Abstract
Background
Despite obesity being closely associated with two common risk factors for albuminuria, namely type 2 diabetes mellitus (T2DM) and hypertension, information on the impact of weight loss on albumin excretion rate in morbidly obese (MO) subjects is scarce.
Objective
To evaluate the independent contribution of weight loss following bariatric surgery (BS) to the improvement of the albumin-to-creatinine ratio (ACR) in MO subjects with T2DM.
Subjects and methods
Observational prospective study, including consecutive (n = 255) patients undergoing Roux-en-Y gastric bypass (GBP) or sleeve gastrectomy (SG) of whom 37.6 % (n = 96) presented with T2DM. Stepwise logistic regression analysis was used to assess the contribution of T2DM-related, hypertension-related, and weight loss-related variables, and type of surgery to normalization of ACR (<30 mg/g) at 12 and 24 months follow-up.
Results
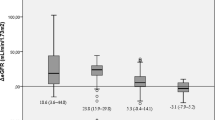
In T2DM subjects, baseline ACR was 85.7 ± 171 mg/g with ACR ≥30 mg/g being present in 45.7 % of the cohort. At 12 months, the ACR significantly decreased in T2DM subjects (42.2 ± 142.8 mg/g; p < 0.005) with no further reduction at 24 months after surgery (44.4 ± 227.7; p = 0.862). Among T2DM subjects with ACR ≥30 mg/g at baseline, the ACR became <30 mg/g in 58.5 % and 76.9 % at 12 and 24 months, respectively (p < 0.001 relative to baseline). Body mass index (BMI) change from baseline was the only independent predictor of ACR normalization at 12 months [Exp(B) 1.373, 95 % confidence interval 1.075–1.703; p < 0.05]. None of the evaluated variables appeared as an independent predictor of ACR normalization at 24 months.
Conclusions
Our data suggest that, in MO subjects with T2DM, interventions aiming at slowing the progression of nephropathy should not only focus on optimization of glucose and blood pressure control but also include effective weight loss strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Optimized glucose and blood pressure control are recommended to reduce the risk or slow the progression of nephropathy in subjects with type 2 diabetes mellitus (T2DM) [1]. It has been shown that, in morbidly obese (MO) subjects, the likelihood of achieving glycated hemoglobin level ≤6.0 % or remission of hyperglycemia is greater following bariatric surgery (BS) than following medical therapy alone [2, 3]. Likewise, the proportion of subjects achieving remission of hypertension following BS is larger than that following a lifestyle intervention aiming at weight loss [4]. Nonetheless, the impact of bariatric surgery on kidney disease in subjects with T2DM has seldom been reported [4–6].
Obesity is a strong and potentially modifiable risk factor for development and progression of kidney disease [7]. Despite obesity being closely associated with two common risk factors for albuminuria, namely T2DM and hypertension, studies have shown that the impact of obesity on kidney disease risk is independent of these major risk factors [7, 8]. Of note, Kramer et al. demonstrated that, in subjects with T2DM, larger BMI and waist circumference are associated with increased risk of albuminuria, with these associations remaining significant even after adjustment for hypertension and use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers [8]. Thus, the paucity of available data supports the need for studies evaluating the implications of weight loss for albumin excretion rate in MO subjects with T2DM.
Against this background, the primary aim of our study is to evaluate the impact of BS on albumin excretion rate in MO subjects with T2DM and to evaluate the independent contribution of weight loss to the improvement of albuminuria up to 24 months after surgery.
Subjects and methods
An observational prospective study was carried out, including consecutive patients undergoing BS at a university hospital. Eligibility for BS was based on current established criteria [9]. The selection criteria for Roux-en-Y gastric bypass (GBP) or sleeve gastrectomy (SG) at our institution have previously been reported and were based on the presence of a larger BMI, an increased estimated operative risk, or the presence of an enlarged liver [10]. Patients with proteinuria in the nephrotic range, previously diagnosed with glomerulonephritis, with estimated glomerular filtration rate <60 ml/min, or with history of renal transplantation at baseline examination were excluded from the study. The Ethics Committee at our institution approved the study, and written informed consent was obtained from all participants.
