Abstract
Background
Current evidence suggests that local anesthetic wound infiltration should be employed as part of multimodal postoperative pain management. There is scarce data concerning the benefits of this anesthetic modality in laparoscopic weight loss surgery. Therefore, we analyzed the influence of trocar site infiltration with bupivacaine on the management of postoperative pain in laparoscopic bariatric surgery.
Methods
This retrospective randomized study included 47 patients undergoing primary obesity surgery between January and September 2014. Laparoscopic gastric bypass was performed in 39 cases and sleeve gastrectomy in 8 cases. Patients were stratified into two groups depending on whether preincisional infiltration with bupivacaine and epinephrine was performed (study group, 27 patients) or not (control group, 20 patients). Visual analogue scale (VAS), International Pain Outcomes questionnaire, and rescue medication records were reviewed to assess postoperative pain.
Results
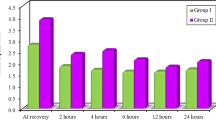
VAS scores in the study group and sleeve gastrectomy group were lower than those in the control and gastric bypass groups in the first 4 h postoperatively without reaching statistical significance (p > 0.05). VAS scores did not differ in any other period of time. No statistically significant differences in pain perception were registered according to the patient’s pain outcomes questionnaire or the need for rescue medication.
Conclusions
The present study did not conclusively prove the efficacy of bupivacaine infiltration by any of the three evaluation methods analyzed. Nevertheless, preincisional infiltration provides good level of comfort in the immediate postoperative period when analgesia is most urgent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgery for morbid obesity presents both surgeon and anesthesiologist a technical challenge. Physiologic responses to laparoscopic surgery, pneumoperitoneum, surgical complications, postoperative pain, and medical complications are some of the perioperative factors that affect recovery after bariatric surgery [1–4]. In this regard, pain after surgical procedures constitutes one of the most common causes of postoperative morbidity and renders the most important limitation for rehabilitation after a surgical intervention. Over the last decades, important advances in perioperative analgesia have been developed due to the evolution of laparoscopic surgery (less pain, shorter hospital stay, facilitation of multimodal rehabilitation programs) [3, 5] and novel strategies proposed to diminish postoperative pain (patient-controlled epidural analgesia, patient-controlled epidural analgesia, elastomeric pain pumps, etc.) [6–8]; thus, nowadays, a variety of inpatient analgesia protocols exist. Wound infiltration with local anesthetics proved to be a simple, effective, and inexpensive means of providing good analgesia in laparoscopic cholecystectomy, laparoscopic inguinal hernia repair, and gynecologic laparoscopic surgery [9–12]. However, few studies have investigated its role in major laparoscopic surgery [13]. To date, no study has investigated the benefits of wound infiltration in the setting of laparoscopic weight loss surgery. Therefore, the objective of the present study was to analyze the influence of trocar site infiltration with bupivacaine on the management of postoperative pain in laparoscopic bariatric surgery.
Materials and Methods
Forty-seven patients were included in this retrospective randomized study. All of them were operated consecutively in our hospital over a 9-month period (January 2014 to September 2014). Laparoscopic gastric bypass (YRGB) was performed in 39 cases and sleeve gastrectomy (SG) in 8 cases. Patients were stratified into two groups depending on whether trocar site infiltration with bupivacaine was performed (study group, 27 patients) or not (control group, 20 patients). The study was approved by the Ethics Committee of the hospital (CUN-BUP-2014-01).
Surgery was indicated following the National Institutes of Health Consensus Development Statement of 1991 [14] for patients with body mass index (BMI) ≥35 kg/m2 with obesity-related comorbidities or BMI >40 kg/m2. Data collected included demographic characteristics (age, gender, BMI, weight, waist circumference, and body fat percentage), preoperative comorbid metabolic conditions (type 2 diabetes mellitus, dyslipidemia, hypertension), and clinical outcomes (postoperative complications, mortality, readmissions) (Table 1).
Pain assessment was performed by three different methods:
-
1.
Visual analogue scale (VAS, 0: no pain, 10: the worst imaginable pain) [15]. Preoperatively, the nurse introduced the concept of VAS scale to the patients. VAS data were recorded four times on the first postoperative day (0–4, 4–8, 8–12, and 12–24 h) followed by one measurement on the second and third days.
-
2.
