Abstract
Background
This study aimed to investigate the influence of new biliopancreatic diversion (NBPD) and duodenal-jejunal bypass (DJB) surgery on blood glucose, lipids, gastrointestinal hormones, and insulin in Goto-Kakizaki (GK) rats, an animal model for type 2 diabetes, in order to elucidate the mechanisms underlying the therapeutic effect of these types of surgery on this clinical condition.
Methods
Thirty 30 male GK rats (SPF) aged 12 weeks were randomly assigned into three groups (n = 10 per group): sham group, NBPD group, and DJB group. Body weight, random plasma glucose, fasting plasma glucose (FPG), oral glucose tolerance (OGT), blood lipids, plasma insulin, glucagon like peptide-1 (GLP-1), and gastric inhibitory polypeptide (GIP) were measured before and after surgery.
Results
NBPD surgery improved glucose tolerance, decreased fasting free fatty acids, triglycerides, and cholesterol. It also increased fasting and postprandial GIP, but caused no change in GLP-1. DJB surgery produced results similar to NBPD surgery except for causing a decrease in postprandial GLP-1 and insulin, and a larger increase in fasting GIP.
Conclusions
Moving the biliopancreatic duct outlet to the mid-jejunum (NBPD surgery) improves glucose tolerance and increases GIP, but does not change GLP-1. Adding duodenal bypass (DJB surgery) increases fasting GIP and decreases postprandial GLP-1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has long been known to be a risk factor for the development of type 2 diabetes mellitus (T2DM), and is one of the components of the pre-diabetic condition known as “metabolic syndrome.” A variety of different types of bariatric surgery for severe obesity have been shown to alleviate or cure T2DM [1–6] However, the improvement in glucose homeostasis and T2DM that occurs after these surgeries seen before weight loss is noticeable, and this sequence of events suggests that an alteration in gastrointestinal hormones caused by by-passing portions of the digestive tract is also involved in the anti-diabetic effect.
In recent years, the Roux-en-Y gastric bypass (RYGB) has been accepted by the International Diabetes Federation and the American Diabetes Association as a treatment for T2DM [7]. In this surgical technique, the duodenum, the proximal portion of the jejunum, and approximately 90 % of the stomach are removed from the nutrient flow. The remaining portion of the jejunum is connected to the stomach remnant, and bile acids and pancreatic digestive enzymes then flow into the digestive chain through the incised duodenal-jejunal portion of the intestine, which has been connected to the jejunum at a level distal to the ligament of Treitz. A variety of studies confirm that GB can improve T2DM and reduce mortality in T2DM patients [8, 9]. However, the exact mechanisms underlying the therapeutic effect of RYGB on T2DM are not clear, because the effects of the weight loss caused by the decrease in stomach size, the changes in nutrient absorption caused by removal of the duodenal-jejunal portion of the intestine, and the changes in GI hormone release and carbohydrate/fat/protein absorption caused by introducing bile and digestive enzymes downstream from the normal site have not been clearly separated from each other.
Goto-Kakizaki (GK) rats are a polygenic non-obese model for spontaneous T2DM that was developed from selective inbreeding of Wistar rats [10]. In a previous study, we showed that moving the biliopancreatic duct entrance from the duodenum to the jejunum, without any other changes to the digestive tract, improved glucose tolerance in GK rats, that is, improved tolerance without the reduction in gastric capacity and alterations in the intestinal digestive route seen when the Roux-en-Y procedure is combined with gastric bypass [11, 12].
In the current study, we compare the effects of moving the pancreatic-biliary fluid entry to the distal jejunum, both with and without bypass of an incised duodeno-jejunal segment, on glucose metabolism, plasma lipids, insulin sensitivity, and GI hormone release.
