Abstract
Background
A standard approach for postoperative analgesia in laparoscopic surgery is to infiltrate the incisions with local anesthetic in combination with systemic opioids. The intraperitoneal introduction of local anesthetic in this setting has the potential to provide appropriate analgesia without the side effects of systemic opioids. We performed a randomized clinical trial of the On-Q pump delivery system to determine the safety and efficacy of this device for this novel purpose.
Methods
Thirty patients undergoing laparoscopic adjustable gastric banding were randomly assigned to one of two groups. The treatment group received On-Q pump systems filled with 0.375% bupivacaine, while the control group received pumps filled with 0.9% normal saline. The pump’s catheter was introduced intraperitoneally, and bupivacaine or saline was then delivered for the first 48 h after surgery. Patient’s subjective pain scores were evaluated at preset intervals. In addition, shoulder pain, morphine requirements, and anti-emetic requirements were tabulated.
Results
A statistically significant decrease in patient’s subjective reports of pain by visual analog score was noted in the On-Q group 1.8 ± 1.93 vs. control 3.5 ± 2.4, p < 0.046 and remained significant until the end of the study (48 h). No statistical difference was noted in shoulder pain, morphine requirements, or anti-emetic requirements at any time point.
Conclusion
Our trial was able to provide evidence of significant reduction in postoperative pain as measured by subjective pain scores with the use of continuous intraperitoneal bupivacaine using the On-Q pain pump system. Further investigation is warranted to evaluate the cost effectiveness of this technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Postoperative pain control, even in this age of minimally invasive surgery, is considered by many to be inadequate. A standard approach has been to use a local long-acting anesthetic at the trocar sites in combination with systemic opioids. Although subcutaneous local anesthetics have been shown to diminish pain scores and the use of opioid supplemental analgesics [1, 2], their duration is relatively brief (4–6 h). Furthermore, they do little to control shoulder, subscapular, and generalized visceral pain [3]. On the other hand, systemic opioid analgesics are often associated with insufficient pain control, drowsiness, postoperative nausea and vomiting (PONV), ileus (POI), dry mouth, urinary retention, and pruritus. Inadequate pain control leads to delayed return to full activities and reduces patient satisfaction.
The intraperitoneal introduction of local anesthetic would seem to be an ideal pain control option in laparoscopic surgery. By blocking visceral nociceptive signals, intraperitoneal bupivacaine (IPB) has the potential of producing long-lasting analgesia without the side effects of systemic opioids. Unfortunately, the literature in this area is conflicted: reports of varying routes, dosages, and sites of intraperitoneal injection in a myriad of different procedures has demonstrated inconsistent results [4–12].
The On-Q PainBuster™ (I-Flow Corporation, Lake Forest, CA, USA) consists of an elastomeric bulb containing local anesthetic. The bulb is coupled to a catheter via a capillary flow-restricting orifice that maintains a constant soaking effect at the catheter tip. The On-Q system can run for up to 5 days and has been shown to decrease opioid use, patient’s perception of the level of pain, and length of hospital stay when used in a preperitoneal position to block somatosensory nerve fibers [13].
Although there are no reports in the medical literature of using the On-Q system for IPB, this device has all the characteristics that would make it the ideal delivery vehicle for this purpose. We performed a prospective, randomized, double-blind, placebo-controlled study of patients undergoing laparoscopic adjustable gastric banding (LAGB) to test the hypothesis that IPB via the On-Q system would be associated with significant reduction in pain scores, referred pain, and the need for opioids and antiemetics after surgery.
Materials and Methods
Study Design
The research protocol for this prospective, randomized, double-blind controlled clinical trial was approved by the Maimonides Medical Center institutional review board. Written informed consent was obtained from all patients enrolling in the study. This study was registered with clinicaltrials.gov identifier # NCT00533845.
Eligibility and Randomization
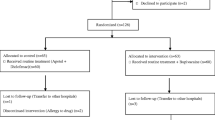
All patients fulfilled the National Institute of Health (NIH) criteria for bariatric surgery and successfully completed extensive preoperative screening and counseling as part of the routine Maimonides Bariatric Center protocol. Additional inclusion criteria were patients between the ages of 18 and 65 and an American Society of Anesthesiologists score of equal to or less than 3. Any patient allergic to bupivacaine, morphine, ondansetron, or ketorolac was excluded. Of the 32 consecutive patients screened, 30 patients gave consent to take part in the study (Fig. 1).
