Abstract
Background
Increased lipopolysaccharide (LPS) translocation due to altered intestinal permeability has been suggested as a mechanism for obesity-associated insulin resistance. The goal of this study was to assess the effect of sleeve gastrectomy (SG) on intestinal barrier permeability in diet-induced obese mice.
Materials and Methods
Four weeks after surgery, the effects of SG on intestinal permeabilities were assessed ex vivo and in vivo in male C57Bl/6J mice fed a high-fat diet. Gene expression of tight junction proteins and inflammatory cytokines was measured in jejunum, colon, liver, and inguinal adipose tissue. Plasma LPS was quantified by HPLCMS/MS spectrometry.
Results
SG significantly reduced body weight and improved glucose homeostasis, as expected. SG decreased paracellular (p = 0.01) and transcellular permeability (p = 0.03) in the jejunum; and increased mRNA levels of the tight junction proteins Jam A (p = 0.02) and occludin (p = 0.01). In contrast in the distal colon, paracellular permeability tended to be increased (p = 0.07) while transcellular permeability was significantly induced (p = 0.03) after SG. In vivo, the paracellular permeability was significantly increased 3 weeks after SG (p = 0.02). Plasma LPS level were increased after SG (p = 0.03), as well as mRNA levels of adipose and hepatic inflammatory markers (p = 0.02).
Conclusions
SG significantly modifies intestinal permeability in a differential manner between the proximal and distal intestine. These changes promote LPS translocation in plasma, induce a low-grade pro-inflammatory state in adipose tissue and liver, but do not impair the SG-induced glucose homeostasis improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morbid obesity is frequently associated with metabolic complications, such as type 2 diabetes mellitus (T2DM). Among bariatric procedures, sleeve gastrectomy (SG) is the most frequently used in many countries. Randomized prospective studies have demonstrated that bariatric surgery is an efficient strategy to improve glycemic control or even cure T2DM [1]. Several molecular mechanisms sustain these beneficial effects of bariatric surgery, including stimulation of incretin secretion and modulation of bile acid signaling [2]. Previous works suggested that alterations in intestinal permeability could underlie the metabolic complications of obesity. In rodents, several studies built the concept that the consumption of a high-fat diet induces microbiome modifications, decreases the expression of tight junction proteins, and increases intestinal permeability that favors plasma LPS translocation [3–8]. Accordingly, increased intestinal permeability induces a chronic inflammatory response and promotes systemic insulin resistance [3, 8–10].
In humans, the effects of obesity and bariatric surgery on intestinal permeability have been poorly explored and have produced varying results [11]. In healthy subjects, a positive correlation was observed between the permeability of the colon and the amount of visceral fat [12]. One team reported that no change in intestinal permeability occurs in the presence of obesity [13], but did observe a slight increase in intestinal permeability in obese patients in a subsequent study [14]. A third study showed that obese patients display increased gastroduodenal permeability but no differences in the permeability of the small intestine or the colon [15]. Intestinal permeability, as measured by the lactulose/mannitol ratio, was not significantly altered in patients 6 months after RYGB [16]. However, a significant increase in claudin 3 and 4 levels was found 8 months after RYGB, whereas occludin and ZO-1 levels decreased in this timeframe [17].
Based on these discordant results, our study aims to assess the short-term effects of SG on intestinal paracellular and transcellular permeabilities and the subsequent effects on LPS translocation, white adipose tissue and hepatic inflammation, and glucose homeostasis in diet-induced obese mice.
Materials and Methods
Animals
Ten-week-old C57Bl/6 mice (Charles River) had free access to water and high-fat diet for 8 weeks prior to surgery (DIO diet 35% kcal from fat, Safe). As the maximal metabolic benefit appears to be reached 3 to 4 weeks after bariatric procedures [18], we did measure the functional consequence of SG on intestinal permeability during this period. All experiments were approved by the Ethics Committee for Animal Experimentation of Pays de la Loire (study n° 01953.01).