Methods
Experimental animals
Thirty male GK rats (SPF) aged 10 weeks and weighing 290–320 g were purchased from Shanghai Slack Experimental Animal CO., Ltd, and maintained in the Experimental Animal Center of Fujian Medical University. All animals were housed in the barrier system of the Experimental Animal Center of Fujian Medical University in a 12-h light/12-h dark cycle. Animals were allowed to accommodate to the environment for 2 weeks before entering the study and given ad libitum access to water and food. Rats were fed with food containing 5 % fat at 20–25 g/day. At the age of 12 weeks, GK rats were randomly assigned into three groups: group A: duodenal-jejunal bypass (DJB) surgery; group B: new biliopancreatic diversion (NBPD) surgery; group C: sham surgery. This study was approved by the Institutional Animal Care and Use Committee of Fujian Medical University,
Surgical procedures
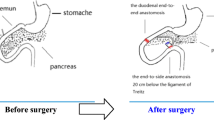
All animals were anesthetized by inhalation of sevoflurane. The two procedures used are similar to those previously reported [1, 11], and a diagram of these procedures is shown in Fig. 1. [Reviewer 2, comment 2]
A schematic diagram of new biliopancreatic diversion and duodenal bypass surgeries. a New biliopancreatic diversion. End-to-end duodenoduodenostomy at 0.5 cm proximal to and 0.5 cm distal to the major duodenal papilla, followed by transfer of the intestinal segment containing the major duodenal papilla to the proximal jejunum (20 cm from the ligament of Treitz) [11]. b Duodenal-jejunal bypass. The duodenum was separated from the stomach, and bowel continuity was interrupted at the level of the distal jejunum, (10 cm from the ligament of Treitz). The distal of the two limbs was directly connected to the stomach (gastrojejunal anastomosis) and the proximal limb carrying the biliopancreatic to the alimentary limb at a distance of 10 cm from the gastrojejunal anastomosis (Roux-en-Y reconstruction) [1]
DJB group
In this group, a segment including the bilioenteric confluence (containing the biliopancreatic duct), duodenum, and proximal jejunum was incised and reattached to the distal jejunum. To obtain this segment, the duodenum was cut 0.5 cm below the pylorus, the jejunum was cut at 10 cm distal to the ligament of Treitz, and the duodenal end of the segment was closed. The remaining end of the jejunum was anastomosed with the remaining end of the duodenum (end-to-end anastomosis), and the jejunal end of the duodenal-jejunal segment was reconnected with the remaining jejunum by end-to-side anastomosis at 20 cm distal to the ligament of Treitz (Fig. 1b). The operation time was 45 ± 5 min.
NBPD group
In this group, only the bilioenteric confluence was incised from the duodenum and attached to the distal jejunum. The segment of the duodenum containing the biliopancreatic confluence was incised by transections made 0.5 cm proximal to and 0.5 cm distal to the this confluence. The proximal end of the segment was closed, and the proximal and distal ends of the remaining duodenum anastomosed end-to-end. The incised [Reviewer 2, comment 1] duodenal segment containing the bilioenteric confluence was anastomosed with the jejunum 20 cm distal to the ligament of Treitz (end-to-side anastomosis) (Fig. 1a). The operation time was 40 ± 5 min.
Sham group
In the sham group, the duodenum was cut at 0.5 cm below the pylorus and then anastomosed in situ. The operation time was 20 ± 3 min.
Detection
Fasting body weight
At 1 week before surgery and 4 and 12 weeks after surgery, rats were fasted overnight and then weighed.
Postprandial body weight
At 1 week before surgery and 1, 6, and 12 weeks after surgery, rats were also weighed without fasting.
Fasting plasma glucose
At 1 week before surgery and 4 and 12 weeks after surgery, rats were fasted overnight and blood was collected from the tail vein for the measurement of plasma glucose with a glucometer.
Random plasma glucose
At 1 week before surgery and 1, 6, and 12 weeks after surgery, blood was collected from the tail vein, and plasma glucose was measured with a glucometer.
Oral glucose tolerance test
At 1 week before and 4 and 12 weeks after surgery, rats were fasted over night and intragastrically treated with oral 50 % glucose at 2 g/kg. Blood was collected at 30, 60, 120, and 180 min for the measurement of plasma glucose.
Blood lipid measurement
At 2 weeks before surgery and 4 weeks after surgery, rats were fasted overnight, and blood was collected from the orbital vein. At 1 week before surgery and 6 and 12 weeks after surgery, blood was collected from the orbital vein without previous fasting. The blood samples were centrifuged, and the serum harvested for the measurement of triglycerides (TG), cholesterol (Chol), and free fatty acids (FFA) with a P800 automatic biochemical analyzer and supporting reagents.
