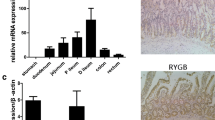
Abstract
Background
Bariatric surgery rapidly induces improvements in type 2 diabetes (T2D) in concert with reduction in systemic markers of inflammation. The impact of bariatric surgery on local intestinal immunity is not known. We hypothesize that sleeve gastrectomy (SG) and gastric bypass (RYGB) surgeries resolve obesity-induced intestinal inflammation, thereby promoting T2D resolution.
Methods
SG and RYGB, or control surgery was performed in SD rats (n = 4–6/group). Key cytokines involved in insulin resistance (TNF-α, IFN-γ), inflammasome activation (IL-1β, IL-18), inflammation resolution (IL-10, IL-33), and Th17 cell responses (IL-17, IL-23) were measured by qPCR in mucosal scrapings of jejunum at 4 weeks post-surgery. Intestinal cytokine expressions were correlated with weight change, systemic and portal glucose, and insulin levels in response to an enteral glucose load.
Results
SG downregulated IL-17 and IL-23 in both proximal and distal jejunum, and IFN-γ was reduced only in distal jejunum (p < 0.05). Jejunal IL-17 and IL-23 expression correlated positively with weight changes after SG (0.93 and 0.98, respectively; p < 0.05). Changes in IFN-γ correlated strongly with insulin levels in portal and systemic circulation (0.99 and 0.95, respectively, p < 0.05). As with SG, IFN-γ, IL-17, and IL-23 were significantly reduced by RYGB. RYGB also reduced TNF-α and IL-18 and increased IL-33 levels (p < 0.05).
Conclusions
RYGB and SG reduce expression of pro-inflammatory cytokines IL-17, IL-23, and IFN-γ in the jejunum. RYGB showed attenuation of additional pro-inflammatory cytokines and enhanced expression of IL-33. Post-surgical changes in intestinal IL-17, IL-23, and IFN-γ correlate strongly with changes in weight and glucose-triggered insulin responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes (T2D) affects nearly 422 million people worldwide (http://www.who.int/diabetes/en/), leading to significant morbidity and mortality. Bariatric surgery, including Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG), is now recognized as our most effective therapy for T2D. Both RYGB and SG lead to significant improvement and often remission of T2D, within days to weeks after surgery [1,2,3]. Changes in intestinal physiology after bariatric surgery are believed to play a key role in its anti-diabetic effects [4]. Bariatric surgery leads to improvements in gut incretin hormone secretion, changes in intestinal metabolism and nutrient receptors, altered bile acid metabolism, and favorable shifts in the intestinal microbiome [5]. Nevertheless, each of these changes, in isolation, do not explain the full anti-diabetic effects of bariatric surgery.
Recent research has highlighted the influence of the immune system in T2D pathogenesis. In obese diabetics, dysregulation of the immune system directly triggers insulin resistance [6]. Low-grade intestinal inflammation is an early sequela of high-fat diet and obesity, leading both to metabolic dysfunction of the intestinal epithelia, as well as translocation of gut microbiota products that trigger systemic insulin resistance [7]. For example, high-fat diet induces TNF-α, MIF, and IFN-γ-induced gene subsets in the small intestine [8, 9]. In obese children, biomarkers of intestinal inflammation are also associated with glucose intolerance [10]. Moreover, therapies that target aberrant inflammation in the intestine improve glucose tolerance and insulin sensitivity [11, 12].
Limited studies suggest that bariatric surgery may reverse the aberrant inflammation of T2D. For example, both RYGB and SG positively influence serum cytokine levels and lead to improved immune cell function [13,14,15,16]. Bariatric surgery reduces circulating levels of IL-18 and MCP-1 in concert with improvements in glucose levels and HOMA-IR [17]. SG also significantly reduces CRP and IL-6 in the short term [18].
Multiple recent studies have demonstrated that the intestinal immune system (the largest reservoir of immune cells in adults), by maintaining healthy interaction with the microbiome and allowing normal epithelial metabolic function, can influence systemic insulin resistance and glucose metabolism [19,20,21,22]. Nevertheless, the underpinnings of intestinal immunity in mediating T2D resolution following bariatric surgery is not elucidated yet.
