Abstract
Background
The prevalences of obesity and chronic kidney disease (CKD) have increased simultaneously. Should a pathophysiological relationship exist between the two conditions, bariatric surgery and associated weight loss could be an important intervention in extremely obese individuals to slow the progression of CKD.
Methods
We conducted a retrospective analysis of 25 patients who had undergone biliopancreatic diversion (BPD) surgery for extreme obesity (body mass index >40 kg/m2), with mean follow-up of 4 years. We assessed pre- and post-surgery renal function, body weight and blood pressure (BP) obtained from electronic hospital and primary care records.
Results
There was a significant reduction in mean body weight at 4 years by 50.3 kg (SD = 20.65). The creatinine and estimated glomerular filtration rate (eGFR) also improved significantly: serum creatinine reduced by 16.2 μmol/l (SD = 19.57) while the eGFR improved by 10.6 ml/min/m2 (SD = 15.45). The greatest improvement in eGFR was in the group (n = 7) with eGFR ≤60 ml/min/m2. A subset of patients (n = 11) had evaluable BP readings and had a reduction in BP of 17/10 mmHg (SD = 33/12).
Conclusions
This retrospective study demonstrates a clinically significant improvement in renal function following BPD. Several mechanisms including weight loss could account for the positive impact on renal function. The physiology underlying this improvement requires further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of obesity has increased at an alarming rate. In the USA, from 2002 to 2008, obesity increased from 27.6 to 33.2 % for men and 33.2 to 35.5 % for women [1, 2]. The prevalence of chronic kidney disease (CKD) has increased simultaneously with increasing levels of obesity [3, 4]. Several observational studies have demonstrated a consistent association between CKD risk and elevated body mass index (BMI) [5]. In the Framingham Heart Study, in multivariable models, elevated baseline BMI was significantly associated with development of CKD, with an odds ratio of 1.23 (95 % CI 1.08–1.41) for each standard deviation in BMI [6]. Obesity as a predictor for the development of CKD was also demonstrated in two large studies with 5,897 and 11,104 patients, respectively [7, 8]. The Hypertension Detection and Follow-up Program found that the risk of developing CKD at 5 years after adjustment for age, sex, race and diabetes for overweight and obese patients was 20 % (95 % CI 1.04–1.39) and 40 % (95 % CI 1.21–1.65), respectively, compared to normal weight individuals [7]. In a large prospective study consisting of healthy male physicians, the risk of developing CKD after 14 years follow-up was approximately 30 % in those who were overweight (1.32; 95 % CI 1.09–1.61) and obese (1.26; 95 % CI 1.03–1.54) after controlling for multiple factors [8].
The relationship between obesity and CKD is complex with both associated with factors including hypertension and diabetes mellitus [9]. A number of studies have examined a potential independent link between obesity and kidney disease. In 1974, it was reported that obese patients have a propensity to nephrotic-range proteinuria [10]. Subsequent renal haemodynamic studies revealed the presence of glomerular hyperfiltration and increased glomerular filtration rate (GFR) in obese patients, with associated increased renal blood flow [11]. Renal biopsy has shown that patients with a raised BMI are more likely to develop a specific pattern of kidney damage with glomerulomegaly and focal segmental glomerulosclerosis [12, 13]. This histological finding has been termed obesity-related glomerulopathy [5].
If obesity were a pathophysiological predictor of CKD, then it would be expected that weight loss would help prevent and/or delay CKD. Several small studies, including randomised controlled trials (RCTs) of dietary weight loss, have demonstrated the anti-proteinuric effect of weight reduction [14]. A systematic review of weight loss interventions reported the benefits of bariatric surgery on renal function [15]. Currently, bariatric surgery is the most effective intervention for long-term weight loss in extreme obesity [16]. We carried out a retrospective analysis of renal function in extremely obese patients undergoing biliopancreatic diversion (BPD), a procedure associated with significant weight loss, at a regional bariatric centre in the West Midlands, UK. The aim was to examine the medium- to long-term effect on renal function.
Subjects and Methods
The analysis was carried out as part of our service evaluation and thus did not require formal ethical committee approval as recommended by the UK National Research Ethics Service (www.nres.npsa.nhs.uk/EasySiteWeb/GatewayLink.aspx?alId=320). All data were analysed anonymously. From our clinical dataset, all adult patients aged ≥18 years who underwent BPD between 2002 and 2005 were identified. All procedures were BPDs with a 1-m common channel length and 2-m alimentary and biliopancreatic limbs. The stomach was resected to the level of the short gastrics and divided transversely, producing a gastric reservoir of approximately 300 cc. All procedures in this historic series were performed by open surgery. Of the 42 patients identified, 15 were male and 27 female. The indications for surgery was extreme obesity defined as BMI >40 kg/m2 or BMI >35 kg/m2 with co-morbidities based on international guidelines. Demographic data, blood pressure readings and serial serum creatinine results were obtained from hospital case notes and electronic patient records.
