Abstract
Background
Gastric bypass (GBP) is one of the most effective surgical procedures to treat morbid obesity and the related comorbidities. This study aimed at identifying preoperative predictors of successful weight loss and type 2 diabetes mellitus (T2DM) remission 1 year after GBP.
Methods
Prospective longitudinal study of 771 patients who underwent GBP was performed at four Italian centres between November 2011 and May 2013 with 1-year follow-up. Preoperative anthropometric, metabolic and social parameters, the surgical technique and the previous failed bariatric procedures were analyzed. Weight, the body mass index (BMI), the percentage of excess weight lost (% EWL), the percentage of excess BMI lost (% BMIL) and glycated haemoglobin (HbA1c) were recorded at follow-up.
Results
Univariate and multivariate analysis showed that BMI <50 kg/m2 (p = 0.006) and dyslipidaemia (p = 0.05) were predictive factors of successful weight loss. Multivariate analysis of surgical technique showed significant weight loss in patients with a small gastric pouch (p < 0.001); the lengths of alimentary and biliary loops showed no statistical significance. All diabetic patients had a significant reduction of HbA1c (p < 0.001) after surgery. BMI ≥ 50 kg/m2 (p = 0.02) and low level of preoperative HbA1c (p < 0.01) were independent risk factors of T2DM remission after surgery.
Conclusions
This study provides a useful tool for making more accurate predictions of best results in terms of weight loss and metabolic improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a growing problem in developed countries, as a result of the adoption of a sedentary lifestyle and the increase in the intake of energy-dense types of food, which are also high in fat. According to the World Health Organization’s (WHO) definition, the term obesity indicates “abnormal or excessive fat accumulation that presents a risk to health.” Obesity therefore is not merely a cosmetic concern, but rather—and more importantly—a health issue: indeed, obesity is often associated with life-threatening diseases, such as cardiovascular diseases, type 2 diabetes mellitus (T2DM), stroke, sleep apnoea and cancers [1, 2]. It is not infrequent for obese people to be unable to either lose weight at all or maintain the weight loss simply by increasing physical exercise and/or applying changes to their diet and behaviour, hence their resorting to bariatric surgery. Laparoscopic gastric bypass (GBP) is one of the most effective bariatric surgical procedures, when aiming at achieving long-term weight loss and metabolic results [3]. But, do all obese patients achieve the same results after undergoing GBP? Literature shows that GBP surgery does not invariably—albeit in the majority of cases—result in weight loss and metabolic improvements: more than 30 % of GBP patients fail to achieve this goal, up to 50 % report a weight gain within 24 months after GBP [4, 5] and about 60 % of T2DM patients do not reach diabetes remission 1 year after bariatric surgery [6]. Such outcomes are multi-factorial, i.e. they involve surgical as well as patient-related factors. Research has shown that bariatric surgery may result in a modification of the complex network of peptides within the gastrointestinal tract, as well as central nervous system, thus altering the levels of satiety and energy expenditure [7, 8]. However, studies in this field are far from conclusive, and further research with the elaboration of new, more reliable approaches is needed.
In our opinion, the analysis of the potential predictors of success could be useful to improve the outcome of diabetic and non-diabetic obese patients, while allowing for a more accurate selection of patients undergoing GBP.
The primary goal of this study was to identify preoperative predictors of successful weight loss 1 year after GBP; subsequently, the study aimed at predicting the achievement of good glycaemic control after surgery among obese and diabetic patients.
Materials and Methods
A prospective longitudinal study of patients undergoing elective GBP between November 2011 and May 2013 was performed in four Italian centres. Informed consent was obtained from all participants included in the study. Consenting patients were included in the study in accordance with the international guidelines: well-informed and motivated patients with acceptable operative risks, failure of non-surgical treatments, declared compliance to follow lifelong medical surveillance, aged 18 to 65 years, BMI of 40 kg/m2 or between 35 and 40 kg/m2 with obesity-related comorbidities [9]. Anthropometric, metabolic and social parameters and smoking were recorded during baseline visits: anthropometric variables included height, body weight, sex, blood pressure (mmHg) and BMI (kg/m2). Ideal body weight (IW) was calculated as the equivalent to the ideal BMI of 25 kg/m2. Excess weight (EW) and excess BMI (EBMI) were defined as the difference between initial weight and ideal weight and between initial BMI and ideal BMI, respectively. Metabolic parameters included the following: diabetic status, according to information provided by the patients; therapy for diabetes and baseline HbA1c % value; high-density cholesterol (HDL); triglycerides. Metabolic syndrome (MS) was diagnosed according to the new International Diabetes Federation (IDF) consensus worldwide definition [10]. Social parameters consisted of marital (single, married/cohabiting, divorced, widower) and employment status (employed, unemployed).