A Spanish version of the Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) questionnaire [16] was filled out by the patients on the first day of their hospital stay.
-
3.
The need for rescue medication was recorded in detail by experienced inpatient nursing staff.
Statistical Analysis
Parametric data were compared between the groups by analysis of variance (ANOVA). Nonparametric data were analyzed by chi-squared tests between the groups. Statistical significance was assumed if p < 0.05. The analysis was performed with the Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL, USA).
Operative Technique
Anesthetic and operative techniques were applied in all cases by the same bariatric team. General anesthesia included ramped head-up intubation avoiding long-acting opioids by using remifentanil and propofol followed by rocuronium and desflurane to maintain it. Volume-controlled ventilation was used together with high positive end-expiratory pressure (PEEP) (6–8 cm H2O). Multimodal analgesia included intravenous paracetamol, diclofenac, and metamizol. Benzodiazepines and corticosteroids were not utilized intraoperatively. In the study group, before the skin incision and trocar placement, local infiltration of the port sites was carried out throughout all layers with 40 ml 0.25 % bupivacaine and 1:200,000 epinephrine. Patients were placed in a 45° reverse Trendelenburg position to ensure optimal intraabdominal space with 10 mm and 45°-view endoscope. Pneumoperitoneum with warmed carbon dioxide was insufflated at a pressure of 14 mmHg, and five laparoscopic trocars were introduced in all cases positioned with one 12-mm trocar in the midline above umbilicus for the endoscope, two 12-mm trocars two to three fingerbreadths below the right and left costal margins in the midclavicular line, 12-mm trocar placed left to the umbilicus for the assistant surgeon and 5-mm trocar 2 cm below the xiphoid process for Nathanson liver retractor. Gastric bypass was performed in an antecolic-antegastric fashion (typically 60 cm biliopancreatic limb, 150–190 cm Roux limb length) using a 30 mm end-to-side linear stapled gastrojejunal anastomosis and 45 mm side-to-side jejunojejunal anastomosis technique. Sleeve gastrectomy was sized using a 34 F orogastric tube and staple line reinforcement with 3/0 absorbable running suture. Intraoperative air leak test was carried out with methylene blue and ephedrine was used at the end of the operation for its sympathomimetic effects in order to allow intraabdominal bleeding to be detected before trocar removal. Nasogastric tubes and urinary catheters were not routinely used and surgical drains were reserved for patients who were considered at increased postoperative risk of bleeding by the surgeon and the anesthesiologist. Patients typically received 1.5–2 l of crystalloid infusion intraoperatively.
Postoperative Management
Postoperative interventions followed hospital bariatric unit protocol based on mobilization within 4 h after the end of the surgical procedure, intermittent pneumatic compression boots, hourly use of incentive spirometry, and the administration of a regular multimodal analgesia protocol with intravenous paracetamol 1 g every 8 h and ketorolac 30 mg every 12 h, antiemetics (ondansetron 4 mg every 8 h) and antihypertensive urapidil to maintain blood pressure under 140 mmHg. Contrast swallow studies were not routinely performed, and oral liquid diet was started on the first postoperative day, advanced to semisolid diet on day 2.
Results
Baseline demographics, clinical characteristics, and comorbid disease state were similar between the groups exhibiting no statistical differences in the variables studied except for gender (Table 1). Operations were completed successfully without any intraoperative complications or major postoperative complications. The average duration of the operation was 130 min for gastric bypass and 100 min for sleeve gastrectomy. The average duration of hospital stay was 2.5 days (range 2–5). Length of stay did not differ significantly between the two groups or type of surgery. No toxic effect was associated with the local use of bupivacaine.
VAS scores in the study group and SG group were lower than those in the control group and YRGB group during the first 4 h postoperatively without reaching statistical significance (p > 0.05), whereas they did not differ in any other period of time (Figs. 1 and 2). During the first 4 h after surgery, morphine was used in 14 patients of the study group (52 %) and in 13 patients of the control group (63 %) with no difference in average morphine doses (p > 0.05). Similarly, there were no significant differences in the nursing data recorded for rescue medication in the remaining periods of time. The administration of rescue medication depended on the patient’s description of persistent pain despite the established analgesia.
