Abstract
Background
Recent studies have suggested that obesity is associated with an increased intestinal permeability as well as an altered microbiota profile. These conditions can promote the translocation of lipopolysaccharide into the circulation and, subsequently, contribute to the observed systemic inflammation. Our aim was to assess gut permeability in patients with obesity compared to non-obese subjects as well as after excessive weight loss following laparoscopic sleeve gastrectomy (LSG).
Methods
We analyzed the dietary intake, metabolic and inflammatory markers, gut permeability (four-probe sugar test), and microbiota composition in 17 morbidly obese patients before and after LSG as well as in 17 age- and gender-matched non-obese subjects. Additionally, we compared gut permeability and inflammatory markers in patients of different stages of obesity.
Results
Patients with obesity showed elevated levels of C-reactive protein and lipopolysaccharide-binding protein as compared to non-obese subjects, but no differences were noted for gut permeability between these two groups. LSG led to improvements in metabolic and inflammatory parameters in the obese patients. Moreover, gastroduodenal as well as small intestinal permeability decreased, whereas colonic permeability increased after surgery. Regarding gut microbiota, differences were noted for main phyla and alpha-diversity between non-obese and obese subjects. After surgery, the composition of the microbiota showed a tendency toward the pattern of the non-obese group.
Conclusions
Gut permeability is not dependent on body mass index, whereas weight loss after LSG initiates distinct changes in gastroduodenal, intestinal, and colonic permeability. These changes do not seem to be associated with changes in the microbiota composition.
Clinical Trial Registry Number and Website
The trials were registered at https://www.drks.de/drks_web/ with the number DRKS00009008 and DRKS00006210.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is associated with disturbances in glucose and lipid metabolism as well as a pro-inflammatory status and often entails comorbidities such as type 2 diabetes and cardiovascular diseases [1]. Bariatric surgery has become a common treatment option for patients with morbid obesity as it effectively reduces body weight and rapidly alleviates obesity-associated disorders [2].
Previous work has identified intestinal permeability as a potential trigger for obesity-associated inflammation and alterations in glucose metabolism. Studies in rodents have shown that high-fat feeding provokes an impairment of gut barrier function and alterations in gut microbiota, thereby increasing the translocation of bacterial products such as lipopolysaccharide (LPS) across the epithelial barrier [3,4,5]. Elevated concentrations of LPS in the circulation can trigger inflammatory processes and contribute to obesity-associated weight gain and insulin resistance [6].
Human studies measuring intestinal permeability in obesity, however, are rare, and their results inconsistent [7,8,9,10]. Hence, the role of intestinal permeability in the pathogenesis of obesity-associated metabolic diseases remains unclear. One study that investigated the effect of Roux-en-Y gastric bypass (RYGB) on intestinal permeability observed a decrease in transcellular permeability 1 month after surgery and a subsequent increase after 6 months. These changes may be due to surgery-induced alterations followed by intestinal adaptations [11]. The effects of other less invasive bariatric surgery procedures have so far only been studied in mice [12].
Considering our limited knowledge of the situation in humans, the aim of the present study was to investigate whether gut barrier function is altered in different stages of obesity compared to lean subjects, and if substantial weight loss after laparoscopic sleeve gastrectomy (LSG), the most common bariatric surgical procedure, modulates permeability in these patients. In addition to gut permeability, gut microbiota composition and inflammatory markers were assessed.
Materials and Methods
Study Population
Seventeen obese individuals (body mass index (BMI) ≥ 40 kg/m2) undergoing LSG at one of the three cooperating hospitals (including Adipositaszentrum München-Bogenhausen, Krankenhaus Landshut-Achdorf, and Kreiskrankenhaus Schrobenhausen) as well as 17 age- and gender-matched non-obese subjects (BMI < 30 kg/m2) were included in this pilot experimentation. Exclusion criteria included the presence of any severe disease (e.g., cancer); acute infection; insulin-dependent diabetes mellitus; drug or alcohol abuse; intolerance to lactulose, sucrose, sucralose, or fructose; antibiotic treatment within the last 2 months; and regular intake of laxative agents, immunosuppressive drugs, and diarrhea medications. For the comparison of gut permeability and inflammatory markers within different BMI categories, data from a former study were included [13]. Both studies were performed in the same study unit using identical methods. This study was reviewed and approved by the ethics committee of the university and was registered in the German Clinical Trial Register.