Laparoscopic GBP was performed in all four centres, with the three variables differing as follows: volume of the gastric pouch (40 and 60 ml); length of the alimentary limb (100, 120, 150 cm); length of the biliary limb (50, 80, 100 cm). Gastric pouch volume and limb length were measured intraoperatively. Once the gastric pouch was completely separated from the bypassed stomach, an intraoperative test with coloured water irrigation through a naso-gastric tube was routinely performed. The calculation of pouch size was the equivalent to a quantity of water necessary to obtain an initial tension on the suture line. The alimentary and biliary limbs were measured using a single use plastic ruler. This device was introduced in the abdomen and scrolled on the limbs.
An additional parameter, namely previous failed bariatric procedures (air-filled intragastric baloon, laparoscopic adjustable gastric banding, sleeve gastrectomy, vertical gastroplasty), was also included in the evaluation.
All patients were enrolled in a follow-up programme consisting of clinical examination and visit with dietician every 3 months and extensive blood tests every 6 months during the first year after surgery. At 1-year follow-up, the following parameters were evaluated: final weight (FW), final BMI, percentage of excess body weight loss (%EWL), percentage of BMI loss (%BMIL) and the final HbA1c percentage. Parameters were calculated as follows: %EWL = [(initialW–FW) / EW] * 100; %BMIL = [(initial BMI–final BMI) / EBMI] * 100.
Success of bariatric surgery was set to EWL greater than 50 % and good glycaemic control of T2DM to HbA1c lower than 6 %.
Statistical Analysis
The database was created, shared and approved by the all four centres, and data were collected anonymously using Microsoft Excel 2007 (Microsoft Excel 2007, Redmond, WA, USA).
Data were analysed using median (min–max) or mean ± standard deviation for continuous variables, and number (n) and percentage (%) for categorical variables. Categorical data were compared using a chi-squared test and Fisher exact test. Differences in baseline variables among patients with EWL > 50 % kg/m2 and <50 % kg/m2 and patients with preoperative HbA1c % value between 6 and 7.8 % (mean HbA1c %) and >7.8 % at 1 year were analysed with Student’s t test and nonparametric test (Wilcoxon and Mann–Whitney) when appropriate.
Preoperative independent risk factors for success of surgery in terms of weight loss and improvement of glycaemic control were subsequently identified using multivariate models to which logistic regression was applied. Linear correlation analysis (Spearman’s test) was used to quantify cross-sectional relationships between EWL and the age- and BMI-related variables.
The significance level was defined as p < 0.05. All analyses were conducted using “R version 3.1.0” statistical software.
Results
We studied a population of 771 patients who underwent GBP, of whom 588 female (76.3 %) and 183 male (23.7 %). Patients had median age of 42 years, with mean age 42.3 years (±9.9 years) and range between 20 and 67 years: 384 (49.8 %) patients were younger than 42 years, 361 (46.8 %) were between 42 and 60 years and 26 (3.4 %) were over 60 years. Mean preoperative BMI was 45.2 (±6.3), with a range comprised between 30.8 and 72.2 kg/m2; 146 out of 771 (18.9 %) patients were suber-obese (BMI ≥ 50 kg/m2). Smokers were 171 (22.2 %). Metabolic syndrome (MS), hypertension, dyslipidaemia and T2DM were found in 215 (27.9 %), 140 (18.2 %), 118 (15.3 %) and 24 (3.1 %) patients, respectively, while obesity without comorbidities was found in 274 patients (35.5 %). Among the obese patients affected by metabolic syndrome, the association between T2DM and hypertension was present in 57 patients (26.5 %), T2DM and dyslipidaemia in 28 patients (13 %) and hypertension and dysplipidaemia in 62 patients (28.8 %). The association of T2DM, hypertension and dyslipidemia was recorded in 68 patients (31.7 %). Considering the parameter T2DM, 177 participants out of a total of 771 participants (22.9 %) were affected by diabetes; 105 subjects (59.3 %) had a baseline HbA1c % > 6, four (<6 %) had a normal range of HbA1c % and 68 (38.4 %) lacked any preoperative or postoperative HbA1c % data. The last two groups were included in the statistical analyses regarding weight results exclusively, i.e. did not qualify for glycaemic outcomes. The volume of the gastric pouch was 40 ml in 284 patients (36.8 %) and 60 ml in 487 (63.2 %); the length of the alimentary limb was 100, 120 and 150 cm in 300 (38.9 %), 128 (16.6 %) and 343 (55.7 %) patients, respectively; the length of biliary limb was 50 cm in 308 (39.9 %), 80 cm in 46 (6.0 %) and 100 cm in 417 (54.1 %) patients, respectively. Previous bariatric procedures were performed in 110 patients (14.3 %): intragastric balloon was positioned in 28 patients (25.5 %), while laparoscopic gastric banding, sleeve gastrectomy and vertical gastroplasty were performed in 41 (37.3 %), 3 (2.7 %) and 38 (34.5 %) patients, respectively.
