-en-Y gastric bypass reverses renal glomerular but not tubular abnormalities in excessively obese diabetics. Surgery. 2010;147(2):282–7.
Chagnac A, Weinstein T, Herman M, et al. The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol. 2003;14(6):1480–6.
Luaces M, Martinez-Martinez E, Medina M, et al. The impact of bariatric surgery on renal and cardiac functions in morbidly obese patients. Nephrol Dial Transplant. 2012;27(Suppl 4):iv53–7.
Navaneethan SD, Kelly KR, Sabagh F, et al. Urinary albumin excretion, HMW adiponectin, and insulin sensitivity in type 2 diabetic patients undergoing bariatric surgery. Obes Surg. 2010;20(3):308–15.
Navaneethan SD, Yehnert H. Bariatric surgery and progression of chronic kidney disease. Surg Obes Relat Dis. 2009;5(6):662–5.
Agrawal V, Khan I, Rai B, et al. The effect of weight loss after bariatric surgery on albuminuria. Clin Nephrol. 2008;70(3):194–202.
Mohan S, Tan J, Gorantla S, et al. Early improvement in albuminuria in non-diabetic patients after roux-en-Y bariatric surgery. Obes Surg. 2012;22(3):375–80.
Fenske WK, Dubb S, Bueter M, et al. Effect of bariatric surgery-induced weight loss on renal and systemic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis. 2013;9(4):559–68.
Hou CC, Shyu RS, Lee WJ, et al. Improved renal function 12 months after bariatric surgery. Surg Obes Relat Dis. 2013;9(2):202–6.
Palomar R, Fernandez-Fresnedo G, Dominguez-Diez A, et al. Effects of weight loss after biliopancreatic diversion on metabolism and cardiovascular profile. Obes Surg. 2005;15(6):794–8.
Getty JL, Hamdallah IN, Shamseddeen HN, et al. Changes in renal function following Roux-en-Y gastric bypass: a prospective study. Obes Surg. 2012;22(7):1055–9.
Jose B, Ford S, Super P, et al. The effect of biliopancreatic diversion surgery on renal function—a retrospective study. Obes Surg. 2013;23(5):634–7.
Ruiz-Tovar J, Giner L, Sarro-Sobrin F, et al. Laparoscopic sleeve gastrectomy prevents the deterioration of renal function in morbidly obese patients over 40 years. Obes Surg. 2015;25(5):796–9.
Schuster DP, Teodorescu M, Mikami D, et al. Effect of bariatric surgery on normal and abnormal renal function. Surg Obes Relat Dis. 2011;7(4):459–64.
Ngoh CL, So JB, Tiong HE, et al. Effect of weight loss after bariatric surgery on kidney function in a multiethnic Asian population. Surg Obes Relat Dis. 2016;12(3):600–5.
Reid TJ, Saeed S, McCoy S, et al. The effect of bariatric surgery on renal function. Surg Obes Relat Dis. 2014;10(5):808–13.
Serra A, Granada ML, Romero R, et al. The effect of bariatric surgery on adipocytokines, renal parameters and other cardiovascular risk factors in severe and very severe obesity: 1-year follow-up. Clin Nutr. 2006;25(3):400–8.
Amor A, Jimenez A, Moize V, et al. Weight loss independently predicts urinary albumin excretion normalization in morbidly obese type 2 diabetic patients undergoing bariatric surgery. Surg Endosc. 2013;27(6):2046–51.
Imam TH, Fischer H, Jing B, et al. Estimated GFR before and after bariatric surgery in CKD. Am J Kidney Dis. 2017;69(3):380–8.
Golomb I, Winkler J, Ben-Yakov A, et al. Laparoscopic sleeve gastrectomy as a weight reduction strategy in obese patients after kidney transplantation. Am J Transplant. 2014;14(10):2384–90.
Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38(10):1933–53.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Wang C, He B, Piao D, et al. Roux-en-Y Esophagojejunostomy ameliorates renal function through reduction of renal inflammatory and fibrotic markers in diabetic nephropathy. Obes Surg. 2016;26(7):1402–13.
Chan G, Garneau P, Hajjar R. The impact and treatment of obesity in kidney transplant candidates and recipients. Can J Kidney Health Dis. 2015;2:26.
MacLaughlin HL, Hall WL, Patel AG, et al. Weight loss, adipokines, and quality of life after sleeve gastrectomy in obese patients with stages 3-4 CKD: a randomized controlled pilot study. Am J Kidney Dis. 2014;64(4):660–3.
Turgeon NA, Perez S, Mondestin M, et al. The impact of renal function on outcomes of bariatric surgery. J Am Soc Nephrol. 2012;23(5):885–94.
Longitudinal Assessment of Bariatric Surgery (LABS) Consortium, Flum DR, Belle SH, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361(5):445–54.
Rao BB, Bhattacharya A, Agrawal V. Renal outcomes of bariatric surgery in obese adults with diabetic kidney disease. J Nephrol. 2014;27(4):361–70.
Nasr SH, D'Agati VD, Said SM, et al. Oxalate nephropathy complicating Roux-en-Y gastric bypass: an underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol. 2008;3(6):1676–83.
Knight EL, Stampfer MJ, Hankinson SE, et al. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003;138(6):460–7.
Kang JG, Park CY. Anti-obesity drugs: a review about their effects and safety. Diabetes Metab J. 2012;36(1):13–25.
Li K, Zou J, Ye Z, et al. Effects of bariatric surgery on renal function in obese patients: a systematic review and meta analysis. PLoS One. 2016;11(10):e0163907.
Funding
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest Disclosure
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Bilha, S.C., Nistor, I., Nedelcu, A. et al. The Effects of Bariatric Surgery on Renal Outcomes: a Systematic Review and Meta-analysis. OBES SURG 28, 3815–3833 (2018). https://doi.org/10.1007/s11695-018-3416-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3416-4