Anthropometric Measurements
The participants were instructed to avoid indulging in sports on the previous day. Measurements were made with light closing, and 1 kg was subtracted to account for residual clothing. Height was determined using a stadiometer and body weight with a calibrated standard scale (Seca, Hamburg, Germany) and BMI (kg/m2) was calculated. Waist circumference was gauged with a soft tape mid-way between the lowest rib margin and the iliac crest. Hip circumference was measured with a soft tape at the widest part of the gluteal region.
For the quantification of weight loss after surgery, following parameters were used:
-
Percent excess weight loss (% EWL) = [(operative body weight (kg) − follow-up body weight (kg))/(operative body weight (kg) − ideal body weight (kg))] × 100
-
Ideal weight (kg) = 25 kg/m2 × (height (m))2
-
Percent total weight loss (%TWL) = (operative body weight (kg) − follow-up body weight (kg))/operative body weight (kg)) × 100
Blood Samples and Biochemical Analyses
In the obese patients, blood was drawn during surgery and at 6 months later in a fasting state. Blood sample of the non-obese subjects was also drawn in a fasting state. The blood samples were collected in a 9-ml EDTA tube and a 7.5-ml serum monovette (Sarstedt, Nümbrecht, Germany). Insulin, zonulin, lipopolysaccharide-binding protein (LBP), and C-reactive protein (CRP) were measured in plasma with commercially available ELISAs (insulin: DRG Diagnostics, Marburg, Germany; zonulin: Immundiagnostik AG, Bensheim, Germany; LBP and CRP: R&D, Wiesbaden, Germany). Non-esterified fatty acids (NEFAs) were measured in plasma using a commercial test kit (Wako Chemicals GmbH, Neuss, Germany). Blood glucose of the patients with obesity was recorded from the lab reports of the respective clinic, and the non-obese group’s levels were determined in serum by Synlab (Munich, Germany). Insulin resistance was estimated using the homeostasis model assessment (HOMA-IR = insulin (μU/mL) × glucose (mmol/L)/22.5) [14].
Dietary Protocols
Patients documented their diet in a 7-day protocol before and at 6 months after surgery. The total energy and macronutrient content of the diet was calculated using the OptiDiet Plus software (Version 5.1.2.046, GOE mbH, Linden, Germany).
Gut Permeability
Gut permeability was measured using an established four-probe sugar test, as described elsewhere [15]. Briefly, after overnight fasting, patients collected their morning urine as a baseline sample and subsequently ingested a solution containing 10 g lactulose, 5 g mannitol, and 20 g sucrose along with six pills containing in total 2 g sucralose. Subsequently, the participants collected their total urine for 26 h in two sampling periods (5 h and another 21 h). Sugars were analyzed in the urine using high-performance liquid chromatography with pulsed electrochemical detection (chromatography module 250, Dionex, Idstein, Germany) [15].
Collection of Fecal Samples
The participants were instructed to collect their stool in a sterile plastic container (1000 ml; VWR International, Munich, Germany). One spoonful of the sample was taken in a stool collection tube containing DNA stabilizer (Stratec Molecular GmbH, Berlin, Germany). Samples were brought to the laboratory at room temperature and frozen and stored at − 80 °C until further analysis.
High-Throughput 16S rRNA Gene Sequencing
Fecal samples were processed as described previously [16]. Raw sequence reads were processed using IMNGS (https://www.imngs.org/) [17] with a pipeline based on UPARSE [18]. Operational taxonomic units (OTUs) were clustered at a threshold of 97% sequence similarity. To exclude artificial OTUs, only those with a relative abundance > 0.25% of total sequences in at least one sample were analyzed further.
For downstream analysis of OTUs, the Rhea pipeline was utilized. Detailed description of the microbiota analysis can be found in the appendix (High-throughput 16S rRNA gene sequencing).
Statistical Analysis
All data are presented as median (25th percentile; 75th percentile), with P < 0.05 regarded as statistically significant. The Wilcoxon matched-pairs test was used to compare obese patients before and after surgery (LSG V1, LSG V2) and the Mann–Whitney test to compare obese patients with non-obese controls. For the comparison of gut barrier function in dependence of BMI, Kruskal–Wallis and Dunn’s multiple comparison tests were employed with *P < 0.05, **P < 0.01, and ***P < 0.001. Sample size calculation for gut permeability was based on recently published data [13] using t test assessing differences before and after the intervention for lactulose and zonulin. Regarding lactulose a study with an effect size of 1.1 will require a total sample number of 11. An effect size of 0.9 for zonulin revealed a subject number of 8. These numbers would give a power of 80% and an association at 5%. Power calculations were performed with the R-package pwr.