Presence of T2DM was based on self-reported prior diagnosis, medical records, use of antidiabetic medications, or fasting plasma glucose, 2-h post 75 g oral glucose tolerance test (OGTT) glucose, and hemoglobin A1c in the diagnostic range [1]. In nondiabetic subjects, diabetes was ruled out based on plasma glucose during a 75-g OGTT and hemoglobin A1c in the normal range [1]. Hypertension and cardiovascular disease diagnosis, and smoking status were self-reported or based on medical records or use of corresponding medications.
Anthropometrics including body weight, height, waist circumference, and systolic and diastolic blood pressure were measured as previously described [10]. Body mass index (BMI) was calculated as weight (kg) divided by height (meters) squared. Blood samples were obtained following an overnight fast. Fasting plasma glucose, serum creatinine, and glycosylated hemoglobin (HbA1c) were measured as previously described [11]. Random spot midstream urine sample collected between 8 and 10 am, on the same day as blood sample withdrawal, was used to measure urinary albumin [immunoturbidimetric assay adapted to a Siemens ADVIA 1800 analyzer, with interassay coefficient of variation (CV) of 8.14 %] and creatinine (Jaffe method adapted to a Siemens ADVIA 2400 analyzer, with interassay CV of 2.19 %). Subjects were advised to refrain from physical exercise in the 24 h prior to urine collection. Urine samples were considered valid only if red and white blood cells were absent in sediment examination. Albumin-to-creatinine ratio (ACR) ≥30 mg/g was considered as abnormal [1].
After surgery, the same anthropometric and laboratory data as at baseline were prospectively collected at 12 and 24 months. Diabetes remission was defined as fasting plasma glucose and HbA1c below the diabetes diagnostic thresholds (<126 mg/dl and <6.5 %, respectively) in the absence of antidiabetic medication [12]. Remission of hypertension was defined as blood pressure <140/90 mmHg in the absence of antihypertensive medication.
Statistical analysis
Statistical analyses were performed using the SPSS 17.0 statistical package (Chicago, IL). Results are expressed as mean ± standard deviation or frequencies and percentages unless otherwise stated. Normal distribution of continuous variables was assessed by means of Kolmogorov–Smirnov test. Differences between groups were evaluated using parametric or nonparametric test as appropriate. Independent predictors of presence of ACR ≥30 mg/g at baseline in the whole cohort (n = 255) or in the subgroup of subjects with T2DM (n = 96) were evaluated by means of logistic regression analysis. Predictors of normalization of ACR at 12 and 24 months after surgery were evaluated by means of logistic regression analysis in the group of subjects with T2DM (n = 96). Variables included in the model were selected based either on results of the comparison of the distribution of each variable in subjects with or without normalization of ACR at 12 or 24 months, or on potential clinical significance based on current knowledge on diabetic nephropathy. Variable collinearity was excluded prior to inclusion of any variable in the regression analysis. Statistical significance was set at p < 0.05.
Results
The clinical features of the 255 study participants at baseline are presented in Table 1. T2DM was present in 37.6 % (n = 96) of the whole cohort. Hypertension, smoking habit, and cardiovascular disease were present in 43.5 % (n = 111), 21.6 % (n = 55), and 5.5 % (n = 14) of the study participants, respectively. The proportion of patients with hypertension (p < 0.001) or cardiovascular disease (p < 0.01) was larger in the subgroup of patients with T2DM. Based on our selection criteria for surgery type, SG was more commonly performed in T2DM subjects (30.2 %) as compared with the nondiabetic subgroup (15.8 %; p < 0.05).