Patients were randomly assigned to either the study group or the control group. The study group (group 1) underwent LAGB with insertion of an intraperitoneal On-Q pump containing 0.375% bupivacaine. The control group (group 2) underwent LAGB with insertion of an intraperitoneal On-Q pump containing 0.9% normal saline. The surgeon, the patient, and the examiner were blinded as to the contents of the pump.
Complications
One patient was excluded from analysis due to dysphagia requiring urgent re-operation and band replacement with a larger band. Subsequently, the patient was not re-enrolled into the study. No complications related to bupivacaine or the On-Q system were noted throughout the study period.
Treatment and Surgical Technique
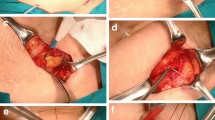
All procedures were performed using a standard technique by one of three experienced bariatric surgeons (DS, JM, HA) [14]. In addition, a dedicated bariatric anesthesia team using a standardized protocol was employed (Table 1). No preemptive morphine was given to patients. Patients at high risk for PONV were given dexamethasone 4 mg and metoclopramide 10 mg. Following Lap-Band (Inamed, Santa Barbara CA, USA) placement, the On-Q infusion catheter was introduced intraperitoneally and placed under laparoscopic guidance adjacent to the site of maximal dissection (Fig. 2). Prior to insertion, the On-Q soaker catheter was separately flushed with 0.9% normal saline. The Lap-Band port was tacked to the fascia on a mesh platform [15]. A total of 20 ml of 0.5% bupivacaine was injected subcutaneously for port site incisional analgesia prior to skin closure. Postoperative analgesia was given in the form of IV ketorolac every 6 h and IV morphine as needed for pain. All On-Q pumps were removed at 48 h postoperatively.
Intraperitoneal bupivacaine was administered via the On-Q system at 7.5 mg/h for a daily dosage of 180 mg and a total of 360 mg over the 48-h study period (0.375% bupivacaine at 2 ml/h for 48 h).
Outcome Measures
Our primary endpoint was postoperative pain as measured by pain scores on a visual analog scale (VAS) as well as total postoperative morphine utilization. Patients subjectively rated their pain from 0 to 10, with 0 being no pain and 10 the worst pain they had ever experienced. The VAS was measured while the patient was at rest (VAS-R) and during cough (VAS-C). Morphine usage was tracked by computer documentation for morphine dispensing. Other variables including the presence of shoulder tip pain, PONV, and the need for antiemetic medication were also recorded. All measurements were obtained at 30 min and 6, 12, 24, and 48 h postoperatively by a blinded investigator.
Sample Size and Power Calculation
Using a combination of historical data from similar studies, as well as a power calculation, we determined that we would recruit 15 patients in each group [16]. Our primary assumption was that using a two-tailed unpaired t test with a probability of a type I error (α) of 0.05 and an assumed SD of ±10 mg in mean morphine utilization at 48 h, we would have an 80% power to detect a difference of approximately 11 mg of morphine between the two groups—a clinically significant result. The power calculation was performed using a commercial software package (StatMate2, GraphPad Software, San Diego, CA, USA).
Statistical Analysis
Comparisons between groups were made with an unpaired Student’s t test unless otherwise specified. An F test was performed to determine whether the assumption of equal standard deviations were found to be unequal, then the more conservative Welch Student’s t test was used. When comparing percentages between groups, we used Fischer exact test. A Mann–Whitney rank sum test was performed when a nonparametric data analysis was required. We considered p < 0.05 to be significant. Data were presented as the mean ± SD unless otherwise specified. All the analysis was performed using a commercial software package (InStat, GraphPad Software, San Diego, CA, USA).
Results
Baseline Demographics and Operating Room Times
Patients in both groups were well matched with regards to age, gender, body mass index (BMI), and presence of medical comorbidities (Table 2). There was also no significant difference in mean operating room time, estimated blood loss, or preoperative baseline VAS between the two groups.