Fasting insulin, gastric inhibitory polypeptide, and glucagon-like peptide-1
At 1 week before surgery and 4 weeks after surgery, rats were fasted overnight, and blood was collected from the orbital vein. After centrifugation at 3000 rpm for 10 min at 4 °C, the plasma was harvested and stored at −20 °C. Fasting insulin (INS), gastric inhibitory polypeptide (GIP), and glucagon-like peptide-1 (GLP-1) were measured with corresponding ELISA kits.
Postprandial INS, GLP-1, and GIP
At 2 weeks before surgery and 2 and 10 weeks after surgery, blood was collected from the orbital vein without fasting and centrifuged at 3000 rpm for 15 min at 4 °C. Plasma was collected and stored at −20 °C. INS, GIP and GLP-1 were measured with corresponding ELISA kits. Rat ELISA kit for insulin was purchased from Alpco (Salem, NH, USA). Rat GIP and GLP-1 ELISA kits were from DRG (Marburg, Germany).
Statistical analysis
All data were presented as mean ± standard deviation. Group difference at a given time point was examined by analysis of variance (ANOVA). Time effect was tested by generalized estimation equation (GEE). Bonferroni’s correction was performed for post hoc tests if any significant result among groups or time points had been revealed. Statistical analysis was considered significant as the two sided p value < 0.05. SPSS 22.0 statistics software (IBM Corp., Armonk, NY, USA) was used for statistical analyses.
Results
Table 1 summarizes differences among the three groups of rats in weight, fasting blood glucose, random blood glucose, fasting GLP-1, fasting GIP, fasting insulin, and OGT measured before surgery. Except for fasting GIP, the baseline parameters in the three groups of rats were similar. The fasting GIP in the DJB group, however, was higher than that in the sham group.
Postoperative changes of weight, fasting blood glucose, random glucose, fasting GLP-1, fasting GIP, and fasting insulin are illustrated in Fig. 2. Body weight in rats increases throughout their lifetime, and body weight in all three groups increased during the 12-week period after surgery, although the increase in weight in the DJB group occurred so slowly that it did not become statistically significant until 12 weeks (Fig. 2a). Increases in fasting insulin and fasting GIP levels were seen in all three groups at 4 weeks (Fig. 2e, f). Reduction in fasting blood glucose in the sham and NBPD groups at 12 weeks and a decrease in random glucose in the DJB and NBPD groups after 1 week were noted as well (Fig. 2b, c).
The effects of surgeries on various parameters. The changes in weight (a), fasting blood glucose (b), random glucose (c), fasting GLP-1 (d), fasting insulin (e), and fasting GIP (f) in the three groups of rat (n = 10 per group). Data were presented as mean ± standard deviation and tested by generalized estimation equation. Asterisks indicate significantly different from baseline. Letters denote significantly different from sham group a, and DJB group b
After surgery, compared to the sham group, the DJB group had a lower body weight, random blood glucose, fasting insulin, and a higher fasting blood glucose and fasting GIP. The NBPD group had lower postoperative random glucose, and fasting insulin, and higher postoperative fasting GIP than the sham group. Of the two experimental groups, the postsurgical fasting blood glucose and fasting GIP were lower in the NBPD group than in the DJB group.
Changes in oral glucose tolerance test (OGTT) plasma glucose levels of the three groups are illustrated in Fig. 3. The time trends of OGTT in three groups were similar. OGTT glucose levels peaked at 60 min and then rapidly declined. No differences between preoperative and postoperative OGTT glucose levels were seen in the sham group (Fig. 3a). However, in both DJB and NBPD groups, OGTT glucose levels became reduced, with lower OGTT levels 12 weeks after surgery (Fig. 3b, c). In terms of the group difference, three groups had similar 60-min OGTT levels before surgery, while a higher value was found in the sham group compared to the NBPD group after 4 weeks, and a higher 60-min OGTT level than the DJB group at 12 weeks.
Fasting FFA, TG, and Chol in the sham group did not change after surgery; however, a significant increase in fasting TG and a decrease in fasting Chol were found in the rats receiving DJB or NBPD. Fasting FFA in two experimental groups changed in divergent ways, with a small but significant decline in the NBPD group and increase in the DJB group (Fig. 4a, c, e). Postprandial FFA, TG, and cholesterol in the sham group were not influenced by the surgery. Postprandial FFA in DJB and NBPD groups did not change after surgery (Fig. 3b), but postprandial TG and cholesterol levels decreased (Fig. 3d and f). Although no differences among the groups were revealed before surgery, rats receiving DJB or NBPD had lower fasting cholesterol, postprandial TG, postprandial cholesterol, and higher fasting TG relative to the sham rats.