We hypothesize that bariatric surgery improves intestinal immune function, thereby improving systemic insulin and glucose sensitivity. To test this hypothesis, we used rodent models of SG and RYGB to (1) quantify and characterize jejunal cytokine profiles and (2) determine whether specific intestinal cytokines correlate with surgery-induced improvements in weight, glucose, and insulin levels.
Materials and Method
Animals
Animal protocols were approved by the Harvard IACUC committee. All applicable institutional and/or national guidelines for the care and use of animals were followed. Nine-week-old male Sprague Dawley rats (Envigo, MD) were used in this study. Animals were acclimatized for 1 week in our facility and maintained at a constant temperature and humidity with 12 h:12 h dark and light cycle. A formula diet (Purina 5008) was provided ad libitum throughout the study except for the initial 5 days post SG and RYGB, when animals were fed a recovery gel diet (ClearH2O, ME).
Humans
This article does not contain any studies with human participants. Therefore, informed consent statement is not required.
Surgery Models
SD underwent SG (n = 5), RYGB (n = 5), or control surgery (n = 4–6). Animals were fasted overnight prior to surgery with ad libitum water access. All the surgeries were performed as described previously by our group ([23]; Aliakbarian et al. 2018 (unpublished)). In summary, for SG, a stapled vertical sleeve gastrectomy was performed with removal of 80% of the rat glandular stomach and 90% of the non-glandular stomach (Fig. 1a).
For SG control surgery, short gastric vessels ligation, which is performed as part of SG, was performed in the control group to mimic the changes in portal circulation after SG. Weights were recorded for all the animals up to 4 weeks post-surgery.
For RYGB, the stomach was divided into two parts, the gastric remnant and gastric pouch (new feeding stomach) using a linear stapler. A 16-cm biliopancreatic limb and 10-cm Roux limb were created (Fig. 1b).
For RYGB control surgery, animals underwent laparotomy and bowel manipulation for RYGB surgery time. Weights were recorded for all the animals up to 4 weeks post-surgery.
Portal and Systemic Blood Collection
At 4 weeks post-surgery, while under 1–3% isoflurane anesthesia, silastic catheters were placed in the right jugular vein to the level of the right atrium, in the superior mesenteric vein (SMV) to the level of the portal vein, and into the duodenum. Baseline portal and systemic fasting glucose levels were measured using a glucometer (One Touch Ultra). Two milligrams of glucose/g of body weight was infused into the duodenum. Portal and systemic blood samples were collected at 0, 10, 30, and 60 min. Glucose and insulin levels were measured using a glucometer and Luminex (EMD Millipore, MA), respectively.
Each group of RYGB and SG rats were compared with respective control group for weight, glucose, and insulin levels.
Tissue Harvest
After above infusions, mucosal scrapings of orthotopically matched jejunal segments were collected from SG (proximal and distal), RYGB (biliopancreatic limb (BP—equivalent to proximal segment) and Roux limb (Rx—equivalent to distal segment)), and control animals (Fig. 1).
RNA Extraction and Quantitation of mRNA Gene Expression
RNA was isolated from mucosal scrapings of proximal and distal jejunum, or equivalent intestinal segments (the Roux (Rx) and biliopancreatic (BP) limbs) using RNeasy mini kits (Qiagen, Germany). cDNA was synthesized using Superscript III First-Strand Synthesis System (Life technologies, CA). qPCR was performed using SYBR green as a reporter. Key cytokines involved in insulin resistance (TNF-α, IFN-γ), inflammasome activation (IL-1β, IL-18), resolution of inflammation (IL-10, IL-33), and Th17 cell responses (IL-17, IL-23) were measured by qPCR. Cyclophilin A was used for standardization. Primer sequences are listed in supplemental Table 1. Results were analyzed by the comparative delta-delta Ct method.
