Abstract
Background
The purpose of this study was to investigate the hypoglycemic effect of new biliopancreatic diversion and duodenal–jejunal bypass in Goto–Kakizaki rats and observe effects of the new surgical procedure on the glucose tolerance of GK rats.
Methods
Twenty-four 10-week-old rats (SPF grade) were randomly divided into groups A, B, and C, each with eight rats. Group A underwent duodenal–jejunal bypass, group B underwent modified biliopancreatic diversion, and group C underwent a sham operation. Median rat body weight, fasting blood glucose, OGTT, and blood lipids were measured in fasting 1 week before surgery and 1, 2, 4, and 8 weeks after surgery. Changes in gastric inhibitory polypeptide, glucagon P-like peptide-1, and insulin levels were measured by ELISA 1 week before surgery and 8 weeks after surgery.
Results
Rats’ mean body weight in groups A and B decreased significantly from 368.025 ± 11.726 and 373.100 ± 9.859 g preoperatively to 345.750 ± 11.403 and 343.260 ± 12.399 g at the early postoperative stage (P < 0.05), and with statistically significant differences compared to the weight of rats in group C (P < 0.05). Comparisons between fasting blood glucose before surgery and 8 weeks after surgery revealed no significant differences between all three groups (P > 0.05). Glucose tolerance in groups A and B decreased from preoperative 21.175 ± 3.684 and 20.820 ± 1.671 mmol/L to postoperative 8.950 ± 0.580 and 10.500 ± 1.509 mmol/L, and both were better than that of group C (P < 0.001).
Conclusions
Both new biliopancreatic diversion and duodenal–jejunal bypass improve glucose tolerance of Goto–Kakizaki rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In 1980, Pories et al. found that weight loss surgery could alleviate type 2 diabetes. Since then, a new way which can treating type 2 diabetes was been hot and a large number of clinical and basic studies have demonstrated the effectiveness of it [1–3]. However, after nearly 30 years research, the mechanism by which weight loss surgery improves type 2 diabetes is still unclear. Until now, most studies have focused on intestinal hypothesis which including the upper and lower intestinal hypothesis. The upper intestinal hypothesis considers which induces decreased secretion of GIP or other unknown pro-diabetic factors, thereby improving blood glucose. The lower intestinal hypothesis considers that postoperative exclusion of the proximal jejunum speeds up the incompletely digested food entering the terminal ileum, which stimulates the secretion of glucagon P-like peptide-1 (GLP-1), and thereby improves blood glucose. However, neither hypothesis can fully explains the mechanism of weight loss surgery can alleviate type 2 diabetes [4, 5].
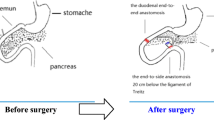
Are there any other possible explanations? We noticed that after surgical treatment, the normal digestion process has been changed, resulting in a delay in the time and the position of food being mixed with bile and pancreatic juice. Bile and pancreatic juice are critical for digestion as the most important digestive juices. This may result in incomplete digestion of carbohydrates and lipids and decreasing blood glucose during surgery. To address this issue, we designed a new biliopancreatic diversion (NBPD) procedure based on the anatomical features of rats (Fig. 1b) [6]. In this experimental study, we observed the hypoglycemic effect of this procedure in Goto–Kakizaki (GK) rats and compared it with that of the duodenal–jejunal bypass (DJB, Fig. 1a), which was previously proven to have hypoglycemic effect in animal studies [3].
Duodenal–jejunal bypass (a). The duodenum was separated from the stomach, and bowel continuity was interrupted at the level of the distal jejunum, (10 cm from the ligament of Treitz). The distal of the two limbs was directly connected to the stomach (gastrojejunal anastomosis) and the proximal limb carrying the biliopancreatic juices was reconnected downward to the alimentary limb at a distance of 10 cm from the gastrojejunal anastomosis (Roux-en-Y reconstruction); Modified biliopancreatic diversion (b). End-to-end duodenoduodenostomy; (above) major duodenal papilla 0.5 cm and (below) major duodenal papilla 0.5 cm, with transfer of the intestinal tract including major duodenal papilla (20 cm from the ligament of Treitz); sham-operation (c). Transection of the gastrointestinal tract and the bowel was immediately reanastomosed after transection
Research Design and Methods
Animals
Twenty-four male 10-week-old GK rats were purchased from Shanghai laboratory animal center. Animals had free access to tap water and were ad libitum fed with a 5 % fat rat chow diet. All animals were housed in individual cages under constant ambient temperature and humidity in a 12-h light/dark cycle. All animals were raised in the Animal Center of Fujian Medical University.