Results
Sleeve Gastrectomy Induced Significant Weight Loss by Causing a Decrease in Energy Intake
Seventeen patients (14 females/3 males) with class III obesity (BMI ≥ 40 kg/m2) undergoing LSG were included in the study together with 17 age- and gender-matched non-obese subjects (BMI ˂ 30 kg/m2). The mean age was 41.8 ± 9.1 years in the obese group and 41.7 ± 9.7 years in the non-obese group. Anthropometric parameters of the patients are presented in Table 1. The patients receiving LSG reduced their body weight by 41.0 kg (33.7 kg; 46.5 kg) until the 6-month follow-up visit. This reduction corresponded to a total weight loss (%TWL) of 25.9% (22.3%; 30.3%) and an excessive weight loss (%EWL) of 50.9% (40.4%; 64.8%). BMI declined by 13.79 kg/m2 (12.10 kg/m2; 15.50 kg/m2) within 6 months after surgery. Both waist and hip circumference decreased significantly. While patients with obesity exhibited a significantly greater waist-to-hip ratio when compared to the non-obese individuals, this difference was not significant any more at 6 months after the surgery (Table 1).
According to the dietary protocols, weight loss after LSG was induced by substantially decreased energy intake. Patients with obesity reduced their total daily energy intake from 1826 kcal (1450 kcal; 2117 kcal) before surgery to 882 kcal (788 kcal; 1087 kcal) at 6 months after surgery (Fig. 1a). The composition of the diet, however, did not change as the percent of calorie intake from the different macronutrients remained unaltered (Fig. 1b–d). The daily energy intake of the non-obese group was 1979 kcal (1726 kcal; 2370 kcal).
Caloric intake of patients with obesity before (LSG V1) and after (LSG V2) laparoscopic sleeve gastrectomy in comparison to non-obese subjects. a Total caloric energy intake per day. b Percentage energy intake of calories from the major macronutrients (carbohydrates, fat, and protein). P values were determined using Wilcoxon matched-pairs test and Mann–Whitney test (n = 17 per group)
Weight Loss Was Accompanied by Improvements in Metabolic and Inflammatory Parameters
Baseline levels of fasting insulin and HOMA-IR were higher in patients with obesity compared to non-obese subjects and dropped significantly after surgery, together with fasting blood glucose levels (Table 1). Before surgery, six patients with obesity were diagnosed with type 2 diabetes mellitus and all of them showed improvement or resolution of the disease 6 months after the procedure.
Baseline blood levels of NEFA were elevated in patients with obesity compared to those of non-obese subjects and decreased significantly after surgery (Table 1). However, the concentrations were still higher than in the non-obese group. A similar trend was noted for CRP. The plasma levels of this inflammatory protein also decreased significantly after surgery, but were still elevated in comparison with those of the non-obese subjects (Fig. 2a). However, levels of LBP, a marker for LPS translocation from the gut into the circulation, were increased in obesity at baseline, but did not decrease significantly after surgery (Fig. 2b and Supplemental Table 2).
Gut Barrier Function Before and After Sleeve Gastrectomy
The permeability of different sections of the gastrointestinal tract was measured with a standardized four-probe sugar test (Fig. 3). Lactulose was used as a marker of the paracellular route of small intestinal permeability. Urinary recovery of lactulose decreased significantly after surgery. The same result was observed for mannitol, a marker of the transcellular route of small intestinal permeability. However, the ratio of lactulose to mannitol excretion, which is often used as a small intestinal permeability index, was unchanged. Urinary recovery of sucrose decreased post-surgery, indicating an improvement in the gastroduodenal permeability. In turn, urinary recovery of sucralose was enhanced, indicating an elevated permeability of the colon. For all four sugars, no significant difference was recorded between the obese patients before surgery and the non-obese group (Fig. 3 and Supplemental Table 2).
Markers of gut permeability of patients with obesity before (LSG V1) and after (LSG V2) laparoscopic sleeve gastrectomy in comparison to non-obese subjects. Lactulose (a) and mannitol (b) represent markers of intestinal permeability. The lactulose/mannitol ratio (c) represents a permeability index of small intestinal permeability. Sucrose (d) reflects gastroduodenal permeability and sucralose (e) colonic permeability. Zonulin concentration in plasma (f) was used as a marker for the tight junctions. P values were determined using Wilcoxon matched-pairs test and Mann–Whitney test (n = 16–17 per group)
In addition to the four-sugar test, the concentration of circulating zonulin, which regulates tight junctions in the intestine, was measured (Fig. 3f). The concentration was not altered after surgery. Moreover, there was no significant difference between obese and non-obese subjects.