As shown in Table 1, baseline ACR in the whole cohort was 55.0 ± 139.1 mg/g, with this ratio being larger in the T2DM subgroup (p < 0.05). ACR ≥30 mg/g was present in 27.3 % of the study participants, with a larger proportion of T2DM subjects presenting with ACR ≥30 mg/g (45.7 %) as compared with the nondiabetic group (16.4 %; p < 0.001). Stepwise logistic regression analysis in the whole cohort showed diagnosis of T2DM as the sole independent predictor of presence of ACR ≥30 mg/g [odds ratio (OR) 4.34, confidence interval (CI) 1.89–9.97; p = 0.01] prior to surgery. Age, gender, smoking habit, time since diagnosis of T2DM, diagnosis and time since diagnosis of hypertension, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, diagnosis of cardiovascular disease, BMI, and waist circumference were not independent predictors of ACR ≥30 mg/g in the whole study group. Fasting plasma glucose and hemoglobin A1c were added to the regression model when restricted to the T2DM cohort. In that subgroup, fasting plasma glucose (OR 1.016, 95 % CI 1.002–1.031; p = 0.028) was the only variable independently associated with ACR ≥30 mg/g at baseline.
Following bariatric surgery, a statistically significant reduction of ACR was observed at 12 months in the whole cohort (31.55 ± 108.5 mg/g; p < 0.001), with no further decrease at 24 months follow-up (30.35 ± 153.7 mg/g, p = 0.710). Likewise, the ACR significantly decreased in the T2DM group at 12 months (42.2 ± 142.8 mg/g; p < 0.005) with no additional reduction at last follow-up assessment (44.4 ± 227.7 mg/g; p = 0.862). The proportion of subjects with T2DM in whom the ACR decreased below the 30 mg/g threshold was 58.5 % and 76.9 % at 12 and 24 months after surgery, respectively (for both p < 0.001 relative to baseline, p = 0.083 for the comparison between 12 and 24 months).
The features of subjects with T2DM and ACR >30 mg/dl at baseline in whom ACR normalized or did not normalize at 12 months after surgery are presented in Table 2. At that time point, weight loss in this group of subjects was 35.64 ± 11.64 kg, corresponding to 28.3 ± 8.6 % of baseline body weight. Normalization of ACR at 12 months was associated with lower ACR at baseline (p < 0.05), larger decrease in waist circumference (p < 0.05), and larger decrease in BMI (p < 0.05). No differences between the two groups were found in variables related to T2DM (changes in fasting plasma glucose or hemoglobin A1c, duration of T2DM) or hypertension (changes in systolic or diastolic blood pressure, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, duration of hypertension). The proportion of subjects presenting with remission of T2DM (ACR normalization: 82.6 %; ACR abnormal: 64.3 %) or hypertension (ACR normalization: 17.4 %; ACR abnormal: 28.6 %) did not differ between the two subgroups (p = 0.208 and p = 0.423, respectively). Likewise, the proportion of subjects who underwent GBP or SG did not differ between the two groups (ACR normalization: GBP/SG 71 %/29 %; ACR abnormal: GBP/SG 65 %/35 %; p = 0.678).
Stepwise logistic regression analysis to evaluate the factors associated with ACR normalization at 12 months after surgery in T2DM subjects was constructed with remission of T2DM, remission of hypertension, baseline ACR value, and change in BMI from baseline as independent variables. BMI change from baseline was the only variable included in the model as a predictor of ACR normalization [Exp(B) 1.373, 95 % confidence interval 1.075–1.703; p = 0.01; R 2 = 0.315]. In alternative models the independent contribution of changes in waist circumference, fasting plasma glucose or hemoglobin A1c, use of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and type of surgery yielded negative results.