Study Protocol
Rats were allowed 2 weeks for acclimation before the start of experiments. Twelve-week-old rats randomly underwent one of the following: (A) DJB, Fig. 1a; (B) NBPD, Fig. 1b; (C) sham operation, Fig. 1c.
DJB group: the duodenum was separated from the stomach, and bowel continuity was interrupted at the level of the distal jejunum (10 cm from the ligament of Treitz). The distal of the two limbs was directly connected to the stomach (gastrojejunal anastomosis) and the proximal limb carrying the biliopancreatic juices was reconnected downward to the alimentary limb at a distance of 10 cm from the gastrojejunal anastomosis (Roux-en-Y reconstruction) [3] .
NBPD group: end-to-end duodenoduodenostomy at up major duodenal papilla 0.5 cm and down major duodenal papilla 0.5 cm, with transfer of the intestinal tract including major duodenal papilla (20 cm from the ligament of Treitz) [6].
The sham operation: transection of the gastrointestinal tract and the bowel was immediately reanastomosed after transection.
Measurements
Body weight was measured 1 week before surgery and 1, 2, 4, and 8 weeks after surgery.
For fasting glycemia, after 12–14 h fasting, blood was collected from the tail in conscious rats (Roche dynamic type blood glucose meter).
For OGTT, after 12–14 h fasting, blood glucose was measured in conscious rats before (baseline) and then 30, 60, 120, and 180 min after the administration of 2 g/kg glucose by oral 50 % glucose solution.
For random blood glucose, 1 week before surgery and 1, 2, 4, and 8 weeks after surgery, blood was collected from the tail in conscious animals. Random blood glucose means rats are ad libitum fed, blood glucose was measured without fasting.
Plasma total cholesterol, triglycerides and free fatty acids (FFA) were measured after 12–14 h fasting, blood from the angular vein of conscious rats was collected in biochemical tube, then analyzed in Clinical Laboratory Department of the First Hospital Affiliated of Fujian Medical University.
For plasma hormones measurements, 1 week before surgery and 8 weeks after surgery, after 12–14 h fasting, blood from the angular vein of conscious rats was collected in EDTA tubes containing the GI preservative. After centrifugation at 3,000 rpm at 4 °C for 10 min, plasma was immediately separated and stored at −80 °C until analyzed. Rat radioimmunoassay kits were used for measurement of insulin, GIP and GLP-1 (R&D Systems, Minneapolis, MN).
Statistical Analysis
Data are expressed as mean ± SD. Areas under curves were calculated by trapezoidal integration. Statistical analysis was performed using multivariate analysis of variance and the Student t test as appropriate. P values < 0.05 were considered to be statistically significant.
Results
Before the surgery, there were no significant differences between all three groups in terms of weight, fasting glycemia, glucose tolerance, blood lipids and plasma hormones.
Weight
One week after the surgery, the rats’ average weight were decreased significantly, from 368.025 ± 11.726 g to 345.750 ± 11.403 g in the DJB group, and 373.100 ± 9.859 g to 343.260 ± 12.399 g in the NBPD group (P < 0.05). From the second week to the eighth week, the weight increased slowly. There was no difference in body weight in sham group pre-operation and post-operation. The NBPD is about to reach preoperative weight soon after the eighth week (Table 1).
Fasting Glycemia
One week after the surgery, fasting blood glucose levels were decreased in all three groups. After the fourth week the trend is to return to pre operative levels in all three procedures. There were no significant differences between all three groups after the surgery (Fig. 2a).
Random Blood Glucose
One week after the surgery, the random blood glucose of DJB group and NBPD group were significantly decreased (P < 0.05), from 21.175 ± 3.684 mmol/L to 10.050 ± 1.330 mmol/L in DJB group, from 20.820 ± 1.671 mmol/L to 13.560 ± 3.224 mmol/L in NBPD group, while still 18.980 ± 1.529 mmol/L in sham operation group (Fig. 2b).