Marital status and employment were evaluated as social parameters in 391 patients with the following results: 102 (26.1 %) single, 261 (66.8 %) married/cohabiting, 22 (5.6 %) divorced and 6 (1.5 %) widower; 272 (69.9 %) employed and 119 (30.4 %) unemployed.
All data regarding patients’ characteristics are shown in Table 1.
Mean postoperative follow-up period was 12.3 ± 0.7 months.
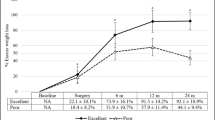
One year after surgery, 688 (89.2 %) patients lost more than 50 % EWL (p < 0.001) while 83 (10.8 %) patients did not reach this goal. We subsequently analysed the relation between age, baseline BMI, sex, presence of comorbidities, smoking, surgical technique, previous bariatric surgery and %EWL. At univariate analysis, we found a significant interaction between %EWL and baseline BMI < 50 kg/m2 (p < 0.001), dyslipidaemia (p = 0.002) and volume of gastric pouch = 40 ml (p < 0.001). Statistically significant interaction between baseline BMI < 50 kg/m2 (p = 0.006), dyslipidaemia (p = 0.05), the volume of gastric pouch = 40 ml (p < 0.001) and %EWL was confirmed at multivariate and linear correlation analysis (Fig. 1). At univariate analysis, negative correlation was found between % EWL and T2DM, metabolic syndrome, hypertension and previous bariatric procedure (p = 0.003, p = 0.03, p = 0.02 and p < 0.001 respectively). The multivariate analysis showed a negative correlation between T2DM (p = 0.005) and metabolic syndrome (p = 0.03) and %EWL. Sex (p = 0.55), age (p = 0.45), length of alimentary (p = 0.59) and biliary limb (p = 0.84) and smoking status (p = 0.4) were not identified as predictors of weight loss.
Of the 105 diabetic patients with HbA1c > 6 %, remission of the T2DM (p < 0.001) was recorded in 61 cases (58 %). The remaining 44 patients (42 %) showed an improvement in their glycaemic control. Mean HbA1c level had significantly decreased from 7.8 ± 1.4 % preoperatively to 5.9 ± 0.8 % 1 year after surgery (−1.9 ± 1.2 %, p < 0.0001); this result was confirmed by the outcome of the linear correlation analysis (p < 0.001) (Fig. 2). Furthermore, surgical success in terms of weight loss was achieved in 147 out 177 diabetic patients (83.1 %), although such result was not deemed statistically significant when compared to data regarding weight loss in non-diabetic patients. Patients whose EWL was still less than or equal to 50 % 12 months after surgery (30 patients, accounting for 16.9 % of cases) displayed a statistically significant good glycaemic control (p < 0.001). For statistical analysis, diabetic patients were divided in two groups, and the cut-off was the mean preoperative HbA1c level equal to 7.8 %. The first group ranged between 6 and 7.8 % and the second with HbA1c value >7.8 %.
At multivariate analysis, preoperative BMI ≥ 50 kg/m2 (p = 0.02) and low-level (6 % < HbA1c < 7.8 %) preoperative HbA1c (p < 0.01) were found as independent risk factors of remission of T2DM after surgery.
The remaining anthropometric, metabolic and social parameters did not show any significant correlation with the improvement of glycaemic control.
Conclusion
Bariatric surgery is the treatment of choice for morbid obesity, but at present, data on potential predictors of success in terms of weight loss and T2DM remission are limited and results frequently controversial.