Correlation of Cytokine Expression with Metabolic Parameters
Cytokines that were significantly different between SG and control rats (p < 0.05) were assessed for correlations with weight loss, portal and systemic glucose levels in response to enteral glucose challenge, and portal and systemic insulin levels after enteral glucose load. Relative weight change of SG animals was calculated as the following:
Glucose and insulin levels were plotted against time. Incremental area under the curve (iAUC) was calculated. The individual ratio of iAUC for SG vs control was calculated as the iAUC for each individual SG rat divided by the mean control iAUC. This relative ratio was used to determine the correlation with metabolic parameters.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism7.0. Comparative analysis of qPCR results was analyzed with one-sample t tests. XY scatter plots were used to create a linear trend analysis, and the Pearson correlation (r) and p value for r were calculated. P ≤ 0.05 were considered significant.
Results
Weight Change After SG and RYGB
While SG control rats steadily gained weight of 149% of baseline throughout the study period, SG rats significantly reduce weight gain and reached 137.7% of baseline weight at 28 post-operative days (p < 0.05).
Similarly, RYGB control rats steadily reached weight of 140% of baseline, RYGB rats lost significant amount of weight initially post-op and reached only 111% of baseline weight at post-operative day 28, as described in our previous study (p < 0.05, [23]).
Sleeve Gastrectomy Selectively Suppresses Pro-inflammatory Cytokine Gene Expression in the Jejunum
Mucosal mRNA expression of TNF-α, IFN-γ, IL-17, IL-23, IL-18, and IL-1β in the proximal and distal jejunum of sleeve gastrectomy (SG) and control rats was measured by qPCR. These cytokines play a critical role in insulin resistance (TNF-α, IFN-γ), inflammasome activation (IL-1β, IL-18), inflammation resolution (IL-10, IL-33), and Th17 cell responses (IL-17, IL-23).
At 4 weeks post-operatively, IFN-γ, IL-17, and IL-23 were 4.33 ± 2.01, 32.3 ± 22.19, and 1.56 ± 0.22-fold downregulated in the distal jejunum of SG animals, respectively (p < 0.05, Fig. 3). Similarly, IL-17 and IL-23 gene expression was 2.15 ± 0.78 and 1.27 ± 0.15-fold downregulated in the proximal jejunum of SG animals, respectively (p < 0.05, Fig. 2). In SG, there was greater suppression of IFN-γ and IL-17 in the distal as opposed to the proximal jejunum. There were no differences in jejunal expression of TNF-α or IL-1β in SG vs. control animals. IL-18 expression in the proximal jejunum was 1.27 ± 0.15-fold higher in SG animals (p < 0.05) but unchanged in the distal jejunum (Figs. 2 and 3). These data demonstrate that SG selectively suppresses pro-inflammatory cytokines in the small intestine.
Cytokine gene expression in the proximal jejunum after SG. a, b Cytokines involved in insulin resistance. c, d Cytokines released due to inflammasome activation. e, f Cytokines involved in Th17 cell axis. g, h Cytokines involved in inflammation resolution. n = 4–5/group; error bars indicate SEM. *p < 0.05
Cytokine gene expression in the distal jejunum after SG. a, b Cytokines linked to insulin resistance. c, d Cytokines released due to inflammasome activation. e, f Cytokines involved in Th17 cell axis. g, h Cytokines involved in inflammation resolution. n = 4–5/group; error bars indicate SEM. *p < 0.05
Effect of SG on Anti-inflammatory IL-10 and IL-33 Gene Expression in the Small Intestine
Expression of the anti-inflammatory cytokines IL-10 and IL-33 was measured in the proximal and distal jejunum of SG and control animals. There was a trend toward increased IL-10 expression in the distal jejunum but not in proximal jejunum of SG animals. In contrast, a decreasing trend in IL-33 expression was present in the proximal jejunum but not in the distal jejunum of SG animals. Overall, anti-inflammatory cytokine expression was not significantly different between SG and control animals (Figs. 2 and 3).