OGTT
One week after surgery, there were no significant differences between all three groups. 8 weeks after surgery, both the DJB and NBPD group’s glucose tolerance were improved, and the mean 60-min peak levels of DJB group was lower than NBPD group’s(P < 0.05) (15.5 ± 2.1 mmol/L vs. 17.8 ± 2.9 mmol/L) (Fig. 3).
Glucose tolerance. a Before surgery, there were no significant difference between all three groups. b–d From 1 to 4 weeks after surgery, there were no significant difference between all three groups. e Eight weeks after surgery, both the DJB and NBPD group’s glucose tolerance were improved, and the mean 60-min peak levels of DJB group was lower than NBPD group’s (P < 0.05)
FFA
Eight weeks after surgery, The FFA of DJB group and NBPD group were significantly decreased (P < 0.05), from 766.25 ± 35.23 μEq/L to 555.75 ± 23.54 μEq/L in DJB group, from 770.6 ± 30.22 μEq/L to 546.2 ± 41.33 μEq/L in NBPD group. There were no significant differences in sham operation group before and after surgery (Fig. 4).
GIP, GLP-1
Despite the minor decrease in GIP and GLP-1 in both the DJB and NBPD groups after surgery, there were no significant differences compared to those of sham operation group (Fig. 5).
INS
Eight weeks after surgery, the DJB group show lower level of INS than preoperative one (P < 0.05). NBPD-treated rats showed similar plasma INS level before and after surgery. The level of INS was significantly increased in sham operation group after surgery (P < 0.05) (Fig. 6).
Triglycerides
Eight weeks after surgery, DJB and NBPD groups show lower level of triglycerides than preoperative one (P < 0.05). The level of triglycerides was not significantly different to that of the sham operation group before and after surgery (P > 0.05) (Fig. 7).
Discussion
Hypoglycemic effect of DJB on GK rats was demonstrated by Rubino et al. [3]. However, we found in the present study that postoperative fasting blood glucose did not improve after DJB surgery in GK rats, contrary to the results of Rubino et al. A possible explanation for this is that the level of fasting blood glucose was not high in the present experiment. In the experiment of Rubino et al., the mean fasting blood glucose of GK rats was higher than 8.5 mmol/L, while the mean fasting blood glucose of GK rats in the current study was only 7.2 mmol/L. For a diabetic patient, postprandial hyperglycemia not only accelerates the occurrence of complications, but it also aggravates diabetes [7–9]. Therefore, it is more important to decrease postprandial blood glucose than to decrease fasting blood glucose.
OGTT is one of the standards of glucose tolerance. There were no significant differences between all three groups until 8 weeks after surgery. It is in contrast to many other studies but consistent with Dr. Rubino [9]. We hypothesize that changes in digestion and absorption maybe an important factor after surgical treatment. But glucose does not need to be digested, so OGTT may make no difference earlier after surgery.
In the present study, GK rats underwent DJB and NBPD surgeries, revealing that even though fasting blood glucose and OGTT did not improve, random blood glucose improved obviously. Our results show that NBPD can decrease blood glucose and improve glucose tolerance in GK rats, which is the first time this result has been reported.
Now that both DJB and NBPD have been shown to improve glucose tolerance in GK rats, then what is the possible mechanism? At present, most scholars consider that the mechanism of surgical treatment for type 2 diabetes consists of changes in the entero-insular axis. Major hypotheses include the upper intestinal hypothesis and the lower intestinal hypothesis. Results of the present study showed that there were no differences in GIP and GLP-1 levels between the three groups (i.e., duodenal–jejunal bypass group, modified biliopancreatic diversion group, and sham operation group) before and after surgery, which agrees with the results of Rubino et al. [10]. Fasting blood glucose did not improve significantly after surgery, while random blood glucose improved obviously. This may be related to the fact that the normal digestive process changes after DJB, which induces changes in hormone secretion when food moves through the intestine. The upper intestinal hypothesis and lower intestinal hypothesis also mention that “decreased secretion of GIP or other unknown pro-diabetic factors, which results in decreased blood glucose,” and “after DJB surgery, food enters the terminal ileum in advance and increases GLP-1 secretion, which decreases postprandial blood glucose” [3, 4].