Our study aimed mainly at identifying preoperative predictors of effective weight loss 1 year after surgery. Our results in terms of weight loss after laparoscopic GBP confirm data currently present in the literature [3, 11–13]: 89.2 % of patients lost greater than 50 % EWL in a mean time of 12 months. In our study, factors significantly associated with GBP success were baseline BMI < 50 kg/m2, dyslipidaemia and small volume of the gastric pouch. Previous studies identified young age as a determinant factor for greater EWL% [14–16] as well, but in our study, this parameter was not identified as predictor of weight loss. Consensus among scholars has yet to be reached on the matter. The same holds for the age-related factor, whereas results regarding the BMI parameter appear less divergent: indeed, a significant number of studies reported a negative association between preoperative BMI and weight loss after bariatric surgery [4, 16, 17]. According to a recent systematic review [4], weight loss in preoperatively non-super-obese patients is greater than in cases of preoperatively super-obesity. In our study, the super-obese group lost a mean of 14 % less EWL compared to its non-super-obese counterpart. Our data showed that initial BMI < 50 kg/m2 and dyslypidaemia are associated with higher EWL% after GBP. Such outcome was obtained through univariate analysis and confirmed at multivariate and at linear correlations analysis.
As far as the size of the gastric pouch is concerned, our data suggest that it is a significant factor influencing weight loss: indeed, between the two sizes of the pouch we considered (40 versus 60 ml), the smallest was appeared to be the most effective. This finding confirms previous studies [18–20], thus corroborating the hypothesis that a large gastric pouch allows for a gradual increased food intake, resulting in a possible gastric dilatation in time.
Previous studies highlighted the correlation between GBP poor results a history of bariatric procedures (intragastric balloon, laparoscopic gastric banding, sleeve gastrectomy, vertical gastroplasty), accounting for 14.3 % (110 patients) in our series. In our study, a history of previous bariatric procedure was associated with the unsuccessful group (30.1 versus 12.3 % in the successful group). According to these authors, revisional surgery was a safe procedure, but weight loss was significantly reduced after primary bariatric surgery [17–21]. Indeed, in their series of 292 patients (66 revisional GBP compared to 226 primary GBP), Slegtenhorst et al. found a lower weight loss 1 year after revisional GBP (37.4 ± 11.5 versus 29.3 ± 17.2 kg; p = 0.001) [22].
Diabetes appeared to negatively influence weight loss in our study: although 83.1 % of diabetic patients achieved weight, such percentage was not statistically significant when compared with the weight loss of the non-diabetic patients. In 16.9 % of cases, EWL was still lower than or equal to 50 % 12 months after surgery, but a statistically significant good glycaemic control was recorded (p < 0.001). We believe that this finding is particularly meaningful, as the benefit of GBP for diabetic patients goes beyond the absolute amount of EWL.
In line with previous studies [23], our data confirmed that for obese patients with T2DM, modest weigh reduction leads to improved glycaemic control. In addition to T2DM, obese patients often have metabolic syndrome, dyslipidaemia or hypertension, which significantly decrease life expectancy. At univariate analysis, our study showed a significant correlation between %EWL and dyslipidaemia (p = 0.002), and a negative correlation with the metabolic syndrome (p = 0.03) and hypertension (p = 0.02).
In our experience, 10.8 % of patients did not reach the Reinhold’s criteria. This finding is consistent with data present in the literature reporting a failure rate up to 40 % [24].
Second aim of our study was to detect baseline factors predicting remission or improvement of T2DM. As “metabolic surgery,” bariatric surgery has been recently endorsed in the clinical practice recommendations of the American Diabetes Association [25] because of its efficacy in improving and even normalizing HbA1c levels in diabetic obese patients. These findings led the International Diabetes Federation to recommend bariatric surgery even for non-obese patients of Asian ethnicity with T2DM [26].
The effect of GBP on diabetes has been extensively discussed in the literature [3, 27, 28]. In our study, of 771 participants, 105 (13.6 %) patients were affected by diabetes. T2DM was completely solved in 61 (58 %) patients (p < 0.001) and improved in the remaining 44 (42 %) at 12 months from surgery. Furthermore, mean HbA1c level had decreased significantly 1 year after surgery in all obese diabetic patients; this finding was confirmed at linear correlation analysis. Preoperative characteristics of patients reaching remission of diabetes were compared with those of patients who failed to reach this goal. In our experience, the majority of patients with T2DM remission were super-obese patients and had low-level (6 % < HbA1c < 7.8 %) preoperative HbA1c. The multivariate analysis showed that preoperative BMI ≥ 50 kg/m2 and low-level (6 % < HbA1c < 7.8 %) preoperative HbA1c appear to be independent risk factors of remission of T2DM after surgery. The relationship between T2DM remission and preoperative BMI is still controversial. A recent meta-analysis showed no significant correlation between baseline BMI and remission rate [29]; other studies identified a BMI ≤ 50 kg/m2 as predictive factor of T2DM resolution. Dixon et al. [30] found higher BMI to be an independent predictor of diabetes remission, which our study also confirms. Previous studies shown the predictive role of preoperative HbA1c appear to further corroborate our results [31–33].