Postoperative Pain
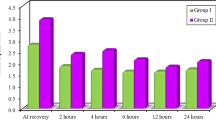
Beginning at 6 h time period, the VAS was lower in the On-Q group (group 1, 1.8 ± 1.93 vs. group 2, 3.5 ± 2.4, p < 0.046) and remained significant until the end of the study (48 h; group 1, 0.93 ± 1.4 vs. group 2, 3.0 ± 2.39; p = 0.0091) (Fig. 3). Similar data were seen with VAS-C, again with the scores becoming significantly different at 6 h (group 1, 2.6 ± 1.86 vs. group 2, 4.6 ± 2.59; p = 0.0280) and remaining statistically separated at 48 h (group 1, 1.7 ± 1.94 vs. group 2, 3.9 ± 2.88; p = 0.0266; Fig. 4). The difference between groups was most marked at 24 h (VAS-R, p < 0.0072; VAS-C, p < .0022).
Although not statistically significant, there was a trend towards decreased patient reporting of shoulder-tip pain in the On-Q group (Fig. 5). The total requirement for morphine used in 48 h in the bupavicaine group was 8.21 ± 6.9 and 9.00 ± 10.03 mg in the control group. These values as well as anti-emetics use and subjective complaints of nausea over the 48-h study period revealed no statistically significant differences between the two groups.
Discussion
We have successfully demonstrated—in a randomized clinical trial—that IPB delivered in a continuous fashion via the On-Q pump can provide safe and effective postoperative analgesia in patients undergoing laparoscopic surgery. In addition, we were also able to document both a clinically and statistically significant improvement in visual analog pain scores—both with and without cough.
Although many methods of IPB administration have been investigated, this study is the first to use a truly continuous IPB infusion technique. The On-Q system is a US Food and Drug Administration-approved device that is simple to place and requires no patient intervention. In addition, it is portable and discrete, making it an excellent choice for laparoscopic surgery in general and ambulatory procedures in particular.
Interestingly, our study did not provide evidence of decreased use of systemic opioids or the incidence of referred shoulder pain. There are multiple possible explanations for these phenomena. First, patient-controlled analgesia (PCA) pumps were not utilized for delivery of systemic opioids in this study; our nursing analgesia protocol may have allowed for patients to receive opioids when they might not have received them had they been using a PCA. Potential confounders such as habits in medication administration, nursing schedules, and patient’s reluctance to request medications may have influenced total morphine usage. Other authors have also described this phenomenon [17–19]. In future studies, we would utilize a PCA to help decrease this heterogeneity. Second, the potential exists that our study may have been underpowered to detect a significant difference in these outcomes (vide supra).
Cunniffe et al. [20] examined a variety of laparoscopic procedures and demonstrated a reduction of shoulder tip pain after irrigation of each dome of the diaphragm with 500 ml of bupivacaine irrigation. Our data did not reproduce these results. Devised for continuous infusion, our study employed a smaller volume and dosage of bupivacaine, which may have led to this lack of difference [21]. In addition, in this trial, IPB did not start until the postoperative period. Some studies have suggested that the ideal IPB timing for prevention of shoulder tip pain is at pneumoperitoneum initiation [22].
In conclusion, we have shown that the use of IPB via the On-Q system is a safe and effective method of providing postoperative analgesia for patients undergoing LAGB, with an associated statistically and clinically significant decrease in subjective measures of pain after surgery. Further future investigation is warranted to evaluate this technique’s effect on morbidity, opioid utilization, and overall cost.
References
Dahl JB, Moiniche S, Kehlet H. Wound infiltration with local anaesthetics for postoperative pain relief. Acta Anaesthsiol Scand 1994;38:7–14.
Moiniche S, Mikkelsen S, Wetterslev J, Dahl JB. A qualitative systemic review of incisional local anaesthesia for postoperative pain relief after abdominal surgery. Br J Anaesth 1998;81:377–83.
Ure BM, Troidl H, Spangenberger W, et al. Pain after laparoscopic cholecystectomy. Surg Endosc 1994;8:90–6.
Wallin G, Cassuto J, Högström S, Hedner T. Influence of intraperitoneal anesthesia on pain and the sympathoadrenal response to abdominal surgery. Acta Anaesthesiol Scand 1988;32(7):553–8.
Elfberg BA, Sjövall-Mjöberg S. Intraperitoneal bupivacaine does not effectively reduce pain after laparoscopic cholecystectomy: a randomized, placebo-controlled and double-blind study. Surg Laparosc Endosc Percutan Tech 2000;10(6):357–9.
Keita H, Benifla JL, Le Bouar V, Porcher R, Wachowska B, Bedairia K, Mantz J, Desmonts JM. Prophylactic ip injection of bupivacaine and/or morphine does not improve postoperative analgesia after laparoscopic gynecologic surgery. Can J Anaesth 2003;50(4):362–7.
