Abstract
Introduction
Obesity is one of the greatest health problems. Bariatric surgery is more effective than non-surgical options; however, postoperative pain is bound to a greater morbidity. Control of postoperative pain is important in facilitating patient convalescence. In this study, we assessed the efficacy of intraperitoneal instillation of bupivacaine after bariatric surgery.
Methods
A hundred patients who underwent bariatric procedures including sleeve gastrectomy, sleeve gastrectomy with cardioplasty, gastric bypass, and gastric mini bypass (one anastomosis gastric bypass) were included in the study. Patients were divided into two groups randomly, 50 patients for each; group I had intraperitoneal instillation of 40 ml bupivacaine 0.25% at the end of the procedure, while group II had normal saline instillation. Monitoring of pain control in the first 24 h after surgery was done using the visual analogue scale (VAS) to assess the efficacy of intraperitoneal bupivacaine instillation and its effect on the overall opioid usage, postoperative nausea and vomiting (PONV), and shoulder tip pain.
Results
Pain scores were significantly lower in group I compared to group II at recovery, 2, 4 and 6 h after surgery, P = 0.004, 0.001, < 0.001, and 0.001 respectively. However, there were no significant differences between 12 and 24 h postoperatively. Additionally, there was a significant difference regarding the need for rescue analgesia at recovery P = < 0.001*. Further analysis revealed lower morphine consumption via PCA in group I compared to group II P = 0.013*. There were no significant differences with the use of intraperitoneal bupivacaine as regards nausea, vomiting, or shoulder tip pain, P = 0.688, 0.249, and 0.487, respectively.
Conclusions
Intraperitoneal instillation of bupivacaine provides a good analgesia in the early postoperative period, reduces the overall consumption of opioid, and decreases the rescue analgesia requirement in the first 24 h after surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity has become one of the fastest-growing and greatest health problems in both developed and developing countries [1]. It is associated with reduced life expectancy, increased morbidity and mortality, and greater healthcare costs [2, 3]. Bariatric surgery is more effective than non-surgical options with a reduction in mortality of 30% demonstrated in surgical recipients [4,5,6].
In minimally accessed surgery, most of the patients experience discomfort in the form of generalized abdominal pain, PONV, and shoulder tip pain. However, uncontrolled postoperative pain has adverse consequences of delayed resumption of normal pulmonary function, restriction of mobility (thus contributing to thromboembolic complications), nausea and vomiting, increase in the systemic vascular resistance, cardiac work, and myocardial oxygen consumption through an increase in the catecholamine release induced by the stress response. [7, 8]
Control of acute postoperative pain is important in facilitating short and long-term patient convalescence. [9] Pain usually occurs on the first day following surgery and it may be a visceral, parietal, or shoulder tip pain. The latter is due to intraperitoneal insufflation of gases like CO2 which stretch the abdominal tissues, in addition to diaphragmatic irritation caused by residual carbon dioxide in the peritoneal cavity.
According to the presumed mechanism, visceral and shoulder tip pain can theoretically be blocked by intraperitoneal instillation, and parietal pain can be blocked by port site infiltration [10,11,12,13].
In this study, we assessed the efficacy of intraperitoneal instillation of long-acting local anesthetic, bupivacaine after bariatric surgery. Local anesthetic agents are widely used, have a good safety profile, and are available in long-acting preparations.
They provide the benefit of anesthesia without the systemic side effects that may result from the use of enterally or parenterally administered drugs. Bupivacaine has a half-life of 2.7 to 3.5 h and has been reported to provide pain control for an average of 6 h [14].
Methods
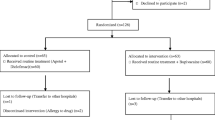
A hundred patients who underwent bariatric procedures including sleeve gastrectomy, sleeve gastrectomy with cardioplasty, gastric bypass, and gastric mini bypass (single anastomosis bypass) in King Hamad University Hospital (KHUH) during the period from July to December 2018 were included in the study. Patients were divided into two groups randomly, 50 patients for each group; the first group had intraperitoneal long-acting local anesthetic instillation, while the second group received placebo in the form of normal saline instillation.
Inclusion Criteria
Patients undergoing bariatric surgical procedures in KHUH who meet the known criteria for bariatric procedures (BMI > 40 or 35 > with comorbidities, obesity for more than 5 years with all efforts to reduce weight failing, etc.).
Exclusion Criteria
-
1-
Sickle cell disease or trait.