IL-17 and IL-23 Gene Expression in the Distal Jejunum Is Positively Correlated with Weight Change in SG Animals
Percent relative weight change of SG to control-operated animals was calculated as described in methods. There was no association between IL-17, IL-23, IFN-γ, and IL-18 proximal jejunal expression and % relative body weight change (data not shown). There was however a positive linear correlation between % relative body weight change of SG animals and IL-17 (r, 0.93) and IL-23 (r, 0.98) expression in the distal jejunum (p < 0.05, Fig. 4a). This data suggests higher expression of IL-17 and IL-23 in the distal jejunum is associated with less weight loss and worse SG weight outcomes.
a Association of jejunal cytokine levels and % relative body weight gain after SG. b Association of jejunal cytokines and systemic insulin level after SG. PJ, proximal jejunum. c Association of jejunal cytokine levels and portal insulin levels after SG. PJ, proximal jejunum; DJ, distal jejunum; r, Pearson correlation. p < 0.05 value indicates correlation significance. Black diamond, DJ and black square, PJ
Pro-inflammatory Cytokine Expression in the Jejunum Is Not Associated with Systemic and Portal Glucose Tolerance
At 4 weeks, jugular and portal catheterization was performed to measure portal and systemic glucose levels at 0 (fasting baseline), 10, 30, and 60 min after duodenal infusion of 2 mg/g of body weight of glucose. Both SG and control rats showed no difference in fasting portal and systemic glucose levels. Incremental AUC for systemic and portal glucose levels following the enteral glucose challenge was calculated. Cytokines significantly altered after SG were tested for any correlation with glucose tolerance. No significant correlation was seen between IL-17, IL-23, IL-18, and IFN-γ levels and systemic or portal glucose levels (data not shown).
IFN-γ Expression in the Distal Jejunum Is Strongly Associated with Insulin Levels
Luminex was used to measure portal and systemic insulin levels in response to enteral glucose load. Plasma samples were collected as in 3.5. While SG rats had significantly increased fasting systemic insulin levels compared to controls, there was no difference in portal insulin levels (p < 0.05; Aliakbarian et al., 2018, unpublished). IFN-γ gene expression in the distal jejunum showed a strong positive correlation with systemic and portal insulin levels of SG animals, with r, 0.95 and 0.99, respectively (p < 0.05, Fig. 4b, c). This data suggests that jejunal IFN-γ expression may influence systemic and portal insulin levels after SG.
Similarity of Gut Cytokine Signatures After RYGB and SG
SG and RYGB both lead to significant weight loss and T2D resolution. Given our hypothesis that gut immune changes reflected in cytokine expression levels mediate metabolic improvement following bariatric surgery, we predicted that RYGB would induce similar intestinal cytokine changes as in SG. Therefore, we compared the SG intestinal cytokine profile with orthotopically matched jejunal segments following RYGB (Rx and BP) and control operations (Fig. 1b).
After RYGB, cytokine expression markedly changed in the distal jejunum (Rx limb) but not in the proximal jejunum (BP limb) (Fig 5and 6). As seen in SG, pro-inflammatory and Th17-related cytokines including TNF-α (6.8 ± 3.34), IFN-γ (96.48 ± 43.32), IL-17 (150.28 ± 66.11), IL-23 (8.14 ± 1.74), and IL-18(1.13 ± 0.54) were significantly downregulated in the distal jejunum of RYGB animals (p < 0.05) when compared to controls (Fig. 6). There was also a 2.78 ± 0.6-fold increase in the anti-inflammatory cytokine, IL-33, in the distal jejunum. The only significant change in pro-inflammatory cytokine expression in the proximal jejunum was a 4.07 ± 1.7-fold reduction in IFN-γ (Figs. 5). IL-10 expression was unchanged in either the proximal or distal jejunum following RYGB.