Changes in fasting free fatty acid (a), fasting triglyceride (b), fasting cholesterol (c), postprandial free fatty acid (d), triglyceride (e), and cholesterol (f) in the 3 groups (n = 10 per group). Data were presented as mean ± standard deviation and tested by generalized estimation equation. Asterisks indicate significantly different from baseline. “a” denotes significantly different from sham group
Postprandial GIP, GLP-1, and insulin are shown in Fig. 5. GIP levels increased significantly in sham rats after surgery, and levels at 10 weeks were still higher than values measured before the surgery though they had decreased slightly. In the DJB and NBPD groups, the postoperative GIP level at 2 weeks was increased compared to the preoperative level and the level in sham rats, and remained high at 10 weeks (Fig. 5a). Although the postprandial GLP-1 in the sham group increased 2 weeks after the operation, the level at 10 weeks decreased to the level lower than the pre-surgical level. A significant reduction in postprandial GLP-1 at week 10 was found in the DJB group compared to the preoperative level, but the postprandial GLP-1 level in the NBPD group was not changed by the operation (Fig. 5b). Postprandial insulin in the three groups changed in divergent ways. After an increase at 2 weeks, the insulin decreased to preoperative level in the sham group. In two experimental groups, the insulin level decreased 2 weeks after the surgery, and was maintained at a stable level in the DJB group, but rose at 10 weeks in the NBPD group (Fig. 5c).
Changes in postprandial GIP (a), GLP-1 (b), and insulin (c) in the 3 groups (n = 10 per group). Data were presented as mean ± standard deviation and tested by generalized estimation equation. Asterisks indicate significantly different from baseline. Letters denote significantly different from sham group a, or DJB group b
Discussion
The effect of NBPD (that is, distal biliopancreatic fluid entry) in T2DM rats on blood glucose and lipids was to decrease random glucose levels, triglyceride, FFA, and cholesterol levels. Of the two gastrointestinal hormones examined, fasting and postprandial GIP levels increased greatly, but fasting and postprandial GLP-1 levels did not change. Glucose homeostasis was improved in these T2DM rats by relocating the biliopancreatic fluid entry site, for random glucose levels decreased, fasting insulin levels increased less with time than in sham-treated rats, and OGTT was improved by 12 weeks after surgery. Fasting and postprandial lipid metabolism also changed, as one might expect from moving the entry of bile acids, which are needed for absorption of dietary lipids, to a location distal to the normal location. The effect of NBPD in this T2DM model (improvement of glucose homeostasis, changes in plasma lipids, and increases in GIP levels) was caused solely by the change in the entry point for biliopancreatic fluid.
The effect of DJB (that is, of adding duodenal-jejunal bypass to distal biliopancreatic fluid entry), was to slow the rat’s normal body weight increase, increase fasting GIP, and decrease postprandial GLP-1 and insulin levels, the other results being the same as to those seen with distal biliopancreatic fluid entry alone. The slower increase in body weight is not surprising because removing the duodenum and proximal jejunum from the nutrient flow removes a considerable area normally available for the absorption of food. The major effect of adding duodenal-jejunal bypass to the distal biliopancreatic fluid entry was a change in endocrine output—increased fasting GIP and decreased postprandial GLP-1 and insulin.
GIP and GLP-1 are the two major GI hormones affecting glucose homeostasis. GIP is secreted by K cells in the proximal duodenum upon stimulation by partially digested food [13, 14]. GLP-1 is secreted by L cells in the lower ileum when stimulated by nutrients. Both increase insulin secretion and delay gastric emptying, but GIP induces and GLP-1 inhibits glucagon secretion.
Both fasting and postprandial GIP were increased by moving the biliopancreatic fluid entry downstream. The increase in postprandial GIP might have occurred because the increase in undigested food in the duodenum that occurred when biliopancreatic fluid entry was diverted to the jejunum stimulated GIP production by duodenal K cells. However, when the duodenum itself was removed from having contact with undigested food, the increase in postprandial GIP still occurred, so this explanation is incorrect. Increased GIP might be involved in the improvement in glucose homeostasis seen in our T2DM model, either by facilitating insulin secretion, an activity compromised in T2DM patients [15], or by delaying stomach emptying, a process found to be accelerated in diabetic rats [16].