Gut Barrier Function in Dependence of BMI
As there was no difference in the gut barrier function between the non-obese and obese group, we further investigated the effect of BMI on gut permeability. We therefore combined our present study group with obese patients from a former study [13], which was performed in the same study unit by identical SOPs and methods. The patients were assigned to four different BMI groups: non-obese group (BMI < 28 kg/m2), obesity class I (BMI 30–34.9 kg/m2), obesity class II (BMI 35–39.9 kg/m2), and obesity class III (BMI ≥ 40 kg/m2). The study characteristics of the subgroups are presented in Supplemental Table 1. Gut permeability was similar in all BMI groups, as there was no difference in the urinary recovery of the four sugars (Fig. 4a–e and Supplemental Table 3). The concentration of zonulin was elevated in class I obesity in comparison with the non-obese group, but decreased again in the higher obesity classes (Fig. 4f).
Markers of gut permeability in dependence of BMI. Lactulose (a) and mannitol (b) represent markers of intestinal permeability. The lactulose/mannitol ratio (c) represents a permeability index of small intestinal permeability. Sucrose (d) reflects gastroduodenal permeability and sucralose (e) colonic permeability. Zonulin concentration in plasma (f) was used as a marker of tight junctions. Non-obese group: BMI < 28 kg/m2, n = 15; obesity class I: BMI 30–34.9 kg/m2, n = 11; obesity class II: BMI 35–39.9 kg/m2, n = 7; and obesity class III: BMI ≥ 40 kg/m2, n = 13. P values were determined using Kruskal–Wallis test and Dunn’s multiple comparison test
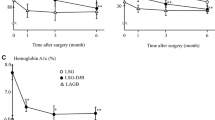
When compared with the non-obese patients, circulating LBP was elevated to a similar extent in all classes of obesity (Fig. 5b and Supplemental Table 3). The inflammatory status, measured by CRP, was also increased in obesity (Fig. 5a and Supplemental Table 3).
LBP (a) and CRP (b) concentrations in dependence of BMI. Non-obese group: BMI < 28 kg/m2, n = 15; obesity class I: BMI 30–34.9 kg/m2, n = 11; obesity class II: BMI 35–39.9 kg/m2, n = 7; and obesity class III: BMI ≥ 40 kg/m2, n = 13. P values were determined using Kruskal–Wallis test and Dunn’s multiple comparison test
Sleeve Gastrectomy Induced Specific Changes in the Gut Microbiota Profile
For the analysis of gut microbiota, we only included patients who did not regularly take proton pump inhibitors (PPIs) at both time points, together with their matched controls (n = 8 per group). PPIs are known to affect the gut microbiota [19, 20], and we could confirm this effect when comparing patients with and without PPI medication (Supplemental Fig. 1).
The analysis delivered 497,737 quality- and chimera-checked reads (20,739 ± 6344 per sample), which clustered in 394 OTUs. The microbiota of subjects with and without obesity differed in their alpha-diversity. Both richness and the Shannon effective number of species were lower in patients with obesity compared to non-obese subjects and increased after surgery (Fig. 6c). Patients displayed high inter-individual variance in beta-diversity, and there was a significant difference between the groups, however not in the pairwise comparison of the groups. Yet, there seemed to be a shift in the microbiota composition of the obese patients post-surgery toward that of the non-obese group (Fig. 6a).
Fecal microbiota analysis of study participants of patients with obesity before (LSG V1) and after (LSG V2) laparoscopic sleeve gastrectomy in comparison to non-obese subjects. a Multidimensional scaling (MDS) plot of phylogenetic distances (beta-diversity). b Phylogram showing hierarchical clustering of samples. c Diversity within samples (alpha-diversity). Numbers in brackets below the x-axis indicate prevalence (number of analyzed samples/number of total samples). d Bacterial taxonomic groups with differences between groups (n = 8 per group)
The phylogram revealed four groups based on the similarity of the samples, with the non-obese samples clustering together mainly in one group (Fig. 6b). The patients P1–05, P1–20, P1–21, and P1–22 showed a change in their microbiota composition after LSG in the direction of the non-obese group. The other four patients with obesity did not show major changes, whereas the microbiota of patient P1–18 was quite similar to the non-obese group even before surgery.
To further investigate differences between the groups, we looked for significant differences in the relative abundance of taxonomic groups. Overall, differences were detected in two phyla, three classes, four orders, five families, and eight genera. Firmicutes and Bacteroidetes were the most abundant phyla in the samples, followed by Proteobacteria and Actinobacteria. Patients with obesity had a higher abundance of Bacteroidetes and a lower abundance of Firmicutes. The abundance of both phyla tended toward the non-obese group after surgery (Fig. 6d).