Table 2 shows results of the APS-POQ-R questionnaire, including five aspects of outcome measurement in acute pain: pain severity, interference with function, affective experience, side effects, and perceptions of care. There were no significant differences between the groups in frequencies and means in any of the five aspects described, while a higher incidence of local itching at the incision sites (nonsignificant) was found in the study group, probably due to the infiltration of the skin causing distension and subsequent dysesthesia.
Discussion
Perioperative care of the obese patient requires a multidisciplinary team, in which surgeon and anesthesiologist play very important roles in preoperative assessment, as well as intraoperative anesthetic management and postoperative care. There is a lack of evidence proving the superiority of one pain treatment modality over others because analgesia for the same type of surgery might be more or less invasive, more or less effective and more or less expensive. Therefore, current approach to pain management is not determined in strict protocols; rather it is team-dependent.
Postoperative analgesia is a major component of perioperative care, and techniques involving the usage of local anesthetics are more effective than systemic analgesia regardless of the operation and mode of delivery [17]. When choosing a “procedure-specific” pain management technique, the simplest, safest, and most effective analgesia should be employed whenever possible [18]. Thus, the meticulous direct application of local anesthetics to each identifiable layer during a surgical procedure has considerable appeal both to the surgeon and the anesthesiologist [19]. Local anesthetic infiltration inhibits the voltage-gated sodium channels resulting in decreased excitability of nerves transmitting pain. It is a simple, effective, and inexpensive means of providing good analgesia for a variety of surgical procedures without any major side effects. This procedure can be performed prior to skin incision (at the beginning of surgery) or after skin incision (at the end of surgery) [20]. Wound infiltration should involve at least skin and subcutaneous tissue but can be performed until the parietal peritoneum [21]. In our study, all the patients undergoing laparoscopic weight loss surgery had infiltration with bupivacaine performed at the beginning and at the end of the operation, throughout all the incision layers including parietal peritoneum. We believe that this approach with a meticulous technique is most beneficial for the patient, as supported by previous published experiences in laparoscopic cholecystectomy [21].
Despite this, the present study did not conclusively prove the efficacy of bupivacaine by any of the three evaluation methods used. There were no statistically significant differences in pain perception by the patient (VAS and APS-POQ-R) or in the rescue medication amounts recorded.
Pain after laparoscopic surgery can arise from different locations: (1) trocar insertion sites, (2) visceral pain from intraabdominal trauma and distension of peritoneum with traumatic traction on blood vessels and nerves, and (3) from irritation of the phrenic nerve and release of inflammatory mediators. We assume that patients in the study group reported less pain in trocar insertion sites but overall VAS score, and patient perception was similar to the control group because of the predominance of visceral pain in the first 24 h.
In any case, as other authors [22–24], we strongly believe that local anesthetic administration is beneficial in the postoperative period by allowing patients to mobilize more quickly and reducing the possible initial difficulties that could hinder the realization of incentive spirometry, ensuring a safe and quick postoperative recovery. Moreover, it is the first four hours after surgery when VAS score was highest. Perception of pain decreased gradually for both operations and groups in the subsequent periods studied. Taking into account these results and considering the elimination half-life of bupivacaine (2.7 h), we also believe that infiltration with bupivacaine at the beginning and at the end of surgery plays an important role in multimodal pain management by providing analgesia when more pain is experienced by the patient.
Our study is not a prospective randomized study, and it is a limitation to draw strong evidence. Among studies investigating wound infiltration in laparoscopic weight loss surgery, no data have been published to date regarding this simple technique. There are numerous recent randomized studies of bariatric patients handled with other strategies proposed to diminish postoperative pain such as bupivacaine pump systems, intraoperative or continuous intraperitoneal bupivacaine application, systemic lidocaine, ultrasound-guided transversus abdominis plane block, etc. [13, 25–29]. These studies render different results, not always reproducible by other authors and expensive to apply in clinical practice in some cases. We believe that wound infiltration with local anesthetic is the easiest, simple, effective, and inexpensive means of providing good analgesia for bariatric patients without any major side effects; it is reproducible and can be standardized by other units.