At 24 months after surgery, weight loss in the group of subjects with T2DM and ACR >30 mg/dl at baseline was 36.39 ± 13.25 kg. Thus, no additional weight loss was observed between 12 and 24 months after surgery (p = 0.543). T2DM and hypertension remission occurred, respectively, in 80.2 % and 27.5 % of patients in this group. At that time point, subjects in whom ACR normalized presented lower ACR at baseline as compared with those in whom ACR remained abnormal (113.9 ± 133.9 mg/g versus 410.8 ± 344.1 mg/g; p = 0.001). Furthermore, the reduction in hemoglobin A1c was larger in those with normalization of ACR compared with those with persistently abnormal ACR (3.1 ± 2.2 % versus 1.7 ± 1.6 %; p = 0.045). However, no difference was found when the proportion of subjects with remission of T2DM (ACR normalization: 79.2 %; ACR abnormal: 50 %; p = 0.112) or hypertension (ACR normalization: 19.2 %; ACR abnormal: 37.5 %; p = 0.287) was compared according to ACR outcome at 24 months after surgery. Nonetheless, in stepwise logistic regression analysis, none of the evaluated variables appeared as an independent predictor of ACR normalization at 24 months.
Discussion
Our observational prospective study shows that, although a high proportion of morbidly obese subjects with T2DM present with abnormal ACR, normalization of urinary albumin excretion occurs in a significant proportion of T2DM subjects following bariatric surgery. Furthermore, weight loss appears as an independent contributor to ACR normalization in morbidly obese subjects undergoing bariatric surgery.
It has been shown that a larger BMI is a risk factor for prevalent and incident altered ACR in T2DM [8, 13]. In fact, the prevalence of elevated urinary albumin excretion in our series tripled that in a survey including 286,791 T2DM subjects with similar duration of disease but mean BMI of 29.6 kg/m2 in the same geographical area [14]. Similarly, the higher prevalence of ACR >30 mg/g as compared with that recently reported by Schauer et al. in a series of subjects with T2DM with morbid obesity could be accounted for by the lower mean BMI in their series [2]. Lower prevalence of elevated urinary albumin excretion has been found in several of the previous studies in MO subjects with BMI distribution similar to that reported herein, but the prevalence of albuminuria specifically in subjects with morbid obesity and T2DM in those series was not reported [4, 6, 15]. Our logistic regression analysis further emphasizes the additional burden of T2DM on renal function in MO subjects. Along these lines, Iaconelli et al. showed an increase in the prevalence of albuminuria from 14.3 % at baseline to 28.6 % after 2-year follow-up in MO subjects with newly diagnosed T2DM and stable body weight [16]. This increase is larger than the 2 % progression rate to microalbuminuria in newly diagnosed T2DM subjects found in the UK Prospective Diabetes Study (UKPDS) [17].
Weight loss achieved by means of lifestyle changes has been associated with a significant reduction of albumin excretion rate, with only part of this relationship being explained by the effect of weight change on blood pressure [18]. However, to date, bariatric surgery has been shown to be the most effective way of achieving and sustaining marked weight loss in MO patients [19]. Previous studies have shown the beneficial effects of bariatric surgery on microalbuminuria in MO subjects, with normalization rates ranging from 57 to 79 % [4, 6, 15]. Furthermore, Agrawal et al. demonstrated a larger percentage decrease in ACR with larger weight loss in MO subjects undergoing gastric bypass [15]. Nonetheless, the relevance of weight loss for ACR normalization in MO subjects with T2DM in previous studies has been limited by the limited proportion of included subjects with diabetes. In the only series restricted to T2DM subjects published to date, Navaneethan et al. reported on the outcome of ACR in 15 subjects undergoing either gastric bypass or restrictive surgery [5]. Interestingly, the resolution rate of ACR at 12 months follow-up in that pilot study was very similar (57.1 %) to that reported herein (58.5 %). However, at variance to our findings, Navaneethan et al. failed to find an association between weight loss and ACR normalization but did report on an association between surgery modality and ACR normalization [5]. The larger series of subjects with T2DM included in our study allowed for stepwise logistic regression analysis in which classical factors associated with progression of albuminuria, namely glucose and blood pressure control, as well as weight loss and surgery type (gastric bypass or sleeve gastrectomy) could be evaluated in the same model. Our data support previous data in nondiabetic subjects on the importance of weight loss as an independent predictor of ACR normalization in MO subjects undergoing BS [15].