-
2-
Cardiac patients.
-
3-
Patients with known allergy to bupivacaine.
-
4-
Prolonged administration of NSAIDS or other analgesics due to chronic pain of any reason.
-
5-
Cirrhosis.
After approval from the Research and Ethics Committee, King Hamad University Hospital. Ref. KHUH/Research/No.234/2018. A randomized controlled double-blinded trial was conducted where a prospective review of pain control in the first 24 h after surgery was done to assess the efficacy of intraperitoneal long-acting local anesthetic instillation on pain control and its effect on the overall opioid use, nausea, vomiting, and shoulder tip pain, in this specific patient group undergoing bariatric surgery.
Management Protocol
The patients were managed according to the following protocol:
Anesthesia Protocol
-
Premedication: metoclopromide 10 mg IV, sodium citrate 30 ml oral, ranitidine 150 mg oral
-
Induction: propofol 2 mg/kg, fentanyl 2 microgram/kg, cisatracurium 0.2 mg/kg, ondansetron 4 mg, dexamethasone 8 mg.
-
Maintenance: desflourane 6%
-
Reversal: neostigmine 2.5 mg + glycopyrrolate 0.5 mg
-
Analgesia: morphine 5 mg + perfalgan 1 g.
-
For sleeve gastrectomy, staple line reinforcement was done by suturing the omentum to the sleeved stomach with plication of the staple line.
-
The first group (group I) had intraperitoneal long-acting local anesthetic instillation (40 ml bupivacaine 0.25%) through the Veress needle or trocar. This solution was instilled in the subdiaphragmatic space in every case and the patients were kept in Trendelenburg’s position for 5 min.
-
The second group (group II) received 40 ml of normal saline and was kept in Trendelenburg’s position for 5 min.
-
Both groups had port site injection of bupivacaine 20 ml 0.25% and received regular paracetamol 1 g intravenously every 6 h as baseline analgesia in addition to patient-controlled analgesia (PCA).
-
Pain score for all patients was measured at recovery then at 2, 4, 6, 12, and 24 h after surgery. This was performed using the visual analogue scale (VAS) consisting of 10-cm scale showing 0 to 10 cm marks. Zero depicted no pain and 10-cm mark indicated the worst pain.
-
PONV and shoulder tip pain were assessed and compared between the two groups. The occurrence of nausea and/or vomiting, shoulder tip pain, and pain scores were assessed by the ward nurses who were not informed to which group the patient belonged. Patients who experienced nausea or vomiting were given ondansetron (4 mg) IV STAT.
-
Any patient from either group with pain (VAS ≥ 6) received rescue analgesia in the form of tramadol 50 mg intravenously STAT dose.
-
The overall amount of analgesics used in the 24 h after surgery was calculated for every patient, including the amount used via PCA.
Data Management and Analysis Plan
A comparison was held between the two groups as regards the amount of opioids used to control the pain, the need for rescue analgesia, the occurrence of nausea, vomiting, and shoulder tip pain. Further comparison was held between patients in the same group to test the effect of gender, BMI, type of surgery and diabetes on the pain scores, opioid consumption, and need for rescue analgesia.
Continuous variables were analyzed with Student t test and categorical variables were analyzed with the chi-square test and Fisher exact test. Statistical significance was taken as P < 0.05. The data was analyzed using SPSS version 22 and Microsoft Excel 2013.
Results
Group I included 50 patients, 16 males and 34 females, with age range from 17 to 65 (mean ± SD 34.14 ± 13.0 and median 30.5). BMI range was from 35 to 68.17 kg/m2 (mean ± SD 44.18 ± 7.11 and median 43.44). Nine of group I were diabetic.
Group II included 50 patients, 17 males and 33 females, with age range from 17 to 61 (mean ± SD 34.14 ± 13.0 and median 32.5). BMI range was from 35.0 to 73.15 kg/m2 (mean ± SD 45.99 ± 8.37 and median 44.05). Eight of group II were diabetic. Procedures are illustrated in Table 1. Operative time varied according to the procedure involved where we included different kinds of procedures. However, for group I, the mean operative time was 62 ± 11 and the median was 60 min. In group II the mean was 61 ± 12 and median was 55 min.
Pain scores were significantly lower in group I (Table 2) compared to group II at recovery, 2, 4, and 6 h after surgery, P = 0.004, 0.001, < 0.001, and 0.001 respectively (Fig. 1). However, there were no significant differences at 12 and 24 h postoperatively P = 0.154 and 0.103, respectively.