Within the phylum Bacteroidetes, the class Bacteroidia and the order Bacteroidales showed significantly lower abundance in the non-obese group and a decreasing trend after surgery. Differences were also observed in the family Prevotellaceae, which was present in six out of eight patients with obesity, but only in a single non-obese subject. Rikenellaceae increased in abundance after surgery.
Regarding the phylum Firmicutes, an overall higher abundance of Clostridia was noted in non-obese compared to obese individuals. This trend was also seen for the order Clostridiales, the family Ruminococcaceae, and several genera within the family Ruminococcaceae (Ruminococcaceae NK4A214, Ruminococcaceae UCG-005, Ruminococcaceae UCG-014, Subdoligranulum). Furthermore, two genera within the family Lachnospiraceae (Lachnospira, Anaerostipes) showed significant differences between the groups. The genera Ruminococcaceae NK5A214 and Anaerostipes were, besides Rikenellaceae, the only taxonomic groups that showed significant differences after surgery. Ruminococcaceae NK4A214 was only present in samples after surgery, while Aanaerostipes disappeared after surgery. The Selenomonadales and Acidaminococcaceae as well as the whole class of Negativicutes showed a higher abundance in the obese group.
No differences were recorded for the phylum Proteobacteria. The relative abundance of Betaproteobacteriales and Burkholderiaceae from the phylum Proteobacteria, however, was identified to be higher in the obese compared to the non-obese subjects, and sleeve gastrectomy did not lead to marked changes.
Among the 394 OTUs analyzed, eight depicted significant differences between patients with and without obesity. Only one OTU revealed varying abundance pre- and post-surgery; OTU44 (Anaerostipes hadrus, 99.07% sequence similarity) was present in five patients before surgery, but did not occur in any patient after the procedure.
Discussion
In the present study, we investigated whether gut permeability of patients with obesity is increased in comparison with non-obese subjects and whether substantial weight loss induced by bariatric surgery has an effect on gut permeability.
The morbidly obese patients recruited for our study reduced energy intake and experienced 50.9% excessive weight loss 6 months after LSG surgery, which is in line with previous reports [21, 22]. This weight loss was also associated with improved metabolic and inflammatory parameters.
As novel findings we observed that weight loss by bariatric surgery resulted in an increased colonic permeability and a decreased gastroduodenal as well as intestinal permeability. The concentration of zonulin did not change. Zonulin interacts with gut tight junctions and thereby increases paracellular intestinal permeability [23]. This result indicates that other factors, such as nutrients, may be involved in the regulation of intestinal paracellular permeability, as measured by lactulose excretion. Several studies have provided evidence for the potential influence of nutrients on gut barrier integrity and LPS translocation [24]. Moreover, it is important to note, that after completion of our data, a study was published [25] that questioned the precision of the widely used zonulin ELISA kit which we also used in our study. Their data suggest that the aforementioned kit does not detect zonulin (pre-haptoglobin 2), but rather a variety of other proteins that are structurally and possibly functionally related to zonulin. The impreciseness of the zonulin ELISA kit could explain the discrepancy between decreased paracellular intestinal permeability and unaltered zonulin levels in our study.
The study by Savassi-Rocha and colleagues is the only other human study so far investigating gut permeability in vivo after bariatric surgery [11]. In this study, a decrease in mannitol excretion rate was identified at 1 month after surgery, which returned to preoperative levels at 6 months post-surgery, whereas gastroduodenal and colonic permeability have not been examined. The observations made in this study are most likely due to the shortening of the intestine by the RYGB surgery and subsequent intestinal adaptations. Sleeve gastrectomy is a less invasive procedure that only reduces stomach size, but does not directly affect the intestine. It is therefore likely, that alterations in gut permeability after LSG are primarily dependent on weight loss and not on the surgical procedure itself. However, accelerated gastric emptying and elevated small bowel transit time have been observed after sleeve gastrectomy [26], which may have contributed to the decrease in small intestinal permeability in our patients. To the best of our knowledge, this is the first human study investigating gut permeability in sleeve gastrectomy. A recent study that investigated the effect of this procedure on gut permeability in diet-induced obese mice largely supports our findings [12]. The paracellular and transcellular jejunal permeability of these mice decreased after surgery, whereas colonic permeability was augmented.