Interestingly, the proportion of subjects in our cohort in whom ACR normalized at 24 months after BS was not significantly larger than that at 12-month evaluation. To our knowledge, no previous studies have reported on the ACR outcome following BS in MO subjects with established T2DM up to that length of follow-up. In support of a potential role of weight loss, the lack of further normalization of ACR in T2DM subjects at 24 months after BS occurred in our study in the context of no statistically significant additional weight loss. However, the change in BMI from baseline to the 24 months observation time point was not a significant predictor of ACR normalization on logistic regression analysis. Of note, a similar observation was reported in a series of nondiabetic MO subjects undergoing BS [20]. The low number of patients with persistent ACR >30 mg/g at 24 months after surgery in our cohort (n = 9) could have limited our ability to detect statistical significance in our logistic regression analysis. Nonetheless, our data suggest that the improvement of glycemic control could also play a role at that length of follow-up.
We acknowledge that our study has several limitations. First, in our study, urinary albumin excretion was defined based on a single urinary specimen. Thus, our diagnostic strategy may have resulted in overdiagnosis of altered urinary albumin excretion rate [1]. Second, daily protein intake was not ascertained in our study cohort. Dietary protein restriction has been suggested to improve measures of renal function in subjects with T2DM and microalbuminuria [1]. Thus, an independent effect of reduced protein intake, such as that occurring following bariatric surgery, cannot be ruled out [21]. Finally, it has been suggested that adipokines involved in the low-grade inflammatory process associated with obesity could be involved in the pathogenesis of altered albumin excretion rate in T2DM subjects with MO [5]. Assessment of the involvement of inflammation or changes in insulin sensitivity in the resolution of elevated ACR was beyond the scope of the present study.
In summary, our data show that weight loss as a result of BS is an effective strategy to improve ACR, not only a marker of renal disease but also a risk factor for cardiovascular disease and early cardiovascular mortality in patients with T2DM [22]. Thus, our data suggest that, in MO subjects with T2DM, interventions aimed at slowing the progression of nephropathy should not only focus on the optimization of glucose and blood pressure control but also include effective weight loss strategies.
References
American Diabetes Association. Standards of medical care in diabetes-2012 (2012) Diabetes Care 35 (Suppl 1):S11–S63
Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL (2012) Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366:1567–1576
Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F (2012) Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 366:1577–1585
Hofsø D, Nordstrand N, Johnson LK, Karlsen TI, Hager H, Jenssen T, Bollerslev J, Godang K, Sandbu R, Røislien J, Hjelmesaeth J (2010) Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol 163:735–745
Navaneethan SD, Kelly KR, Sabbagh F, Schauer PR, Kirwan JP, Kashyap SR (2010) Urinary albumin excretion, HMW adiponectin, and insulin sensitivity in type 2 diabetic patients undergoing bariatric surgery. Obes Surg 20:308–315
Agrawal V, Khan I, Rai B, Zalesin KC, Rocher LL, McCullough PA (2008) The effect of weight loss after bariatric surgery on albuminuria. Clin Nephrol 70:194–202
Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ (2008) Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int 73:19–33
Kramer H, Reboussin D, Bertoni AG, Marcovina S, Lipkin E, Greenway FL 3rd, Brancati FL; Look Ahead Research Group (2009) Obesity and albuminuria among adults with type 2 diabetes: the Look AHEAD (Action for Health in Diabetes) Study. Diabetes Care 32:851-853