Additionally, there was a significant difference between the two studied groups regarding the need for rescue analgesia at recovery as shown in Table 3. Furthermore, the analysis revealed lower morphine consumption via PCA in group I compared to group II, P = 0.013* (Table 4).
Postoperative nausea and vomiting were monitored during the 24 h following surgery, as well as shoulder tip pain. There were no significant differences with the use of intraperitoneal bupivacaine as regards nausea, vomiting, or shoulder tip pain, P = 0.688, 0.249, and 0.487, respectively.
Nausea was reported in 22 patients in group I and 24 patients in group II, while 5 patients from group I had vomiting and 9 from group II. Shoulder tip pain was noted in 3 patients from group I and 6 patients from group II.
Further analysis of data revealed a significant difference between pain scores at recovery and the following scores at 2, 4, 6, 12, and 24 h after surgery within each group (Fig. 2). Only one exception was noticed in group I, where there was no significant difference between pain scores at recovery and scores at 2 h postoperative (Table 5).
Regarding the types of surgery performed, we noticed a slight increase in rescue analgesia requirement and the total amount of the opioid used via PCA in patients who underwent cardioplasty or hiatal hernia repair alongside their weight loss procedures in both groups, however the figures were below the statistical significance, P = 0.351 and 0.054 for group I and P = 0.395 and 0.066 for group II respectively. On the other hand, the type of surgery was not reflected on the pain scores recorded in the 24 h following surgery in both groups. Other variables tested like age, gender, BMI, and diabetic status had no positive correlation with pain scores or analgesia requirements.
Discussion
Postoperative pain after laparoscopic surgeries might reduce the great advantage of laparoscopy as a minimally invasive surgical approach. Given the expanding role of ambulatory surgery and the need to facilitate an earlier hospital discharge, postoperative pain control has become an integral part of any surgical protocol. Furthermore, an effective pain control encourages early ambulation, which significantly reduces the risk of deep vein thrombosis and pulmonary emboli (PE), enhances patient’s ability to take deep breaths to decrease the risk of pulmonary complications (e.g., atelectasis and pneumonia), and decreases the incidence of tachycardia and unnecessary related investigations. Pain management is particularly relevant in the obese population given their higher susceptibility for serious perioperative complications from cardiovascular, thromboembolic, and pulmonary events. These include a high prevalence of obstructive sleep apnea, hypoxia, respiratory depression, and PE, which is the second leading cause of death among bariatric surgery patients [15].
There is still no definite consensus as which technique is superior, but including one of the methods in addition to providing the patient with parenteral and enteral drugs could provide better postsurgical analgesia. Of these, trocar site injection of local anesthetic, transversus abdominis plane block, and administration of IP anesthetics have been proven to be effective adjuncts for pain management [16,17,18,19,20].
Among the various techniques, the benefits of peritoneal local anesthetic have been well documented [17, 18, 21] Despite that, there is still a paucity of studies that have specifically focused on the use of IPLA in the bariatric surgery population.
In our study, we used bupivacaine due to its cheap price and availability. We used 40 ml of 0.25% bupivacaine (100 mg) to achieve an acceptable efficacy within the safe limits to avoid the side effects [22]. Malhotra et al. [23] found the analgesic effect of intraperitoneal instillation of bupivacaine was dose-dependent. Moreover, a review by Mitra et al. pointed out that, larger volumes of local anesthetic solution led to a better pain control than smaller volumes. Also, higher concentrations of the local anesthetic such as 0.25 or 0.5% of ropivacaine or bupivacaine may have a better analgesic effect [24]. Also, we kept the patients in Trendelenburg’s position to prolong the contact time with the diaphragm and surgical sites (stomach and stapler lines).
Our results showed that IP instillation of bupivacaine led to a significant pain control over the first 6 h after surgery, an effect which faded with time as indicated with pain scores at 12 and 24 h postoperatively. This pattern is consistent with the duration of action of bupivacaine.
Additionally, the efficiency of pain control was reflected on the requirement of rescue analgesia and the total consumption of opioid, where there was a significant reduction in the need for rescue analgesia in the group which received IP bupivacaine, as well as the total consumption of morphine via the PCA.