Surgical Procedures
Prior surgery, mice were matched on body weight and plasma cholesterol and divided in three experimental groups. Sham and Pairfed control mice were only subjected to a sham operation (laparotomy and mobilization of the stomach). Sham and SG mice were fed ad libitum when Pairfed mice received the same amount of food consumed by the SG mice 24 h before. A SG was performed as follows on the third group of mice. After laparotomy, the stomach was externalized, and the pylorus vessels were sutured along the greater stomach curvature with 8.0 Prolene single sutures (Ethicon®, Johnson & Johnson). Then, a gastrostomy was performed on the anatomical line present between the pyloric region and the cardiac region of the stomach and the incision site was sutured with 8.0 Prolene using a running suture from the gastro-esophageal junction. Eighty percent of the stomach was removed. The muscle layer of the abdominal wall and the skin was closed using interrupted sutures with 5.0 Prolene. After surgery, isoflurane was stopped and O2 flow (0.8 L/min) was continued until the mice were fully recovered. Mice were individually housed in a 30 °C incubator for the next 5 days. All groups had free access to Highfat Geldiet (lard 10%, liquid sugar 10%, water 57%; Safe) for 5 days and the DIO diet was reintroduced 3 days after surgery. Buprenorphine (0.1 mg/kg, twice daily), meloxicam (1 mg/kg), and marbofloxacin (10 mg/kg) were subcutaneously administrated for 3 days after surgery and metoclopramide (1 mg/kg) was maintained for 5 days.
Food Intake Measurement
The food intake was measured for each mouse daily for up to 4 weeks after surgery by substrating the uneaten food from a pre-weighed amount of solid diet placed in the cage 24 h before.
Oral Glucose Tolerance Test
Two weeks after surgery, 6-h-fasted mice received an oral bolus of d-glucose (2 g/kg) and plasma glucose levels were measured at 0, 15, 30, 60, and 120 min after gavage by tail bleeding (Glucometer-One Touch Verio®).
Total Transit Time and Colonic Motility Measurement
Total transit time (TTT) and colonic motility were evaluated as previously reported [19]. Mouse was housed in individual cage, and produced feces were harvested every 15 min for 2 h. The fecal water content was estimated by subtracting the fresh fecal weight by the dry fecal weight (after feces lyophilization).
Intestinal Permeability Assessment
In vivo intestinal barrier function was assessed by measuring the paracellular transport of fluorescein-conjugated sulfonic acid (F-SA) in plasma [19]. Ex vivo, jejunal, or colonic intestinal biopsies were mounted in Ussing chambers and paracellular and transcellular permeabilities were determined by respectively quantifying the incoming transport of F-SA and horseradish peroxidase (HRP) as described before [19].
mRNA Levels Analysis
Total RNA was extracted from tissues using NucleoSpin RNA II (Macherey Nagel). cDNA was synthesized using SuperScript III Reverse Transcriptase (Life Technologies). Real-time quantitative PCR (qPCR) was performed using primers described in Table 1 using Absolute Blue SYBR Green Fluorescein mix (Life Technologies), and reactions were run on a StepOne + thermocycler (Life Technologies).
Plasma LPS Measurement
Plasma LPS was separated by reverse-phase HPLC and quantitated by MS/MS spectrometry, as described before [20].
Statistical Analysis
Statistical analysis was performed by Prism5 (GraphPad) using non parametric Mann-Whitney test or Kruskal–Wallis test followed by Dunn’s test.
Results
SG is an Efficient Procedure in Obese Mice
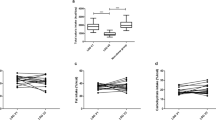
Eight weeks after a HFD, 40 male mice were randomized based on body weight and plasma total cholesterol and divided into 3 experimental groups (Sham, Pair-fed, SG). The average body weight before surgery was 35.8 ± 3.9 g. The average duration of the procedure was 41 ± 7 min. The survival rate was 86% after SG. Compared to baseline, SG led to significant reductions in body weight at 2 (−8%, p = 0.03) and 4 weeks post-surgery (−5.5%, p = 0.13, Fig. 1a). There was a significant reduction in body weight in the SG group compared to the sham group on day 14 (−13%, p = 0.02) and day 21 (−9%, p = 0.02, Fig. 1a). One week after the surgery, we observed a significant difference in food intake between the sham and SG/pair-fed groups (p < 0.0001, Fig. 1b). Four weeks after surgery, epididymal white adipose tissue mass was decreased in the SG group compared to the sham (−22%, p = 0.08) and pair-fed groups (−32%, p = 0.01, Fig. 1c). An oral glucose tolerance test (oGTT) confirmed that glucose homeostasis was significantly improved 2 weeks after SG compared to the control groups (p = 0.002, Fig. 1d).