Mechanick JI, Kushner RF, Sugerman HJ, Gonzalez-Campoy JM, Collazo-Clavell ML, Spitz AF, Apovian CM, Livingston EH, Brolin R, Sarwer DB, Anderson WA, Dixon J, Guven S (2009) American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery medical guidelines for clinical practice for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient. Obesity (Silver Spring) 17(Suppl 1):S1–S70
Vidal J, Ibarzabal A, Romero F, Delgado S, Momblan D, Flores L, Lacy A (2008) Type 2 diabetes mellitus and the metabolic syndrome following SG in in severely obese subjects. Obes Surg 18:1077–1082
Vidal J, Morínigo R, Codoceo VH, Casamitjana R, Pellitero S, Gomis R (2005) The importance of diagnostic criteria in the association between the metabolic syndrome and cardiovascular disease in obese subjects. Int J Obes (Lond) 29:668–674
Buse JB, Caprio S, Cefalu WT, Ceriello A, Del Prato S, Inzucchi SE, McLaughlin S, Phillips GL 2nd, Robertson RP, Rubino F, Kahn R, Kirkman MS (2009) How do we define cure of diabetes? Diabetes Care 32:2133–2135
Afghahi H, Cederholm J, Eliasson B, Zethelius B, Gudbjörnsdottir S, Hadimeri H, Svensson MK (2011) Risk factors for the development of albuminuria and renal impairment in type 2 diabetes-the Swedish National Diabetes Register (NDR). Nephrol Dial Transplant 26:1236–1243
Vinagre I, Mata-Cases M, Hermosilla E, Morros R, Fina F, Rosell M, Castell C, Franch-Nadal J, Bolíbar B, Mauricio D (2012) Control of glycemia and cardiovascular risk factors in patients with type 2 diabetes in primary care in Catalonia (Spain). Diabetes Care 35:774–779
Agrawal V, Krause KR, Chengelis DL, Zalesin KC, Rocher LL, McCullough PA (2009) Relation between degree of weight loss after bariatric surgery and reduction in albuminuria and C-reactive protein. Surg Obes Relat Dis 5:20–26
Iaconelli A, Panunzi S, De Gaetano A, Manco M, Guidone C, Leccesi L, Gniuli D, Nanni G, Castagneto M, Ghirlanda G, Mingrone G (2011) Effects of bilio-pancreatic diversion on diabetic complications: a 10-year follow-up. Diabetes Care 34:561–567
Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR; UKPDS GROUP (2003) Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63:225-232
Bello AK, de Zeeuw D, El Nahas M, Brantsma AH, Bakker SJ, de Jong PE, Gansevoort RT (2007) Impact of weight change on albuminuria in the general population. Nephrol Dial Transplant 22:1619–1627
Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H; Swedish Obese Subjects Study Scientific Group (2004) Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351:2683-2693
Navarro-Díaz M, Serra A, Romero R, Bonet J, Bayés B, Homs M, Pérez N, Bonal J (2006) Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol 17(Suppl 3):S213–S217
Andreu A, Moizé V, Rodríguez L, Flores L, Vidal J (2010) Protein intake, body composition, and protein status following bariatric surgery. Obes Surg 20:1509–1515
van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium (2011) Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79:1341–1352
Acknowledgments
This work was supported by a grant from the Fondo de Investigaciones Sanitarias Instituto Carlos III (PI11/00892), Madrid, Spain.
Disclosures
Authors Antonio Amor, Amanda Jiménez, Violeta Moizé, Lílliam Flores, Ainitze Ibarzabal, and Josep Vidal have no financial relationships to declare with any pharmaceutical or device company specific to the data presented herein. Josep Vidal is the principal investigator of the research project PI11/00892 funded by the Fondo de Investigaciones Sanitarias Instituto Carlos III (Madrid, Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amor, A., Jiménez, A., Moizé, V. et al. Weight loss independently predicts urinary albumin excretion normalization in morbidly obese type 2 diabetic patients undergoing bariatric surgery. Surg Endosc 27, 2046–2051 (2013). https://doi.org/10.1007/s00464-012-2708-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-012-2708-3