Previous studies also identify age [34, 35], female gender [36], duration of T2DM prior to surgery [30, 37], preoperative weight loss [7] and psychosocial factors [38] as independent predictors for glycaemic control after GBP. In our study, age, gender, smoking status and social parameters did not qualify as predictors of weight loss.
The present study has a number of limitations. Our evaluation did not include a number of preoperative factors that have been associated with EWL and T2DM remission/improvement, such as diet or physical activity after surgery, the duration of T2DM prior to surgery, oral medication/or insulin use and C-peptide level [34, 36, 37]. Furthermore, we limited our study to the 12-month outcome, as this appears to be the most consistently reported in the literature. Undoubtedly, both weight loss and weight regain may continue in the following months; however, the first 12 months after surgery have proved particularly significant for gathering clinical information and for establishing the need for further investigation.
In conclusion, our efforts to detect preoperative predictive factors influencing the outcome of surgery focused on weight loss variations and diabetes resolution 12 months after laparoscopic gastric bypass.
Our data suggest that baseline BMI < 50 kg/m2, dyslipidaemia and a small gastric pouch could be considered predictors of success in terms of weight loss: a statistically significant interaction between these factors and %EWL 12 months after surgery was found at univariate, multivariate and linear correlation analysis. Metabolic syndrome and T2DM showed negative correlation with %EWL.
As regards to metabolic parameters, preoperative BMI ≥ 50 kg/m2 and low-level (6 % < HbA1c < 7.8 %) preoperative HbA1c were found to be independent risk factors of remission of T2DM after surgery.
Further studies on external validity may prove useful in corroborating our findings.
References
Residori L, García-Lorda P, Flancbaum L, et al. Prevalence of co-morbidities in obese patients before bariatric surgery: effect of race. Obes Surg. 2003;13(3):333–40.
Allison DB, Fontaine KR, Manson JE, et al. Annual deaths attributable to obesity in the United States. JAMA. 1999;282(16):1530–8.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Livhits M, Mercado C, Yermilov I, et al. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg. 2012;22(1):70–89.
Magro DO, Geloneze B, Delfini R, et al. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008;18:648–51.
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
Alger-Mayer S, Polimeni JM, Malone M. Preoperative weight loss as a predictor of long-term success following Roux-en-Y gastric bypass. Obes Surg. 2008;18(7):772–5.
Barazzoni R, Zanetti M, Nagliati C, et al. Gastric bypass does not normalize obesity-related changes in ghrelin profile and leads to higher acylated ghrelin fraction. Obesity. 2013;21(4):718–22.
Gastrointestinal Surgery for Severe Obesity. NIH Consensus Statement. 1991 Mar 25-27; 9:1-20.
O'Brien PE, McPhail T, Chaston TB, et al. Systematic review of medium-term weight loss after bariatric operations. Obes Surg. 2006;16:1032–40.
Nguyen NT, Goldman C, Rosenquist CJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;23:279–89.
Suter M, Giusti V, Héraief E, et al. Laparoscopic Roux-en-Y gastric bypass: initial 2-year experience. Surg Endosc. 2003;17:603–9.
Coleman KJ, Toussi R, Fujioka K. Do gastric bypass patient characteristics, behavior, and health differ depending upon how successful weight loss is defined? Obes Surg. 2010;20(10):1385–92.
Contreras JE, Santander C, Court I, et al. Correlation between age and weight loss after bariatric surgery. Obes Surg. 2013;23(8):1286–9.
Ortega E, Morínigo R, Flores L, et al. Predictive factors of excess body weight loss 1 year after laparoscopic bariatric surgery. Surg Endosc. 2012;26(6):1744–50.
Robert M, Pelascini E, Disse E, et al. Preoperative fat-free mass: a predictive factor of weight loss after gastric bypass. Obes Surg. 2013;23(4):446–55.
Roberts K, Duffy A, Kaufman J, et al. Size matters: gastric pouch size correlates with weight loss after laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2007;21(8):1397–402.