SG Does Not Alter Intestinal TTT or Fecal Water Content
We first assessed the effects of SG on intestinal function by measuring TTT and fecal water content from the three groups 2 weeks after surgery. As shown in Fig. 2a, b, there were no differences in these parameters among the three groups.
SG Affects Differently Jejunal and Colon Permeabilities
SG decreased both paracellular permeability, as reflected by reduced F-SA transport (−39%, p = 0.01), and transcellular permeability, with reduced HRP flux (−45%, p = 0.03) measured ex vivo in jejunal biopsies (Fig. 3a, b). Consistently, these jejunal alterations were associated with higher mRNA expression of Jam A (+31%, p = 0.02; Fig. 3c) and occludin (+34%, p = 0.01; Fig. 3d) in the SG group, whereas ZO-1 mRNA levels were not significantly altered (p = 0.27; Fig. 3e). In contrast, both ex-vivo paracellular and transcellular permeabilities were respectively increased in the distal colon of SG mice (+ 26%, p = 0.07 and +44%, p = 0.03; Fig. 4a, b), whereas expressions of occludin, Jam A and ZO-1 mRNA were unchanged (Fig. 4c–e).
Ex vivo measurement of the effect of sleeve on jejunal paracellular (a) and transcellular permeability (b). Consequences of sleeve on jejunal mRNA levels of Jam A (c), Occludin (d), and ZO-1 (e). Values are normalized with S6 and reported to values measured in the sham group arbitrarily set to 1. Data are means ± SEM. *p < 0.05
Ex vivo measurement of the effect of sleeve on colonic paracellular (a) and transcellular permeability (b). Consequences of sleeve on colonic mRNA levels of Jam A (c), Occludin (d), and ZO-1 (e). Values are normalized with S6 and reported to values measured in the sham group arbitrarily set to 1. Data are means ± SEM. *p < 0.05
Effects of SG on In Vivo Paracellular Permeability, LPS Translocation, and Pro-inflammatory Markers Expression
We next measured the consequence of SG on in vivo paracellular permeability. While sham surgery has no effect on the paracellular transport of F-SA in plasma, SG increases significantly plasma F-SA concentrations 3 weeks after surgery (Fig. 5a). Thus, SG overall increases paracellular permeability, despite the reduced jejunal permeability observed ex vivo. Consistently, 4 weeks after surgery, plasma LPS levels were significantly increased in the SG compared to the sham control mice (+28%, p = 0.03) (Fig. 5b), suggesting that the increased colonic paracellular permeability favors LPS translocation. There were no modifications in the intestinal gene expression of proinflammatory cytokines (IL6, TNFα, and IFNγ) in the SG compared to the control group (data not shown). In contrast, in inguinal white adipose tissue, there was a significant increase in IL1β mRNA levels in the SG compared to the sham group (+30%, p = 0.02), with a trend for TNFα mRNA levels (+33%, p = 0.11), whereas there was no change in IL1β expression (p = 0.35) (Fig. 6a–c). As LPS also exerts some hepatic pro-inflammatory actions, we measured the expression of several hepatic acute-phase proteins. A significant increase in hepatic SAA2, SAA3, and haptoglobin mRNA levels was observed after SG (Fig. 6e, f, h). A trend for an increase in hepatic SAA4 mRNA levels was also noticed (Fig. 6g).
Discussion
Increased LPS translocation due to altered intestinal permeability has been suggested as a mechanism for the chronic inflammatory state and insulin resistance that are associated with morbid obesity. Using a mouse model, we investigated the effect of SG on intestinal permeability, LPS translocation and inflammation.
First, we observed a significant body weight reduction and a glucose homeostasis improvement, thereby validating our SG surgical model [21, 22]. Then, we more specifically examined intestinal function. Neither TTT nor fecal water content was significantly modified following SG. While it is known that gastric emptying is increased after SG, only one small study has shown a decrease in intestinal transit time 4 months after SG in humans [23].