LBP was measured as a marker of LPS translocation from the gut into the circulation. LBP concentrations were elevated in obesity, but did not decrease with weight loss after surgery. As colonic permeability in contrast to intestinal permeability increased after surgery, there was no overall decrease in paracellular permeability that could restrain the translocation of LPS. However, other studies have recorded reduced LPS and LBP levels after surgery [27]; but it can take up to 12 months until this decrease becomes significant [28]. Although LBP levels were unaltered, systemic inflammation observed in the obese patients, as measured by CRP levels, decreased after surgery. Therefore, other factors, such as a reduction of adipocyte size with a decrease of inflammatory cytokine production, may have contributed to the overall decline in the inflammatory load [29].
Surprisingly, no significant differences in gut permeability between patients with morbid obesity before surgery and non-obese subjects were found. Other human studies have reported conflicting results. While some studies described an elevated small intestinal permeability in obesity [8, 9], others did not [7, 10]. We therefore further investigated gut permeability at different stages of obesity. However, we could not identify any effect of BMI on gut permeability. One explanation for the conflicting results between different studies may be that gut permeability is not directly dependent on body weight, but rather on diet, especially high-fat diets, and associated changes in gut microbiota [3, 4], which might have differed between the study groups. In our study, the dietary composition was similar in non-obese and obese subjects before surgery, which supports this hypothesis.
Interestingly, the concentration of zonulin was increased in obesity class I in comparison with the non-obese group and decreased again in the higher classes of obesity, while small intestinal permeability was unaltered. The decrease of zonulin at higher BMI may be a result of unknown compensatory mechanisms that counteract elevated zonulin levels at high BMI. However, as mentioned before, the data may not be meaningful due to the inaccuracy of the zonulin ELISA [25].
Despite unaltered gut permeability, the levels of LBP were elevated in obesity. The inflammatory status, as measured by CRP, was also raised. Besides LPS, the increased adipose tissue mass and its pro-inflammatory secretion pattern may contribute to this surge in inflammation [30].
Overall, we could not find evidence for an impaired gut barrier function with increasing BMI. However, excessive weight loss after bariatric surgery induced changes in gut barrier function. A recent study that investigated gut barrier function in obese women before and after caloric restriction with a subsequent moderate weight loss of 7 kg revealed a decline in gastroduodenal, small intestinal, and colonic permeability [13]. Moreover, a study in obese patients with steatosis could also show effects of weight reduction on intestinal permeability [31]. Together, these results suggest that body weight per se does not influence gut barrier function, whereas interventions such as caloric restriction and consecutive weight loss can initiate distinct changes in permeability.
Changes in gut permeability have been associated with alterations in gut microbiota composition [3, 4]. However, this finding has not been confirmed so far in humans [7]. In our study, taking into account co-medication with PPIs, subjects with and without obesity showed separate clusters of microbiota composition. Alpha-diversity was significantly lower in the obese patients than in the non-obese subjects. Similar results have also been reported in previous studies [7, 32]. Regarding taxonomic composition, the relative abundance of Bacteroidetes was enhanced in obesity, whereas Firmicutes were more abundant in the non-obese group. This finding is in contrast to the proposed hypothesis of an increased Firmicutes/Bacteroidetes ratio in obesity [33]. However, with the introduction of the enterotypes concept, microbiologists came to the conclusion that the individual composition of the gut microbiota is not dependent on factors such as ethnicity, age, or BMI, but rather fits into one of three clusters of well-balanced, defined, and stable microbial communities [34, 35]. As most investigations conducted so far did not select study groups on the basis of enterotypes, the comparisons of taxonomic differences are not conclusive. Indeed, in our study group, it seems that patients with and without obesity derived from different enterotypes. While non-obese subjects resembled enterotype 3 with a high abundance of Firmicutes, especially Ruminococcus, patients with obesity are more likely to have originated from enterotype 1 with a low richness and a high abundance of Bacteroidetes. We therefore cannot draw reliable conclusions by comparing the gut microbiota of our study groups.
While investigating the effects of LSG on the gut microbiota, we observed an increase in alpha-diversity after surgery. Similar results have also been attained in a previous study on sleeve gastrectomy [36] in comparison with a more pronounced increase in bacterial richness after RYGB. Taxonomic changes tended toward the composition of the non-obese group, yet were in most cases minor and not significant. It is particularly remarkable that LSG had a varying impact on the gut microbiota of individual patients. While some experienced a considerable shift in the direction of the non-obese controls, the microbiota of others remained quite stable after surgery. This shift was not dependent on changes in weight, diet, metabolic parameters, or gut permeability. Thus, the factors triggering microbiota changes after weight loss are still unknown. Other studies on gut microbiota changes after restrictive bariatric surgery have also not yielded consistent results so far [36,37,38,39]. It is possible that the different enterotypes vary in their resilience as well as their recovery after interventions such as weight loss. In the future, larger study groups that are stratified according to the enterotype status of the patients and controls are needed.