Cytokine gene expression in the proximal jejunum (equivalent to BP limb) after RYGB. a, b Cytokines involved in insulin resistance. c, d Cytokines released due to inflammasome activation. e, f Cytokines involved in Th17 cell axis. g, h Cytokines involved in inflammation resolution. n = 4–5/group; error bars indicate SEM. *p < 0.05
Cytokine gene expression in the distal jejunum (equivalent to Rx limb) after RYGB. a, b Cytokines involved in insulin resistance. c, d Cytokines released due to inflammasome activation. e, f Cytokines involved in Th17 cell axis. g, h Cytokines involved in inflammation resolution. n = 5/group; error bars indicate SEM. *p < 0.05
We also examined whether RYGB-altered intestinal cytokines were associated with post-surgical weight outcomes. No correlation between % relative body weight changes and cytokine expression was seen in RYGB (data not shown). There was no difference at steady-state plasma glucose and insulin levels between RYGB and control group of normoglycemic SD rats.
Discussion
An emerging body of evidence indicates that insulin resistance is caused by a dysregulated systemic inflammatory state, which originates from obesity-induced dysregulation of intestinal immune defense against enteral antigens. Given the known profound physiologic changes that bariatric surgery induces in the intestine, we hypothesized that SG and RYGB also resolve intestinal inflammation, therefore leading to downstream improvement in glucose regulation. We find that SG specifically attenuated the expression of the pro-inflammatory cytokines IL-17, IL-23, and IFN-γ in the jejunum. Interestingly, RYGB suppressed these same cytokines, but even more strongly. RYGB also reduced expression of additional pro-inflammatory cytokines while enhancing levels of the pro-resolution cytokine IL-33. Together, this study represents the first systematic characterization of bariatric surgery’s influence on intestinal immune pathways.
A conserved pattern of reduction in IL-17, IL-23, and IFN-γ was seen after both SG and RYGB. These cytokines are the product of the Th17/Th1 axis of the adaptive immune response and are primarily secreted by CD4+ Th1 and Th17 cells (IL-17, IFN-γ), macrophages (IFN-γ, IL-23), and dendritic cells (IL-17, IL-23). Previous studies have found elevated Th17 cytokines in obesity, and that Th17 cytokine production is a hallmark of obese diabetics [24,25,26,27]. In mouse models of diet-induced obesity, Th17 and Th1 cells and their products are elevated in the small and large intestine [21, 28, 29]. Importantly, targeted inhibition of the Th17/Th1 axis in the intestine improves insulin resistance in animal models [12]. We now show that both SG and RYGB both reduce Th17 and Th1 cytokines. Moreover, the cytokine changes correlated tightly to weight change in glucose-stimulated insulin responses after SG. Together, these data suggest that bariatric surgery may influence intestinal Th1 and Th17 cell biology, and that these changes may contribute, in part, to surgery’s anti-diabetic effect.
Remarkably, two anatomically and technically distinct operations lead to highly similar pattern of cytokine changes (Table 1). These changes are not likely to be related to surgical manipulation or healing, as these intestinal cytokine changes were also seen after sleeve gastrectomy, where the intestine is left surgically undisturbed. The similar pattern of cytokine changes in the small intestine after both operations suggests that the cytokines are linked to the similar weight loss and anti-diabetic effects of the operation. It also argues that a conserved mechanism induced by surgery triggers these cytokine changes. Both SG and RYGB have been demonstrated to alter bile acids, microbiota, and intestinal incretin hormones. Each of these factors are known to directly or indirectly signal in immune cells, through leukocyte expressed bile acid receptors (TGR5), incretin receptors (GLP-1 receptor), and innate and adaptive bacterial receptors (Toll-like receptors, aryl hydrocarbon receptor, T cell receptors TCR), and can potentially contribute to the cytokine changes we observe. Future studies are needed to define the factors and mechanisms by which SG and RYGB cause intestinal cytokine changes, and their importance to the outcomes of weight loss and diabetes.