For the first time, we showed that ex vivo jejunal intestinal permeability decreased 4 weeks after SG. The increased jejunal mRNA expression of tight junction proteins such as Jam A and occludin might have contributed to the reduced proximal intestinal permeability. One striking and novel finding is the fact that SG differentially affects intestinal permeability all along the gastrointestinal tract, with an increase ex vivo in the permeability of the colon after SG. It seems that the alteration of the colonic permeability plays a predominant role since there is an overall increase in vivo of the paracellular permeability 3 to 4 weeks after SG. In contrast to what was observed in the jejunum, no change was observed in the intestinal mRNA expression of tight junction proteins suggesting that alteration occurs at the protein level. Further studies are warranted to decipher the underlying mechanisms sustaining these permeability modifications. As the gut permeability in human could be different from laboratory rodents [24], it would be interesting to test the effect of SG in guinea pigs that share more similarities with humans [24]. One limitation of our study was the fact that the weight loss was less severe in the pair fed group than in the SG group. Indeed, we cannot rule out the possibility that an age- and body weight-matched group of mice would present similar gut permeability change than the SG group. To date, the effect of caloric restriction has been poorly explored. In 1992, one study showed that caloric restriction does not affect and prevent the aging induced increased intestinal permeability [25].
Few clinical studies have examined the impact of bariatric surgery on intestinal permeability. Based on the lactulose/mannitol ratio, it was reported that intestinal permeability was not modified 6 months after RYGB surgery in 16 obese patients [16]. In accordance with our mice data, one study performed 8 months after RYGB showed increased expression of tight junction proteins and decreased paracellular permeability in the proximal intestine in humans [17].
In accordance with an increased in vivo permeability, plasma LPS levels were significantly increased in SG-operated compared to control mice. In contrast, it has been reported in humans that plasma LPS levels decrease rapidly after bariatric surgery, with a concomitant decrease of CRP levels [26, 27]. The reason for this discrepancy remains unclear and requires further investigation. Importantly, we found that this increase in plasma LPS following SG was associated with a pro-inflammatory state in inguinal adipose tissue and in the liver. Importantly, despite this low-grade inflammation, glucose homeostasis was improved after SG, suggesting that the other beneficial metabolic effects of SG overcome the increased colonic permeability. If it is well admitted that bariatric surgeries induce a rapid glucose homeostasis improvement, the precise molecular mechanisms responsible for this beneficial effects are not fully characterized. Indeed, it was reported by several studies (reviewed in [28]) that reduced caloric intake, modification of gastro-intestinal peptides, alteration of bile acid fluxes, and microbiota changes can contribute to improvement in glucose homeostasis. Thus, it could be emphasized that the overall effect of SG on glucose homeostasis is the result of several positive and negative parameters and that the increased LPS influx by itself is not sufficient to alter glucose homeostasis.
Conclusion
SG induces significant modifications of intestinal paracellular and transcellular permeability. These changes potentially favor LPS translocation in plasma, thus promoting a low-grade pro-inflammatory state in adipose tissue and liver. However, they do not negate the beneficial effects of SG on glucose homeostasis. As a future work, it would be interesting to assess the long-term effect of SG on intestinal permeability and also the consequence of other bariatric procedures, i.e., RYGB.
References
Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2008;351:2683–93.
Wu Q, Zhang X, Zhong M, et al. Effects of bariatric surgery on Serum bile acid composition and conjugation in a diabetic rat model. Obes Surg. 2016;26:2384–92.
Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81.
Medzhitov R, Horng T. Transcriptional control of the inflammatory response. Nat Rev Immunol. 2009;9:692–703.
De La Serre CB, Ellis CL, Lee J, et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–8.
Suzuki T, Hara H. Dietary fat and bile juice, but not obesity, are responsible for the increase in small intestinal permeability induced through the suppression of tight junction protein expression in LETO and OLETF rats. Nutr Metab (Lond). 2010;7:19.
Saad MJ, Santos A, Prada PO. Linking gut Microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda). 2016;31:283–93.