Conclusions
In summary, the results of our study imply that gut permeability is not dependent on BMI, but weight loss by LSG results in distinct changes in gastroduodenal, intestinal as well as colonic permeability. Regarding microbiota composition, sleeve gastrectomy may induce only minor changes, with some patients showing a more stable microbiota than others. Therefore, we could not confirm a clear link between microbiota composition and gut permeability in obese patients after LSG.
Change history
29 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11695-021-05439-1
References
Han TS, Lean ME. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis. 2016;5:1–13. https://doi.org/10.1177/2048004016633371.
Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol. 2017;14:160–9. https://doi.org/10.1038/nrgastro.2016.170.
Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83. https://doi.org/10.1007/s00125-007-0791-0.
Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. https://doi.org/10.2337/db07-1403.
Lam YY, Ha CWY, Campbell CR, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233. https://doi.org/10.1371/journal.pone.0034233.
Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. https://doi.org/10.2337/db06-1491.
Verdam FJ, Fuentes S, de JC, et al. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring). 2013;21:E607–15. https://doi.org/10.1002/oby.20466.
Teixeira TFS, Souza NCS, Chiarello PG, et al. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr. 2012;31:735–40. https://doi.org/10.1016/j.clnu.2012.02.009.
Gummesson A, Carlsson LMS, Storlien LH, et al. Intestinal permeability is associated with visceral adiposity in healthy women. Obesity (Silver Spring). 2011;19:2280–2. https://doi.org/10.1038/oby.2011.251.
Brignardello J, Morales P, Diaz E, et al. Pilot study: alterations of intestinal microbiota in obese humans are not associated with colonic inflammation or disturbances of barrier function. Aliment Pharmacol Ther. 2010;32:1307–14. https://doi.org/10.1111/j.1365-2036.2010.04475.x.
Savassi-Rocha AL, Diniz MTC, Vilela EG, et al. Changes in intestinal permeability after Roux-en-Y gastric bypass. Obes Surg. 2014;24:184–90. https://doi.org/10.1007/s11695-013-1084-y.
Blanchard C, Moreau F, Chevalier J, et al. Sleeve gastrectomy alters intestinal permeability in diet-induced obese mice. Obes Surg. 2017;27:2590–8. https://doi.org/10.1007/s11695-017-2670-1.
Ott B, Skurk T, Hastreiter L, et al. Effect of caloric restriction on gut permeability, inflammation markers, and fecal microbiota in obese women. Sci Rep. 2017;7:11955. https://doi.org/10.1038/s41598-017-12109-9.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Norman K, Pirlich M, Schulzke J-D, et al. Increased intestinal permeability in malnourished patients with liver cirrhosis. Eur J Clin Nutr. 2012;66:1116–9. https://doi.org/10.1038/ejcn.2012.104.
Lagkouvardos I, Kläring K, Heinzmann SS, et al. Gut metabolites and bacterial community networks during a pilot intervention study with flaxseeds in healthy adult men. Mol Nutr Food Res. 2015;59:1614–28. https://doi.org/10.1002/mnfr.201500125.
Lagkouvardos I, Joseph D, Kapfhammer M, et al. IMNGS: a comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Sci Rep. 2016;6:33721. https://doi.org/10.1038/srep33721.
Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–8. https://doi.org/10.1038/nmeth.2604.
Jackson MA, Goodrich JK, Maxan M-E, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–56. https://doi.org/10.1136/gutjnl-2015-310861.
Imhann F, Bonder MJ, Vila AV, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–8. https://doi.org/10.1136/gutjnl-2015-310376.
Hoogerboord M, Wiebe S, Klassen D, et al. Laparoscopic sleeve gastrectomy: perioperative outcomes, weight loss and impact on type 2 diabetes mellitus over 2 years. Can J Surg. 2014;57:101–5.
Roa PE, Kaidar-Person O, Pinto D, et al. Laparoscopic sleeve gastrectomy as treatment for morbid besity: technique and short-term outcome. Obes Surg. 2006;16:1323–6.
Fasano A, Fiorentini C, Donelli G, et al. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Invest. 1995;96:710–20. https://doi.org/10.1172/JCI118114.
Teixeira TFS, Collado MC, Ferreira CLLF, et al. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res. 2012;32:637–47. https://doi.org/10.1016/j.nutres.2012.07.003.
Scheffler L, Crane A, Heyne H, et al. Widely used commercial ELISA does not detect precursor of haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front Endocrinol (Lausanne). 2018;9:22. https://doi.org/10.3389/fendo.2018.00022.