Mraović B, Jurisić T, Kogler-Majeric V, Sustic A. Intraperitoneal bupivacaine for analgesia after laparoscopic cholecystectomy. Acta Anaesthesiol Scand 1997;41(2):193–6.
Szem JW, Hydo L, Barie PS. A double-blinded evaluation of intraperitoneal bupivacaine vs saline for the reduction of postoperative pain and nausea after laparoscopic cholecystectomy. Surg Endosc 1996;10(1):44–8.
Symons JL, Kemmeter PR, Davis AT, Foote JA, Baker RS, Bettendorf MJ, Paulson JE. A double-blinded, prospective randomized controlled trial of intraperitoneal bupivacaine in laparos copic Roux-en-Y gastric bypass. J Am Coll Surg 2007;204(3):392–8.
Gupta A, Thörn SE, Axelsson K, Larsson LG, Agren G, Holmström B, Rawal N. Postoperative pain relief using intermittent injections of 0.5% ropivacaine through a catheter after laparoscopic cholecystectomy. Anesth Analg 2002;95(2):450–6.
Ozer Y, Tanriverdi HA, Ozkocak I, Altunkaya H, Demirel CB, Bayar U, Barut A. Evaluation of a local anaesthesia regimen using a subphrenic catheter after gynaecological laparoscopy. Eur J Anaesthesiol 2005;22(6):442–6.
Alkhamesi NA, Peck DH, Lomax D, Darzi AW. Intraperitoneal aerosolization of bupivacaine reduces postoperative pain in laparoscopic surgery: a randomized prospective controlled double-blinded clinical trial. Surg Endosc 2007;21(4):602–6.
Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. JACS 2006;203:6.
Ren CJ, Fielding GA. Laparoscopic adjustable gastric banding: surgical technique. J Laparoendosc Adv Surg Tech 2003;13(4):257–63.
Geiss AC, Katz L. The use of a stabilizing mesh platform and ultrasound adjustment of the Lap Band. Plenary session presentation. ASBS nineteenth annual meeting. 2003.
Savel RH, Balasubramanya S, Lasheen S, Gaprindashvili T, Arabov E, Fazylov RM, Lazzaro RS, Macura JM. Beneficial effects of humidified, warmed carbon dioxide insufflation during laparoscopic bariatric surgery: a randomized clinical trial. Obes Surg 2005;15(1):64–9.
Szem JW, Hydo L, Barie PS. A double-blinded evaluation of intraperitoneal bupivacaine vs saline for the reduction of postoperative pain and nausea after laparoscopic cholecystectomy. Surg Endosc 1996;10:44–8.
Jiranantarat V, Rushatamukayanunt W, Lert-akyamanee N, et al. Analgesic effect of intraperitoneal instillation of bupivacaine for postoperative laparoscopic cholecystectomy. J Med Assoc Thai 2002;85(Suppl 3):S897–903.
Joris J, Thiry E, Paris P, Weerts J, Lamy M. Pain after laparoscopic cholecystectomy: characteristics and effect of intraperitoneal bupivacaine. Anesth Analg 1995;81:379–84.
Cunniffe MG, McAnena OJ, Dar MA, Calleary J, Flynn N. A prospective randomized trial of intraoperative bupivacaine irrigation for management of shoulder-tip pain following laparoscopy. Am J Surg 1998;176:258–61.
Tsimoyiannis EC, Siakis P, Tassis A, Lekkas ET, Tzourou H, Kambili M. Intraperitoneal normal saline infusion for postoperative pain after laparoscopic cholecystectomy. World J Surg 1998;22:824–8.
Pasqualucci A, de Angelis V, Contardo R, Colò F, Terrosu G, Donini A, Pasetto A, Bresadola F. Preemptive analgesia: intraperitoneal local anesthetic in laparoscopic cholecystectomy. A randomized, double-blind, placebo-controlled study. Anesthesiology 1996;85(1):11–20.
Acknowledgement
The authors wish to thank I-Flow Corporation for donating the On-Q pump device for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sherwinter, D.A., Ghaznavi, A.M., Spinner, D. et al. Continuous Infusion of Intraperitoneal Bupivacaine after Laparoscopic Surgery: A Randomized Controlled Trial. OBES SURG 18, 1581–1586 (2008). https://doi.org/10.1007/s11695-008-9628-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-008-9628-2