This goes with the literature, as indicated by many meta-analyses. In a systematic review involving five randomized trials of laparoscopic gastric procedures, intraperitoneal LA was shown to decrease both abdominal and shoulder pain [25]. Another meta-analysis of seven randomized controlled trials comparing pain scores after intraperitoneal analgesic with placebo during gynecological laparoscopic surgery indicated that the pain was significantly reduced in the first 6 h after surgery [26].
Other similar studies which were specifically verifying the efficacy of IP local anesthetic instillation in bariatric surgical procedures indicated similar results. Ruiz-Tovar J et al. found that, the intraoperative peritoneal infusion with ropivacaine in patients undergoing bariatric surgery was associated with a reduction in postoperative pain, lower morphine needs, earlier mobilization, earlier oral intake of fluids after surgery, and a shorter hospital stay [27].
In another review [28] of 289 patients who underwent RYGB (Roux-en-Y gastric bypass), the treatment group received a continuous infusion of 0.375% bupivacaine administered by an intraperitoneal catheter for 48 h via an infusion pump, while the control group did not receive a pump or local anesthetic.
Morphine equivalents over the postoperative time period studied were significantly lower in the bupivacaine group than the control group (133 vs 106 mg, respectively; P = 0.001). However, there was no significant difference in VAS scores between the two groups (P = 0.80). Finally, the length of hospitalization between the two groups did not differ (P = 0.77).
Although some studies which examined the effect of IP instillation of local anesthetic on postoperative nausea, vomiting, and shoulder tip pain, found a significant reduction in these variables with IP instillation of local anesthetic [25, 29, 30] we did not reproduce the same. This in part might be due to the nature of the procedures included which comprised manipulation of the stomach and small bowel, a fact which led to nausea in many cases within the treatment and control groups, 44% and 48% respectively. Vomiting was reported in 5 patients in group I and 9 patients in group II, again the figures are under statistical significance P = 0.249. Regarding shoulder tip pain, it was only noticed in 3 patients in group I and 6 patients in group II, P = 0.487, this could be attributed to the proper desufflation of pneumoperitoneum after completion of surgery in all patients which led to a reduced incidence of diaphragmatic irritation by the residual CO2.
Study Limitations
-
The procedures included were heterogeneous, including sleeve gastrectomy, gastric bypass, sleeve with hiatal hernia repair, and some revisional surgeries. However, all procedures included the same mechanisms of pain induction like pneumoperitoneum creation, stapler usage, and trocars insertion.
-
The relatively small number of patients included.
Conclusions
We conclude that intraperitoneal instillation of bupivacaine provides a good analgesia in the early postoperative period after laparoscopic bariatric procedures. Moreover, intraperitoneal instillation of bupivacaine reduces the overall consumption of opioid in the first 24 h after surgery and decreases the rescue analgesia requirement in the early postoperative period.
References
Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9:13–27.
Yan LL, Daviglus ML, Liu K, et al. Midlife body mass index and hospitalization and mortality in older age. JAMA. 2006;295:190–8.
Vlad I. Obesity costs UK economy 2bn pounds sterling a year. BMJ. 2003;327:1308.
Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52.
Zhou X, Yu J, Li L, et al. Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients: systematic review and meta-analysis. Obes Surg. 2016 Nov;26(11):2590–601. https://doi.org/10.1007/s11695-016-2144-x.
Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61.
Babu R, Jain P, Sherif L. Intraperitoneal instillation: ropivacaine vs bupivacaine for post operative pain relief in laparoscopic cholecystectomy. Int J Health Sci Res. 2013;3(12):42–7.
Rawal N, Allvin R, Amilon A. Postoperative analgesia at home after ambulatory hand surgery: a controlled comparison of tramadol, metamizole and paracetamol. Anesth Analg. 2001;92:347–51.
Egan TD. Miller’s anesthesia. 6th ed. Anesthesiology 2005;103(3):673.
Bisgaard T, Klarskov B, Rosenberg J, et al. Characteristics and prediction of early pain after laparoscopic cholecystectomy. Pain. 2001;90(3):261–9.
Kandil TS, El Hefnawy E. Shoulder pain following laparoscopic cholecystectomy: factors affecting the incidence and severity. J Laparoendosc Adv Surg Tech A. 2010;20(8):677–82.
Tolver MA, Strandfelt P, Rosenberg J, et al. Pain characteristics after laparoscopic inguinal hernia repair. Surg Endosc. 2011;25(12):3859–64.
Bisgaard T, Stockel M, Klarskov B, et al. Prospective analysis of convalescence and early pain after uncomplicated laparoscopic fundoplication. Br J Surg. 2004;91(11):1473–8.