In addition to the influence of intestinal hormones, are there any other possible explanations? After gastrointestinal surgeries like DJB, not only the normal digestive process changes, but the time and location of food being mixed with bile and pancreatic juice also changes. NBPD only delays the time and location of food being mixed with bile and pancreatic juice and does not change the digestive process, therefore it also can improve glucose tolerance in GK rats. Bile and pancreatic juices are very important digestive juices that play a key role in the digestion of lipids, and a high-fat diet happens to be a high risk factor for diabetes. It has been reported that there is a positive correlation between a high-fat diet and insulin resistance [11, 12], while the key point in the treatment of type 2 diabetes is to resolve insulin resistance. Is it finally possible to reduce digestion and absorption of lipids to improve insulin resistance and restore glucose tolerance? While the surgical procedure designed in the present study changed the location and time of food being mixed with bile and pancreatic juice, it did not carry out exclusion of the intestine. Our results showed that serum FFA and triglycerides levels decreased in the DJB group and NBPD group 8 weeks after surgery. Fasting plasma insulin levels decreased significantly in the DJB group while levels did not change in the NBPD group and increased in the sham operation group. This shows that absorption of lipids was reduced and insulin resistance improved after DJB and NBPD. This can possibly be explained by the delay and change in location of food being mixed with bile and pancreatic juice, which resulted in incomplete digestion and absorption of lipids to reduce lipid absorption and improve insulin resistance. It also improved the sensitivity of the human body to insulin, which resulted in the recovery of glucose tolerance. Therefore, changes in the digestion and absorption after surgical treatment may be one of the mechanisms of surgical treatment for diabetes. As stated above, NBPD as proposed by the current experiment delayed the time and changed the location of food being mixed with bile and pancreatic juice, while exclusion of the intestine was not carried out. This method can be applied for studying changes in digestion and absorption after surgical treatment.
After DJB, exclusion of the proximal intestine not only affects food digestion and absorption but also reduces GIP secretion just as it does in the upper intestinal hypothesis and lower intestinal hypothesis. Moreover, exclusion of some parts of the intestine accelerates the entrance of chyme into the terminal ileum and increases GLP-1 secretion. All of these actions together may contribute to improvement in glucose tolerance.
Conclusions
Results of our study show the modified biliopancreatic diversion can improve the glucose tolerance of GK rats. Changes in the digestion and absorption after surgical treatment may be one of the mechanisms of surgical treatment for diabetes.
References
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37.
Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222(3):339–50.
Rubino F, Marescaux J. Effect of duodenal–jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11.
Mason EE. Ilial transposition and enteroglucagon/GLP-1 in obesity (and diabetic?) surgery. Obes Surg. 1999;9:223–8.
Patriti A, Aisa M, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto–Kakizaki rats through an enhanced proglucagon gene expression and L-cell number. Surgery. 2007;142(1):74–85.
Weng S-G. Modified biliopancreatic diversion for GK rats: a proposal for a simpler technique and mechanism research. Obes Surg. 2012;22(6):997–8.
Hanefeld M, Temelkova-Kurktschiev T. The postprandial state and the risk of atherosclerosis. Diabet Med. 1997;14 Suppl 3:S6–S11.
Coutinho M, et al. The relationship between glucose and incident cardiovascular events. Diabetes Care. 1999;22(2):223–40.
O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100(5):899–904.
Speck M, Cho YM, Rubino F, et al. Duodenal–jejunal bypass protects GK rats from beta-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells co-expressing GIP and GLP-1. Am J Physiol Endocrinol Metab. 2011;300(5):E923–32.
Maedler K, Spinas GA, Dyntar D, et al. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50(1):69–76.
Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276(18):14890–5.
Acknowledgments
We are thankful to the Fujian Medical University for providing place for housed animal. We also thank the First Hospital Affiliated of Fujian Medical University and Fujian Province Institute of Abdominal Surgery for providing place for our research.
Conflict of interest
The authors declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by: the Fujian Provincial Natural Science Foundation, No. 2010J01152
Rights and permissions
About this article
Cite this article
Weng, Sg., Zhang, B., Feng, S. et al. Effects of Modified Biliopancreatic Diversion on Glucose Tolerance of GK Rats. OBES SURG 23, 522–530 (2013). https://doi.org/10.1007/s11695-012-0830-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-012-0830-x