While many of the cytokine changes were conserved, RYGB induced a stronger overall anti-inflammatory small intestinal signature, including significant reductions in TNF-α and IL-18, as well as upregulation of IL-33. Intestinal TNF-α has been previously demonstrated to be increased in high-fat diet-induced obesity and to directly contribute to insulin resistance. IL-18 is associated with inflammasome activation, which has also been shown to contribute to insulin resistance. Therefore, reductions in these intestinal cytokines by RYGB are likely to directly contribute to the improved insulin sensitivity seen post-operatively. Less is known about the actions of intestinal IL-33, an anti-inflammatory and pro-resolution cytokine, in the pathogenesis of insulin resistance. IL-33 was one of the key genes found to be broadly upregulated in multiple tissues in a gene expression screen after RYGB [30]. Further studies into the pathways engaged by RYGB that lead to its stronger intestinal anti-inflammatory effect may offer a therapeutic window for augmenting the efficacy of SG.
Interestingly, cytokine changes after RYGB were confined to the Roux limb, with limited to no significant changes in the BP limb. This pattern of cytokine changes mirrors the previously reported intestinotrophic localized to the Roux limb after RYGB. The mechanism of this localized change is unknown, but incretin, growth factors, and enhanced exposure of the alimentary Roux limb to chyme are speculated to play a role. RYGB also specifically enhances Roux limb glucose uptake, and it will be of interest in the future to define whether our observed immune changes drive this enhanced glucose uptake or are a secondary, associated finding.
Our study has several limitations. The animal models utilized are normal weight, insulin sensitive rats, which may explain why we found limited correlation with markers of glucose or insulin sensitivity. Future studies in animal diabetic models and, eventually, humans are needed to confirm our findings and hypothesis. Secondly, our studies were performed on the intestinal mucosa alone. Distinct immune populations are found in the deeper intestinal lamina propria and Peyer’s patches, and characterization of surgery’s effects on the immune populations is indicated in the future. Lastly, as noted above, we were not able to determine in these studies whether these intestinal cytokine changes are a cause or consequence of weight loss induced by bariatric surgery, nor define the mechanisms leading to these cytokine changes. As weight loss was also different in our SG and RYGB models, it is possible that the procedure-specific changes in cytokine profiles we observe are due to differences in weight loss rather than the surgical procedure. Future studies using weight-matched controls may be helpful in addressing this question.
In summary, we find that SG and RYGB induce broadly anti-inflammatory changes in key small intestinal cytokines that have been linked to obesity and diabetes. RYGB has stronger and broader anti-inflammatory effects, but the patterns of changes are similar in SG and RYGB, suggesting that the intestinal immune response may play a role in the weight loss and diabetes remission seen after both operations. These findings support the hypothesis that bariatric surgery improves intestinal immune physiology, with downstream benefits on systemic inflammation and insulin resistance. Future studies need to focus on defining the cellular sources and signaling molecules that lead to these cytokine changes in the intestine, with the goal of exploiting these pathways therapeutically to mimic or augment the effects of bariatric surgery.
References
Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med. 2017;376:641–51.
Wallenius V, Dirinck E, Fändriks L, et al. Glycemic control after sleeve gastrectomy and Roux-en-Y gastric bypass in obese subjects with type 2 diabetes mellitus. Obes Surg. 2017;28:1461–72.
Heshmati K, Harris DA, Aliakbarian H, et al. Comparison of early type 2 diabetes improvement after gastric bypass and sleeve gastrectomy: medication cessation at discharge predicts 1-year outcomes. SOARD.2019; S1550–7289(18)31259-0.
Yarmush ML, D'Alessandro M, Saeidi N. Regulation of energy homeostasis after gastric bypass surgery. Annu Rev Biomed Eng. 2017;19:459–84.
Murphy R, Tsai P, Jüllig M, et al. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg. 2017;27:917–25.
Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–74.
Delzenne NM, Cani PD. Gut microbiota and the pathogenesis of insulin resistance. Curr Diab Rep. 2011;11:154–9.
De Wit NJ, Bosch-Vermeulen H, de Groot PJ, et al. The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med Genomics. 2008;1:14.
Ding S, Chi MM, Scull BP, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191.
Spagnuolo MI, Cicalese MP, Caiazzo MA, et al. Relationship between severe obesity and gut inflammation in children: what’s next? Ital J Pediatr. 2010;36:66.
Febbraio MA. Role of interleukins in obesity: implications for metabolic disease. Trends Endocrinol Metab. 2014;25:312–9.