American Society of Hospital Pharmacists, Committee on Pharmacy and Pharmaceuticals. American hospital formulary service; a collection of drug monographs and other information. Hamilton: Hamilton Press; 1996.
Fernandez AZ, Demaria EJ, Tichansky DS, et al. Multivariate analysis of risk factors for death following gastric bypass for treatment of morbid obesity. Ann Surg. 2004;239:698–703.
Huang S, Mi S, He Y, et al. Analgesic efficacy of trocar sites local anaesthetic infiltration with and without transversus abdominis plane block after laparoscopic hysterectomy: a randomized trial. Int J Clin Exp Med. 2016;9(3):6518–24.
Yadava A, Rajput SK, Katiyar S, et al. A comparison of intraperitoneal bupivacaine – tramadol with bupivacaine – magnesium sulphate for pain relief after laparoscopic cholecystectomy: prospective, randomised study. Indian J Anaesth. 2016;60(10):757–62.
Badawy AM. Intraperitoneal analgesia to reduce pain after laparoscopic hysterectomy. Int J Reprod Contracept Obstet Gynecol. 2017;6:3235–40.
Kim AJ, Yong RJ, Urman RD. The role of transversus abdominis plane blocks in ERAS pathways for open and laparoscopic colorectal surgery. J Laparoendosc Adv Surg Tech A. 2017 Published online 2017 Jul 25;27:909–14. https://doi.org/10.1089/lap.2017.0337.
Oh TK, Lee SJ, Do SH, et al. Transversus abdominis plane block using a short-acting local anaesthetic for postoperative pain after laparoscopic colorectal surgery: a systematic review and meta-analysis. Surg Endosc. 2018;32(2):545–52.
Hamill JK, Rahiri JL, Hill AG. Analgesic effect of intraperitoneal local anaesthetic in surgery: an overview of systematic reviews. J Surg Res. 2017;212:167–77. https://doi.org/10.1016/j.jss.2017.01.022.
Scarth E, Smith S. Drugs in anaesthesia and intensive care. 5th ed. Oxford University Press; 2016. https://doi.org/10.1093/med/9780198768814.001.0001.
Malhotra N, Chanana C, Roy KK, et al. To compare the efficacy of two doses of intraperitoneal bupivacaine for pain relief after operative laparoscopy in gynaecology. Arch Gynecol Obstet. 2007;276(4):323–6.
Mitra S, Khandelwal P, Roberts K, et al. Pain relief in laparoscopic cholecystectomy--a review of the current options. Pain Pract. 2012;12(6):485–96.
Kahokehr A, Sammour T, Srinivasa S, et al. Systematic review and meta-analysis of intraperitoneal local anaesthetic for pain reduction after laparoscopic gastric procedures. Br J Surg. 2011;98(1):29–36.
Marks JL, Ata B, Tulandi T. Systematic review and meta-analysis of intraperitoneal instillation of local anaesthetics for reduction of pain after gynecologic laparoscopy. J Minim Invasive Gynecol. 2012;19(5):545–53.
Ruiz-Tovar J, Gonzalez J, Garcia A, et al. Intraperitoneal Ropivacaine irrigation in patients undergoing bariatric surgery: a prospective randomized clinical trial. Obes Surg. 2016 Nov;26(11):2616–21.
Cohen AR, Smith AN, Henriksen BS. Postoperative opioid requirements following Roux-en-Y gastric bypass in patients receiving continuous bupivacaine through a pump system: a retrospective review. Hosp Pharm. 2013;48(6):479–83.
Bindra TK, Chawla D, Kumar P, et al. Comparison of intraperitoneal instillation of ropivacaine with normal saline in laparoscopic cholecystectomy. Int J Res Med Sci. 2017;5:4924–8.
Choi GJ, Kang H, Baek CW, et al. Effect of intraperitoneal local anaesthetic on pain characteristics after laparoscopic cholecystectomy. World J Gastroenterol. 2015;21(47):13386–95.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Approved by the ethical committee.
Consent
Written informed consent was obtained from the patient for participation in the study before allocation to either arms of the study, in addition to publishing the results.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Omar, I., Abualsel, A. Efficacy of Intraperitoneal Instillation of Bupivacaine after Bariatric Surgery: Randomized Controlled Trial. OBES SURG 29, 1735–1741 (2019). https://doi.org/10.1007/s11695-019-03775-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-03775-x