In a recent review [30] of randomized controlled trials investigating analgesic regimens applied in surgery, three studies analyzing the administration of subcutaneous/subfascial or intraperitoneal local anesthetics in gastric bypass were found. Two studies investigated the administration of intraperitoneal bupivacaine. Symons et al. achieved reduction in postoperative oral narcotic use, with no differences in pain scores or outcome variables between the groups [26]. Alkhamesi et al., in contrast, reported lower pain scores in the treatment group, while maintaining similar levels of postoperative rescue medication use [27]. A study by Cottam et al. showed that continuous postoperative subfascial/subcutaneous infusion of bupivacaine via subcostal catheters allowed switching patient-controlled meperidine to oral analgesics quicker in the treatment group while maintaining similar pain scores as in the control group [13]. Andersen summarizes that local anesthetics might be effective for patients undergoing laparoscopic gastric bypass, but the conclusions of the recent studies in the field are severely limited by their heterogeneous quality and design [30].
One of the limitations of local anesthetics in the postoperative setting is their relatively short duration of action. In search of increased duration of action, multivesicular liposomes containing bupivacaine have been utilized [31]. Hu reports that liposome bupivacaine exhibits bimodal kinetics with rapid uptake observed during the first few hours and prolonged release through 96 h after administration [32]. There is already a case published of liposome bupivacaine used in laparoscopic sleeve gastrectomy with promising outcomes [33].
Conclusion
Concisely, wound infiltration with local anesthetics is a simple and inexpensive means of providing good analgesia for a variety of surgical procedures without any major side effects. A great challenge in weight loss surgery is to ensure good analgesia and both safe and rapid mobilization and recovery. Wound infiltration with local anesthetics offers a means of postoperative analgesia that is practically harmless, easily reproducible, and can well be standardized. Current evidence suggests that this strategy should be employed in bariatric patient population as part of a multimodal postoperative pain management.
References
Schumann R. Anaesthesia for bariatric surgery. Best Pract Res Clin Anaesthesiol. 2011;25(1):83–93.
Schumann R, Jones SB, Ortiz VE, et al. Best practice recommendations for anesthetic perioperative care and pain management in weight loss surgery. Obes Res. 2005;13(2):254–66.
Lamvu G, Zolnoun D, Boggess J, et al. Obesity: physiologic changes and challenges during laparoscopy. Am J Obstet Gynecol. 2004;191(2):669–74.
Levi D, Goodman ER, Patel M, et al. Critical care of the obese and bariatric surgical patient. Crit Care Clin. 2003;19(1):11–32.
Korolija D, Sauerland S, Wood-Dauphinee S, et al. Evaluation of quality of life after laparoscopic surgery: evidence-based guidelines of the european association for endoscopic surgery. Surg Endosc. 2004;18(6):879–97.
Kaye AD, Ali SI, Urman RD. Perioperative analgesia: ever-changing technology and pharmacology. Best Pract Res Clin Anaesthesiol. 2014;28(1):3–14.
Palmer PP, Royal MA, Miller RD. Novel delivery systems for postoperative analgesia. Best Pract Res Clin Anaesthesiol. 2014;28(1):81–90.
Banks A. Innovations in postoperative pain management: continuous infusion of local anesthetics. AORN J. 2007;85(5):904. 14; quiz 915–8.
Gupta A. Local anaesthesia for pain relief after laparoscopic cholecystectomy—a systematic review. Best Pract Res Clin Anaesthesiol. 2005;19(2):275–92.
Pavlidis TE, Atmatzidis KS, Papaziogas BT, et al. The effect of preincisional periportal infiltration with ropivacaine in pain relief after laparoscopic procedures: a prospective, randomized controlled trial. JSLS. 2003;7(4):305–10.
Gerges FJ, Kanazi GE, Jabbour-Khoury SI. Anesthesia for laparoscopy: a review. J Clin Anesth. 2006;18(1):67–78.
El Hachem L, Small E, Chung P, et al. Randomized controlled double-blind trial of transversus abdominis plane block versus trocar site infiltration in gynecologic laparoscopy. Am J Obstet Gynecol. 2014.
Cottam DR, Fisher B, Atkinson J, et al. A randomized trial of bupivicaine pain pumps to eliminate the need for patient controlled analgesia pumps in primary laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17(5):595–600.
NIH conference. gastrointestinal surgery for severe obesity. consensus development conference panel. Ann Intern Med. 1991;115(12):956–61.
Johnson C. Measuring pain visual analog scale versus numeric pain scale: what is the difference? J Chiropr Med. 2005;4(1):43–4.