All patients who underwent a BPD procedure and had evaluable data for serum creatinine and weight prior to and at time of analysis were included in the analysis. Of the cohort of 42 patients, 25 patients met the criteria and had complete data available over a follow-up period of 2–6 years. Eleven patients had follow-up blood pressure (BP) readings available, and these were analysed separately.
Estimated glomerular filtration rates (eGFR) were calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) equation using serum creatinine values, age and ethnicity [17]. Serum creatinine on the day of or immediately prior to the surgery was considered as baseline. The latest creatinine following surgery was taken for end point analysis.
Statistical analysis was carried out using STATA (version 12; StataCorp, USA). Results are expressed as mean (±standard deviation) or median (range). The variables were analysed for normality using visual inspection and Shapiro–Wilk test of normality. Body weight and BMI were log transformed. eGFR, but not change in GFR, was not normally distributed. For normally distributed and transformed data, paired sample t tests were carried out. For eGFR, the Wilcoxon matched pairs signed-rank test was carried out. We compared eGFR change between those with GFR of ≤60 ml/min/m2 (n = 7) vs those with GFR >60 ml/min/m2 (n = 18) using a two-sample independent t test. A p value of <0.05 was considered significant.
Results
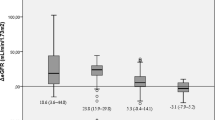
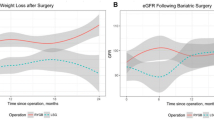
Table 1 shows the patient characteristics. Patients were followed up for a mean of 3.9 years, ranging from 2–6 years. Eleven patients had BP readings at baseline and at study end. Table 2 shows the changes observed in the parameters examined. The mean weight loss was 50.3 ± 20.65 kg at 3.9 years, from 166.2 ± 37.89 to 115.87 ± 33.89 kg (p < 0.001). BMI decreased by 17.04 ± 6.79 kg/m2 at study end (p < 0.001). Figure 1 shows serum creatinine levels for patients at baseline (pre-surgery) and at follow-up. The mean serum creatinine value decreased by 16.23 ± 19.57 μmol/l (p < 0.001) from 86.71 ± 15.57 to 70.48 ± 14.28 μmol/l at study end (p < 0.001). eGFR values improved significantly—a mean increase of 10.6 ± 15.45 ml/min/m2 [median, +10 ml/min/m2; range, −24 to +43 ml/min/m2] at study end (p = 0.048). Of note, those with GFR of ≤60 ml/min/m2 (n = 7) had the greatest improvement in GFR (mean, +28 ± 10.5 ml/min/m2) vs those with GFR >60 ml/min/m2 (n = 18; mean, +3.8 ± 10.5 ml/min/m2), p < 0.001.
A subset of patients had evaluable BP readings. Mean BP reduction was 17/10 ± 33/12 mmHg, from 141/85 ± 24/9 mmHg before BPD to 125/75 ± 16/8 mmHg at end of follow-up. Although the systolic BP was not significantly reduced, the reduction in diastolic BP was statistically significant (p = 0.019).
In six patients, the creatinine levels either did not change or increased over the follow-up period. Of those who showed elevations in creatinine, the majority had increases in creatinine that were within normal ageing. One patient had a marked elevation in creatinine at the time of follow-up. Further enquiry revealed that this was due to an episode of acute kidney injury that resolved. Four of the patients with diabetes had resolution of diabetes, and the remaining patient remained on tablet treatment for diabetes with good diabetes control.
Discussion
This retrospective observational study demonstrates that BPD is associated with significant and sustained weight reduction over nearly 4 years. BPD is also associated with a decrease in serum creatinine, an increase in eGFR and reduction in diastolic BP. Given that the ageing decline in eGFR is equivalent to 1 ml/min/year and obesity is associated with accelerated ageing, an average improvement of about 10 ml/min is clinically highly significant. Furthermore, the greatest improvement in eGFR was observed in those whose baseline eGFR was ≤60 ml/min/m2. Our study is in agreement with Schuster and colleagues who observed an improvement in creatinine levels after bariatric surgery at up to 2 years follow-up [18].
We chose to study patients who had BPD with the longest follow-up because there is moderate evidence that this procedure, although uncommon, is most successful in weight reduction [16]. There are no RCTs comparing BPD with other forms of weight loss surgery, but a systematic review has reported that BPD resulted in a mean weight loss of 51.9 kg (95 % CI 45.1–58.8) at 12-month follow-up and 53.1 kg (95 % CI 47.4–58.8) at 36-month follow-up and beyond [16]. In our cohort, the mean weight loss following BPD surgery was comparable at 50.33 ± 20.56 kg.