Postprandial GLP-1 levels were unchanged by moving the biliopancreatic fluid entry site, but the addition of duodenal-jejunal bypass decreased postprandial GLP-1 in a manner similar to that seen in sham rats. Han et al. [17] reported that moving the entry point for biliopancreatic fluid and an accompanying by-passed duodenal-jejunal segment to a more distal location increased fasting GLP-1/ Ileal transposition with or without biliopancreatic fluid entry relocation has been reported in other studies to increase glucose-stimulated GLP-1 [6, 17–20]. However, GLP-1 has a 2-min half-life, and results obtained during the 15–30-min period after oral glucose stimulation of fasted rats cannot properly be compared to our results, which were obtained on non-fasted, ad libitum-fed rats.
DJB surgery does not delay gastric emptying and would facilitate the entry of food into the terminal part of the small intestine, where GLP-1 is synthesized and secreted; however, in our study, GLP-1 was decreased rather than elevated. NBPD surgery neither accelerates nor delays gastric emptying, and although it did not alter GLP-1 levels, it improved glucose levels in T2DM rats. Therefore, the hindgut theory that exclusion of the foregut removes foregut hormones that would decrease secretion of GLP-1 cannot fully explain the mechanism through which these surgeries alleviate T2DM. In addition to changes in GIP and GLP-1, alterations in digestion and absorption may be an explanation.
Cholesterol is increased in T2DM and obese patients [21]. In the present study, both NBPD and DJB lowered fasting and postprandial serum cholesterol compared to levels seen in control T2DM rats. The lower postprandial cholesterol levels are due to the reduced duodenal absorption of cholesterol that followed the distal diversion of biliopancreatic fluid. A long-lasting reduction in cholesterol absorption may reduce blood cholesterol and be the reason that the fasting cholesterol was reduced.
Postprandial TG was similar in both treatment groups and was lower than before surgery and than in the sham group. This may be explained as follows: as the duration of interaction between biliopancreatic juice and food is reduced and the gastrointestinal tract is shortened, the absorption of digestion products of TG (FFA + glycerides) is reduced, and therefore, the postprandial synthesis and storage of TG are reduced. The reduction in post-prandial TG in the DJB group was similar to that in the NBPD group. Thus, the diversion of biliopancreatic fluid is the major factor causing to the reduction in postprandial TG and exclusion of the upper small intestine plays a minor, if any, role. However, it is confusing that the fasting TG after surgery in the NBPD group and DJB group was significantly higher than in the sham group and than the postprandial TG. Thus, we speculate that the insulin sensitivity increases after surgery, which reduces the degradation of TG at fasting status and increases the re-esterification of FFA, leading to the increase in serum TG.
FFA levels are closely related to the occurrence and development of T2DM, and re-esterification of FFA is significantly compromised in T2DM patients [22]. Our results showed that, at 4 weeks after surgery, the fasting FFA in NBPD and DJB groups was lower than in control T2MP rats. This may be related to reduced degradation of TG and increased re-esterification of FFA, both of which may increase insulin sensitivity.
The RYGB surgery that is used to treat both obesity and T2DM may affect plasma glucose in three ways: (1) reducing gastric capacity and food intake, leading to an improvement in plasma glucose similar to that seen in the diet control used in the management of early T2DM; (2) diverting biliopancreatic juice and changing the location of the mixing of biliopancreatic juice and food, thus affecting digestion and absorption of food; (3) shortening the small intestine and causing more rapid entry of undigested food into the ileum.
The non-obese T2DM rats used in our study allowed us to study the effects of changing the location of entry of biliopancreatic fluid and shortening the small intestine without the confounding effects from obesity or the reduction in stomach size that is performed during RYGB. Diverting the entry of biliopancreatic fluid to a more distal location improves glucose tolerance and increases GIP. Adding the shortening of the small intestine increased fasting GIP and decreased postprandial GLP-1.