Müller MK, Wildi S, Scholz T, et al. Laparoscopic pouch resizing and redo of gastro-jejunal anastomosis for pouch dilatation following gastric bypass. Obes Surg. 2005;15(8):1089–95.
Gobble RM, Parikh MS, Greives MR, et al. Gastric banding as a salvage procedure for patients with weight loss failure after Roux-en-Y gastric bypass. Surg Endosc. 2008;22(4):1019–22.
Zingg U, McQuinn A, DiValentino D, et al. Revisional vs. primary Roux-en-Y gastric bypass–a case-matched analysis: less weight loss in revisions. Obes Surg. 2010;20(12):1627–32.
Slegtenhorst BR, van der Harst E, Demirkiran A, et al. Effect of primary versus revisional Roux-en-Y gastric bypass: inferior weight loss of revisional surgery after gastric banding. Surg Obes Relat Dis. 2013;9(2):253–8.
Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16(6):397–415.
Reinhold RB. Critical analysis of long term weight loss following gastric bypass. Surg Gynecol Obstet. 1982;155(3):385–94.
American Diabetes Association. Executive summary: standard of medical care in diabetes- 2011. Diabetes Care. 2011;34 Suppl 1:S4–S10.
Dixon JB, Zimmet P, Alberti KG, et al. International Diabetes Federation Taskforce on Epidemiology and Prevention. Bariatric surgery: an IDF statement for obese Type 2 diabetes. Surg Obes Relat Dis. 2011;7(4):433–47.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56.
Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23(4):427–36.
Yan YX, Wang GF, Xu N, et al. Correlation between postoperative weight loss and diabetes mellitus remission: a meta-analysis. Obes Surg. 2014;24(11):1862–9.
Dixon JB, Chuang LM, Chong K, et al. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care. 2013;36(1):20–6.
Dogan K, Betzel B, Homan J, et al. Long-term effects of laparoscopic Roux-en-Y gastric bypass on diabetes mellitus, hypertension and dyslipidaemia in morbidly obese patients. Obes Surg. 2014;24(11):1835–42.
Robert M, Ferrand-Gaillard C, Disse E, et al. Predictive factors of type 2 diabetes remission 1 year after bariatric surgery: impact of surgical techniques. Obes Surg. 2013;23(6):770–5.
Dixon JB, Hur KY, Lee WJ, et al. Gastric bypass in Type 2 diabetes with BMI < 30: weight and weight loss have a major influence on outcomes. Diabet Med. 2013;30(4):e127–34.
Yamaguchi CM, Faintuch J, Hayashi SY, et al. Refractory and new-onset diabetes more than 5 years after gastric bypass for morbid obesity. Surg Endosc. 2012;26(10):2843–7.
Hamza N, Abbas MH, Darwish A, et al. Predictors of remission of type 2 diabetes mellitus after laparoscopic gastric banding and bypass. Surg Obes Relat Dis. 2011;7(6):691–6.
Chikunguwo SM, Wolfe LG, Dodson P, et al. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6(3):254–9.
Hall TC, Pellen MG, Sedman PC, et al. Preoperative factors predicting remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery for obesity. Obes Surg. 2010;20(9):1245–50.
Kinzl JF, Schrattenecker M, Traweger C, et al. Psychosocial predictors of weight loss after bariatric surgery. Obes Surg. 2006;16(12):1609–14.
Acknowledgments
We would like to acknowledge Dr. Eugenia Dal Fovo for translating this article and Dr Fabiola Giudici for statistical analysis.
Informed consent was obtained from all individual participants included in the study.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
Silvia Palmisano: no conflict of interest.
Marta Silvestri: no conflict of interest.
Michela Giuricin: no conflict of interest.
Nicolò de Manzini: no conflict of interest.
Edoardo Baldini: no conflict of interest.
Simone Albertario: no conflict of interest.
Patrizio Capelli: no conflict of interest.
Bernardo Marzano: no conflict of interest.
Giovanni Fanti: no conflict of interest.
Aron Zompichiatti: no conflict of interest.
Paolo Millo: no conflict of interest.
Massimiliano Fabozzi: no conflict of interest.
Riccardo Brachet Contul: no conflict of interest.
Elisa Ponte: no conflict of interest.
Rosaldo Allieta: no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palmisano, S., Silvestri, M., Giuricin, M. et al. Preoperative Predictive Factors of Successful Weight Loss and Glycaemic Control 1 Year After Gastric Bypass for Morbid Obesity. OBES SURG 25, 2040–2046 (2015). https://doi.org/10.1007/s11695-015-1662-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1662-2