Trøseid M, Nestvold TK, Rudi K, et al. Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: evidence from bariatric surgery. Diabetes Care. 2013;36:3627–32.
Caricilli AM, Picardi PK, de Abreu LL, et al. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol. 2011;9:e1001212.
Burcelin R, Garidou L, Pomié C. Immuno-microbiota cross and talk: the new paradigm of metabolic diseases. Semin Immunol. 2012;24:67–74.
Genser L, Poitou C, Brot-Laroche E, et al. Alteration of intestinal permeability: the missing link between gut microbiota modifications and inflammation in obesity? Med Sci (Paris). 2016;32:461–9.
Gummesson A, Carlsson LM, Storlien LH, et al. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity (Silver Spring). 2011;19:2280–2.
Brignardello J, Morales P, Diaz E, et al. Pilot study: alterations of intestinal microbiota in obese humans are not associated with colonic inflammation or disturbances of barrier function. Aliment Pharmacol Ther. 2010;32:1307–14.
Teixeira TF, Souza NC, Chiarello PG, et al. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr. 2012;31:735–40.
Verdam FJ, Fuentes S, de Jonge C, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring). 2013;21:E607–15.
Savassi-Rocha AL, Diniz MT, Vilela EG, et al. Changes in intestinal permeability after roux-en-Y gastric bypass. Obes Surg. 2014;24:184–90.
Casselbrant A, Elias E, Fändriks L, et al. Expression of tight-junction proteins in human proximal small intestinal mucosa before and after roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2015;11:45–53.
Yin DP, Gao Q, Ma LL, et al. Assessment of different bariatric surgeries in the treatment of obesity and insulin resistance in mice. Ann Surg. 2011;254:73–82.
Tasselli M, Chaumette T, Paillusson S, et al. Effects of oral administration of rotenone on gastrointestinal functions in mice. Neurogastroenterol Motil. 2013;25:e183–93.
Pais de Barros JP, Gautier T, Sali W, et al. Quantitative lipopolysaccharide analysis using HPLC/MS/MS and its combination with the limulus amebocyte lysate assay. J Lipid Res. 2015;56:1363–9.
Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–8.
Arapis K, Cavin JB, Gillard L, et al. Remodeling of the residual gastric mucosa after roux-en-y gastric bypass or vertical sleeve gastrectomy in diet-induced obese rats. PLoS One. 2015;10:e0121414.
Melissas J, Leventi A, Klinaki I, et al. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Ann Surg. 2013;258:976–82.
Delahunty T, Hollander D. A comparison of intestinal permeability between humans and three common laboratory animals. Comp Biochem Physiol A Comp Physiol. 1987;86:565–7.
Ma TY, Hollander D, Dadufalza V, et al. Effect of aging and caloric restriction on intestinal permeability. Exp Gerontol. 1992;27:321–33.
Monte SV, Caruana JA, Ghanim H, et al. Reduction in endotoxemia, oxidative and inflammatory stress, and insulin resistance after roux-en-Y gastric bypass surgery in patients with morbid obesity and type 2 diabetes mellitus. Surgery. 2012;151:587–93.
Clemente-Postigo M, Roca-Rodriguez Mdel M, Camargo A, et al. Lipopolysaccharide and lipopolysaccharide-binding protein levels and their relationship to early metabolic improvement after bariatric surgery. Surg Obes Relat Dis. 2015;11:933–9.
Cho YM. A gut feeling to cure diabetes: potential mechanisms of diabetes remission after bariatric surgery. Diabetes Metab J. 2014;38:406–15.
Acknowledgements
We thank Marie Liabeuf and Stephanie Lemarchand-Minde (Animal Facility, l’Institut du Thorax, Nantes, France) for their help in creating the animal care protocol. We thank the American Journal experts for the final editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Source of Funding
Région Pays de La Loire (France), CASDEN, Fondation Genavie.
Rights and permissions
About this article
Cite this article
Blanchard, C., Moreau, F., Chevalier, J. et al. Sleeve Gastrectomy Alters Intestinal Permeability in Diet-Induced Obese Mice. OBES SURG 27, 2590–2598 (2017). https://doi.org/10.1007/s11695-017-2670-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-2670-1