These results may be explained as follows: (1) the distal diversion of biliopancreatic fluid causes incomplete digestion and absorption of food and reduces fasting FFA and postprandial TG, which may improve lipid metabolism and decrease insulin resistance. The increase in insulin sensitivity may reduce the degradation of TG into FFA during fasting status and thus decrease the FFA-induced damage to islet cells, leading to an improvement of insulin sensitivity, which forms a virtual cycle [23]. The increase in insulin sensitivity may improve glucose metabolism and lower plasma glucose; (2) the postsurgical increase in GIP secretion then inhibits gastric emptying, which delays the digestion and absorption of food and improves plasma glucose.
References
Rubino F, Marescaux J. Effect of duodenal–jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11.
Alleotti E, Palma RT, Pinto Jr PE, et al. Biliopancreatic diversion with duodenojenunal exclusion associated with truncal vagotomy. A new proposal for type 2 diabetes mellitus treatment. Acta Cirirgica Brasil. 2012;27:577–84.
Koehestanie P, Dogan K, Berends F, et al. Duodenal-jejunal bypass liner implantation provokes rapid weight loss and improved glycemic control, accompanied by elevated fasting ghrelin levels. Endosc Int Open. 2014. doi:10.1055/s-0034-1365222.
Raffaelli M, Iaconelli A, Nanni G, et al. Effects of biliopancreatic diversion on diurnal leptin, insulin and free fatty acid levels. BJS. 2015;102:682–90.
Gan SS, Talbot ML, Jorgensen JO. Efficacy of surgery in the management of obesity-related type 2 diabetes mellitus. ANZ J Surg. 2007;77(11):958–62.
Pereferrer FS, Gonzalez MH, Rovira AF, et al. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. Obes Surg. 2008;18(1):97–108.
American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33:692.
Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–61.
Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52.
Akash MSH, Rehman K, Chen S. Goto-kakizaki rats: Suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Curr Diabetes Rev. 2013;9:1–10.
Weng S-G. Modified biliopancreatic diversion for GK rats: a proposal for a simpler technique and mechanism research. Obes Surg. 2012;22:997–8.
Weng SG, Zhang B, Feng S, et al. Effects of modified biliopancreatic diversion on glucose tolerance of GK rats. Obes Surg. 2012;23:522–30.
Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–57.
Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–31.
Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–7.
Young AA, Gedulin B, Vine W, et al. Gastric emptying is accelerated in diabetic BB rats and is slowed by subcutaneous injections of amylin. Diabetologia. 1995;38:642–8.
Han H, Wang L, Du H, et al. Expedited biliopancreatic juice flow to the distal gut benefits the diabetes control after duodenal jejunal bypass. Obes Surg. 2015;25:1802–9.
Zhang GY, Wang TT, Cheng ZQ, et al. Resolution of diabetes mellitus by ileal transposition compared with bilioancreatic diversion in a nonobese animal model of diabetes. Can J Surg. 2011;54:243–51.
Patriti A, Aisa M, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced proglucagon gene expression and L-cell number. Surgery. 2007;142(1):74–85.
Speck M, Cho YM, Asadi A, et al. Duodenal-jejunal bypass protects GK rats from b-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1. Am J Physiol Endocrinol Metab. 2011;300:E923–32.
Charles MA, Eschwege E, Thibult N, et al. The role of NEFA in the deterioration of glucose tolerance in Caucasian subjects:result of the pairs prospective study. Diabetologia. 1997;40:1101–9.
Taskinen MR, Bogardus C, Kennedy A, et al. Multiple disturbances of free fatty acid metabolism in noninsulin-dependent diabetes. Effect of oral hypoglycemic therapy. J Clin Invest. 1985;76(2):637–44.
Boden G. Does inhibition of beta cell proliferation by free fatty acid in mice explain the progressive failure of insulin secretion in type 2 diabetes? Diabetes. 2012;61(3):560–1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of informed consent
Not applicable
Statement of human and animal rights
All applicable institutional and/or national guidelines for the care and use of animals were followed.
Additional information
Supported by: The Fujian Provincial Natural Science Foundation, No. 2014J01312. Training Project of Young Talents in Health System of Fujian Province, No.2014-ZQN-ZD-17. Professor Development Fund of Fujian Medical University, No.JS14020.
Rights and permissions
About this article
Cite this article
Weng, S., Zhang, B., Xu, C. et al. Influence of New Modified Biliopancreatic Diversion on Blood Glucose and Lipids in GK rats. OBES SURG 27, 657–664 (2017). https://doi.org/10.1007/s11695-016-2320-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2320-z