Winer DA, Luck H, Tsai S, et al. The intestinal immune system in obesity and insulin resistance. Cell Metab. 2016;23:413–26.
Rao SR. Inflammatory markers and bariatric surgery: a meta-analysis. Inflamm Res. 2012;61:789–807.
Illán-Gómez F, Gonzálvez-Ortega M, Orea-Soler I, et al. Obesity and inflammation: change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes Surg. 2012;22:950–5.
Poitou C, Perret C, Mathieu F, et al. Bariatric surgery induces disruption in inflammatory signaling pathways mediated by immune cells in adipose tissue: a RNA-Seq study. PLoS One. 2015;10:e0125718.
Roberts HM, Grant MM, Hubber N, et al. Impact of bariatric surgical intervention on peripheral blood neutrophil (PBN) function in obesity. Obes Surg. 2017;6:1611–21.
Schernthaner GH, Kopp HP, Kriwanek S, et al. Effect of massive weight loss induced by bariatric surgery on serum levels of interleukin-18 and monocyte-chemoattractant-protein-1 in morbid obesity. Obes Surg. 2006;16:709–15.
Mallipedhi A, Prior SL, Barry JD, et al. Changes in inflammatory markers after sleeve gastrectomy in patients with impaired glucose homeostasis and type 2 diabetes. Surg Obes Relat Dis. 2014;10:1123–8.
Greer RL, Morgun A, Shulzhenko N. Bridging immunity and lipid metabolism by gut microbiota. J Allergy Clin Immunol. 2013;132:253–62.
Rodrigues RR, Greer RL, Dong X, et al. Antibiotic- induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front Microbiol. 2017;8:2306.
Winer DA, Winer S, Dranse HJ, et al. Immunologic impact of the intestine in metabolic disease. J Clin Invest. 2017;127:33–42.
Cani PD. Severe obesity and gut microbiota: does bariatric surgery really reset the system? Gut. 2019;68:5–6.
Bhutta HY, Deelman TE, le Roux CW, et al. Intestinal sweet-sensing pathways and metabolic changes after Roux-en-Y gastric bypass surgery. Am J Physiol Gastrointest Liver Physiol. 2014;307:G588–93.
Sumarac-Dumanovic M1, Stevanovic D, Ljubic A, et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes. 2009;33(1):151–6.
Fatima N, Faisal SM, Zubair S, et al. Emerging role of interleukins IL-23/IL-17 axis and biochemical markers in the pathogenesis of type 2 diabetes: association with age and gender in human subjects. Int J Biol Macromol. 2017;105:1279–88.
Ip B, Cilfone NA, Belkina AC, et al. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFα production. Obesity. 2016;24:102–12.
DeFuria J, Belkina AC, Jagannathan-Bogdan M, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. PNAS. 2013;110:5133–8.
Luck H, Tsai S, Chung J, et al. Regulation of obesity-related insulin resistance with gut anti-inflammatory agents. Cell Metab. 2015;21:527–42.
McLaughlin T, Ackerman SE, Shen L, et al. Role of innate and adaptive immunity in obesity-associated metabolic disease. J Clin Invest. 2017;127:5–13.
Ben-Zvi D, Meoli L, Abidi WM, et al. Time-dependent molecular responses differ between gastric bypass and dieting but are conserved across species. Cell Metab. 2018;28:1–14.
Acknowledgments
This study was supported by a Pilot and Feasibility Grant from the Boston Area Diabetes Endocrinology Research Center (P30 DK057521) and a KL2 Award from Harvard Catalyst (4KL2TR001100-04).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Table S1
(DOCX 56 kb)
Rights and permissions
About this article
Cite this article
Subramaniam, R., Aliakbarian, H., Bhutta, H.Y. et al. Sleeve Gastrectomy and Roux-en-Y Gastric Bypass Attenuate Pro-inflammatory Small Intestinal Cytokine Signatures. OBES SURG 29, 3824–3832 (2019). https://doi.org/10.1007/s11695-019-04059-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04059-0