Gordon DB, Polomano RC, Pellino TA, et al. Revised american pain society patient outcome questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172–86.
Wu CL, Cohen SR, Richman JM, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology. 2005;103(5):1079,88; quiz 1109–10.
Kehlet H, Liu SS. Continuous local anesthetic wound infusion to improve postoperative outcome: back to the periphery? Anesthesiology. 2007;107(3):369–71.
Scott NB. Wound infiltration for surgery. Anaesthesia. 2010;65 Suppl 1:67–75.
Coughlin SM, Karanicolas PJ, Emmerton-Coughlin HM, et al. Better late than never? Impact of local analgesia timing on postoperative pain in laparoscopic surgery: a systematic review and metaanalysis. Surg Endosc. 2010;24(12):3167–76.
Lee IO, Kim SH, Kong MH, et al. Pain after laparoscopic cholecystectomy: the effect and timing of incisional and intraperitoneal bupivacaine. Can J Anaesth. 2001;48(6):545–50.
Mullender A, Melichar G, Schmucker P, et al. Psychological traits, course of surgery and recovery following hernia repair in patients preferring general or local anaesthesia. Anaesthesist. 2006;55(3):247–54.
Anantanarayanan P, Raja DK, Kumar JN, et al. Catheter-based donor site analgesia after rib grafting: a prospective, randomized, double-blinded clinical trial comparing ropivacaine and bupivacaine. J Oral Maxillofac Surg. 2013;71(1):29–34.
Lklouk M, Ismail K. Intra-operative fentanyl gives superior pain relief for patients undergoing laparoscopic bariatric surgery when compared to morphine: 14AP4-6. Eur J Anaesthesiol. 2014;31:225.
Sherwinter DA, Ghaznavi AM, Spinner D, et al. Continuous infusion of intraperitoneal bupivacaine after laparoscopic surgery: a randomized controlled trial. Obes Surg. 2008;18(12):1581–6.
Symons JL, Kemmeter PR, Davis AT, et al. A double-blinded, prospective randomized controlled trial of intraperitoneal bupivacaine in laparoscopic roux-en-Y gastric bypass. J Am Coll Surg. 2007;204(3):392–8.
Alkhamesi NA, Kane JM, Guske PJ, et al. Intraperitoneal aerosolization of bupivacaine is a safe and effective method in controlling postoperative pain in laparoscopic Roux-en-Y gastric bypass. J Pain Res. 2008;1:9–13.
De Oliveira GS, Duncan Jr K, Fitzgerald P, et al. Systemic lidocaine to improve quality of recovery after laparoscopic bariatric surgery: a randomized double-blinded placebo-controlled trial. Obes Surg. 2014;24(2):212–8.
Sinha A, Jayaraman L, Punhani D. Efficacy of ultrasound-guided transversus abdominis plane block after laparoscopic bariatric surgery: a double blind, randomized, controlled study. Obes Surg. 2013;23(4):548–53.
Andersen LP, Werner MU, Rosenberg J, et al. Analgesic treatment in laparoscopic gastric bypass surgery: a systematic review of randomized trials. Obes Surg. 2014;24(3):462–70.
Tong YC, Kaye AD, Urman RD. Liposomal bupivacaine and clinical outcomes. Best Pract Res Clin Anaesthesiol. 2014;28(1):15–27.
Hu D, Onel E, Singla N, et al. Pharmacokinetic profile of liposome bupivacaine injection following a single administration at the surgical site. Clin Drug Investig. 2013;33(2):109–15.
Bertin PM. Liposome bupivacaine for postsurgical pain in an obese woman with chronic pain undergoing laparoscopic gastrectomy: a case report. J Med Case Rep. 2014;8:21,1947-8-21.
Conflict of Interest
The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed Consent
Does not apply.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was performed in the Department of Surgery, Bariatric and Metabolic Surgery, Clínica Universidad de Navarra, Avenida Pío XII, 36, 31008 Pamplona, Navarra, Spain
Rights and permissions
About this article
Cite this article
Moncada, R., Martinaitis, L., Landecho, M. et al. Does Preincisional Infiltration with Bupivacaine Reduce Postoperative Pain in Laparoscopic Bariatric Surgery?. OBES SURG 26, 282–288 (2016). https://doi.org/10.1007/s11695-015-1761-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1761-0