A study by Chagnac et al. reported that in obese patients, there was renal hyperfiltration and pathologically raised measured GFR values (140 ± 14 ml/min pre-surgery), measured by inulin clearance [11]. The authors claimed that all subjects had a ‘normal’ creatinine level prior to weight loss, casting doubt on the efficacy of the MDRD eGFR values in obesity. Glomerular hyperfiltration was postulated to lead to long-term glomerular damage and deterioration in renal function. As per this hypothesis, the development of hyperfiltration is presumably an early event in the natural history of the process, which eventually leads to glomerular damage, resulting in a decrease in GFR. The study by Chagnac et al. showed a decrease in measured GFR, 12 months after weight loss surgery. Our study cohort had an average starting MDRD eGFR of 70.96 ± 15.58 ml/min/m2 suggesting that this cohort did not have renal hyperfiltration. The subsequent rise in eGFR after weight loss could thus reflect a true improvement in renal function.
In the subset of patients with BP readings, BP improved over the follow-up period, although only the reduction in diastolic BP showed statistical significance. A 2003 meta-analysis of previous RCTs also observed a reduction in blood pressure after weight reduction [9]. The authors postulated that an overactive renin–angiotensin–aldosterone system in obesity is corrected following weight reduction, which in turn leads to improved BP. It is possible that the BP improvement, at least in part, contributed to the improvement in renal function.
There are a number of limitations to our study. This is a retrospective study of a small cohort of patients with heterogeneous co-morbidities. As yet MDRD GFR has not been validated in obese subjects. Neither did we have data on urinary albumin excretion or other measures of renal function. We also did not have data on other confounders such as smoking status, co-morbidities or co-existing drug therapy. Despite these limitations, this study clearly demonstrates that biliopancreatic surgery and weight reduction lead to significant improvements in serum creatinine and eGFR. There is now a large body of evidence suggesting that obesity is associated with more rapid progression of CKD. The results of this study encourage us to believe that for obese patients with CKD, weight reduction surgery will help slow down CKD progression. Nevertheless, because of small patient numbers, it was not possible to observe a significant direct association between weight loss and improvement in renal function. Other mechanisms including change in gut hormonal milieu may also be important. A prospective RCT, in obese CKD patients selected for co-morbidities, with measured GFR, is now warranted, including a study of potential long-term risks beyond our follow-up period.
It is promising at this juncture that even in a small patient cohort, the improvement in renal function is significant. Future studies are needed to investigate whether the benefits in renal function and blood pressure can be extrapolated to a population of patients suffering from CKD.
References
Baskin ML, Ard J, Franklin F, et al. Prevalence of obesity in the United States. Obes Rev. 2005;6(1):5–7.
Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41.
Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47.
Snyder JJ, Foley RN, Collins AJ. Prevalence of CKD in the United States: a sensitivity analysis using the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2009;53:218–28.
Ting SMS, Nair H, Ching I, et al. Overweight, obesity and chronic kidney disease. Nephron Clin Pract. 2009;112:c121–7.
Fox CS, Larson MG, Leip EP, et al. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–50.
Kramer H, Luke A, Bidani A, et al. Obesity and prevalent and incident CKD: the hypertension detection and follow-up program. Am J Kidney Dis. 2005;46:587–94.
Gelber RP, Kurth T, Kausz AT, et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46:871–80.
Neter JE, Stam BE, Kok FJ, et al. Influence of weight reduction on blood pressure. A meta-analysis of randomized controlled trials. J Hypertens. 2003;42:878–84.
Weisinger JR, Kempson R, Eldidge L, et al. The nephrotic syndrome: a complication of massive obesity. Ann Intern Med. 1974;81:440–7.
Chagnac A, Weinstein T, Korzets A, et al. Glomerular haemodynamics in severe obesity. Am J Physiol Ren Physiol. 2000;278:817–22.
Kasiske BL, et al. Glomerulosclerosis in patients with massive obesity. Am J Nephrol. 1985;5:45–50.
Jennette JC, Charles L, Grubb W. Glomerulomegaly and focal segmental glomerulosclerosis associated with obesity and sleep apnoea syndrome. Am J Kidney Dis. 1987;10:470–2.
Praga M, Hernandez E, Andres A, et al. Effects of body weight loss and captopril treatment on proteinuria associated with obesity. Nephron. 1995;70:35–41.
Navaneethan SD, Yehnert H, Moustarah F, et al. Weight loss interventions in chronic kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2009;4(10):1565–74. Epub 2009 Sep 17.
Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142(7):547–59.
Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70.
Schuster DP, Teodorescu M, Mikami D, et al. Effect of bariatric surgery on normal and abnormal renal function. Surg Obes Relat Dis. 2011;7(4):459–64.
Acknowledgments
Dr. Shahrad Taheri is funded by the National Institute for Health Research (NIHR) through the Collaborations for Leadership in Applied Health Research and Care for Birmingham and Black Country (CLAHRC BBC) programme. The views expressed in this publication are not necessarily those of the NIHR, the Department of Health, NHS South Birmingham, University of Birmingham or the CLAHRC BBC Theme 8 Management/Steering Group.
Conflict of Interest
None
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Jose, B., Ford, S., Super, P. et al. The Effect of Biliopancreatic Diversion Surgery on Renal Function—a Retrospective Study. OBES SURG 23, 634–637 (2013). https://doi.org/10.1007/s11695-012-0851-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-012-0851-5