Shah S, Shah P, Todkar J, et al. Prospective controlled study of effect of laparoscopic sleeve gastrectomy on small bowel transit time and gastric emptying half-time in morbidly obese patients with type 2 diabetes mellitus. Surg Obes Relat Dis. 2010;6:152–7. https://doi.org/10.1016/j.soard.2009.11.019.
Tuomi K, Logomarsino JV. Bacterial lipopolysaccharide, lipopolysaccharide-binding protein, and other inflammatory markers in obesity and after bariatric surgery. Metab Syndr Relat Disord. 2016;14:279–88. https://doi.org/10.1089/met.2015.0170.
van Dielen FMH, Buurman WA, Hadfoune M, et al. Macrophage inhibitory factor, plasminogen activator inhibitor-1, other acute phase proteins, and inflammatory mediators normalize as a result of weight loss in morbidly obese subjects treated with gastric restrictive surgery. J Clin Endocrinol Metab. 2004;89:4062–8. https://doi.org/10.1210/jc.2003-032125.
Moschen AR, Molnar C, Geiger S, et al. Anti-inflammatory effects of excessive weight loss: potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–64. https://doi.org/10.1136/gut.2010.214577.
Skurk T, Alberti-Huber C, Herder C, et al. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33. https://doi.org/10.1210/jc.2006-1055.
Damms-Machado A, Louis S, Schnitzer A, et al. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am J Clin Nutr. 2017;105:127–35. https://doi.org/10.3945/ajcn.116.131110.
Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. https://doi.org/10.1038/nature07540.
Ley RE, Bäckhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5. https://doi.org/10.1073/pnas.0504978102.
Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. https://doi.org/10.1038/nature09944.
Costea PI, Hildebrand F, Arumugam M, et al. Enterotypes in the landscape of gut microbial community composition. Nat Microbiol. 2018;3:8–16. https://doi.org/10.1038/s41564-017-0072-8.
Murphy R, Tsai P, Jüllig M, et al. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg. 2017;27:917–25. https://doi.org/10.1007/s11695-016-2399-2.
Damms-Machado A, Mitra S, Schollenberger AE, et al. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int. 2015;2015:806248–12. https://doi.org/10.1155/2015/806248.
Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22:228–38. https://doi.org/10.1016/j.cmet.2015.07.009.
Patil DP, Dhotre DP, Chavan SG, et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J Biosci. 2012;37:647–57. https://doi.org/10.1007/s12038-012-9244-0.
Acknowledgments
We highly acknowledge Prof. Dr. Thomas Hüttl, Dr. Peter Stauch, Dr. Otto Dietl, and Hildegard Wood together with their whole team at the Adipositas Zentrum München Bogenhausen; Dr. Georg Dechantsreiter and team at the Krankenhaus Landshut Achdorf; and Dr. Andreas Limberger and team at the Kreiskrankenhaus Schrobenhausen for their support in recruitment and sample collection. Additionally, we are thankful to Martina Werich for the analysis of sugars in the urine samples. We are grateful to Manuela Hubersberger for her excellent technical assistance and to the ZIEL Core Facility Microbiome/NGS at the TU Munich for support with high-throughput 16S rRNA gene amplicon sequencing and analysis.
Funding
The study was founded by the DFG (Deutsche Forschungsgemeinschaft) as part of the Graduiertenkolleg 1482 as well as by the BMBF (Federal Ministry of Education and Research, grant no. 0315674) and in part by the Else Kröner-Fresenius-Foundation, Bad Homburg v. d. H., Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Beate Brandl Née: Ott
Electronic Supplementary Material
Supplemental Figure 1
Multidimensional scaling (MDS) plot of phylogenetic distances (β-diversity). Non-obese: non-obese group, n = 17; PPI V1: obese patients with PPI medication before surgery, n = 5; no PPI V1: obese patients without PPI medication before surgery, n = 12; PPI V2: obese patients with PPI medication 6 months after surgery, n = 7; no PPI V2: obese patients without PPI medication 6 months after surgery, n = 10. (PNG 356 kb)
High resolution image
(EPS 968 kb)
ESM 2
(DOCX 23.0 kb)
ESM 3
(DOCX 26.6 kb)
ESM 4
(DOCX 28.5 kb)
ESM 5
(DOCX 22.5 kb)
Rights and permissions
About this article
Cite this article
Kellerer, T., Brandl, B., Büttner, J. et al. Impact of Laparoscopic Sleeve Gastrectomy on Gut Permeability in Morbidly Obese Subjects. OBES SURG 29, 2132–2143 (2019). https://doi.org/10.1007/s11695-019-03815-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-03815-6