Abstract
Aim
The aim of this study was to find independent perioperative factors predicting unsuccessful weight loss following one anastomosis gastric bypass-mini gastric bypass (OAGB-MGB), a recently developed technique of bariatric surgery.
Methods
Using regression analysis, this prospective cohort study assessed the role of demographic and socioeconomic factors, clinical features, body composition, length of biliopancreatic limb (LBL), nutritional habits, comorbidities, and early post-operative weight reduction values, in predicting 1-year weight loss after OAGB-MGB. All patients at the center who underwent laparoscopic OAGB-MGB between October 2010 and May 2017 were included. The dependent variable was the percent of excess weight loss (%EWL) after 12-month follow-up. Weight loss outcome was defined as successful EWL (≥ 50%) or unsuccessful EWL (< 50%).
Results
Follow-up data at 1 year was available for 1309 (77%) patients. Mean EWL and BMI reduction were 81.63% and 16.82 ± 4.37 kg/m2 at 1 year. In addition, 48 (3.7%) patients had unsuccessful weight loss. Pre-operative higher BMI (OR, 1.34; p = 0.001), type 2 diabetes (OR, 4.26; p = 0.039), pre-surgery volume eating habit (OR, 0.12; p = 0.003), weight reduction value in the first month after surgery (OR, 0.80; p = 0.002), and length of biliopancreatic limb (LBL) (OR, 1.05; p = 0.017) were independently associated with unsuccessful weight loss at 1-year follow-up.
Conclusions
OAGB-MGB provides considerable weight loss for most patients. Initial lower BMI, absence of diabetes, being volume eater, and higher first month weight loss are independently associated with successful weight loss after 1 year.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In fact, among all bariatric surgery procedures with specified survival benefits and reduced total mortality, none of them is an “ideal” option [1].
The Mason loop adjustment in gastric bypass procedures with a longer lesser curvature-based pouch is a recently developed surgical technique which is identified as a single or one-anastomosis gastric bypass (OAGB) or mini-gastric bypass (MGB). In addition to certain advantages like its simplification of technical complexity, shorter operative times, and lower risk of leaks from one less anastomotic site, this technique leads to a considerable reduction in excess weight loss, as well as improved morbidity and mortality rates [2, 3]. According to studies into OAGB-MGB, the mean excess weight loss (EWL) was more than 70% over 5 years [4]. Despite all advantages, any bariatric surgery has its own complications; in addition, the outcomes may be affected by a number of patient-related characteristics [5].
It has been suggested that several factors such as age, sex, marital status, and initial weight might be associated with weight loss in the post-operative period [6]. Also, the presence of comorbidities, such as diabetes mellitus or other obesity-related complications, could be associated with poor weight loss outcomes [6,7,8], as patients with diabetes or obstructive sleep apnea showed considerably less weight loss at 18 months and 24 months after laparoscopic sleeve gastrectomy (SG) [7]. Greater body mass index or being super obese (BMI ≥ 50 kg/m2) is another factor which has been reported to cause poorer weight loss [8, 9]. Inversely, other studies reported no relation between EWL and higher pre-operative BMI or BMI ≥ 50 kg/m2; however, higher waist circumference has been associated with lower weight loss and the duration of weight nadir [6, 10]. Male sex, insurance status, employment, and larger pouch size are other suggested predictive factors [7, 8]. Since gastric pouch size, or the length of the alimentary limb, showed independent association with weight reduction after GBP [6], the length of the biliopancreatic limb, which is decided according to the patient’s BMI, may have a role in weight loss failure rates after OAGB-MGB.
Pre-operative predictors of weight loss after a Roux-en-Y gastric bypass (RYGB) or a SG have previously been investigated in several studies, but few investigations have focused on the laparoscopic OAGB-MGB and possible predictive factors of post-operative weight-loss. Therefore, the aim of this study is to determine the independent effects of demographic, socioeconomic, and clinical factors on the 1-year post-operative weight loss values following OAGB-MGB.
Methods
Study Population
In this retrospective cohort study, data was collected from 1701 consecutive patients who underwent laparoscopic OAGB-MGB between October 2010 and May 2017, at the Surgical Department of Hazrat-e Rasool Hospital (approved as a Center of Excellence for Metabolic and Bariatric Surgery by the International Federation of Surgery for Obesity and Metabolic Disorders: IFSO, 2014), Tehran, Iran. Follow-up data at 12 months were available for 1309 patients.
All selected patients were consistent with the NIH criteria for bariatric surgery [11]. All patients who underwent OAGB-MGB were enrolled in this investigation. Patients who did not complete the 12-month follow-up were excluded from the final analysis. All remaining patients had neither revisional OAGB-MGB procedure nor pregnancy during the 12 months.
Pre-operative Assessment
All needed data were extracted from the previously approved National Obesity Surgery Database and collected then approved by a single researcher, in order to decrease any possible sources of bias. Center of excellence clinical pathways was followed consistently for all patients.
Surgical Procedure
For all patients who underwent OAGB-MGB, a 15–18-cm-long gastric pouch was created, and a side-to-side loop (45 mm) gastrojejunostomy was performed 100–280 cm from the Treitz ligament. The biliary limbs were measured using a scaled atraumatic grasper (@Applied). According to our previous acceptable results, biliopancreatic limb was tailored based on patient’s age and pre-operative BMI. Longitudinal gastrojejunostomy was performed on the posterior aspect of the pouch, and the enterostomy site was sewn with a 2-0 polydioxanone (PDS).
Post-operative Assessment
All patients followed the medical center protocol, including taking a daily multivitamin and mineral supplement, and preventive medication for gastric protection and gallstone formation during weight loss, which were prescribed by the specialists.
Patients were requested to follow up at 10 days; 1, 3, 6, 9, 12, 18, and 24 months; and then on an annual basis, to keep track of their weight loss, improvement in comorbidity conditions, and nutritional status after the surgery. Also, according to biochemical test results or clinical features, other oral supplements were prescribed as needed.
Study Variables
Dependent Outcome Variable: 1-Year Weight Loss
To evaluate weight loss after OAGB-MGB, weight change was quantified as the percentage of EWL, total weight loss (TWL), alterable weight loss (AWL), absolute weight loss, and absolute change in BMI. The %EWL at any given point after surgery was calculated as:
where a is weight at the last pre-surgery visit and b is ideal body weight calculated by BMI = 25 kg/m2.
For multivariate analysis, EWL was studied as a 2-level classification (successful vs unsuccessful). To determine unsuccessful weight loss, an EWL < 50% was considered the cutoff [12].
Independent, Possibly Predictive Variables
The independent variables included age, sex, employment status, marital status, education level, initial weight and body mass index (BMI), diet or balloon history, number of weight loss attempts, nutritional habits, primary body composition (including fat mass, trunk fat, fat free mass, muscle mass (kg), and visceral fat level was determined using a body composition analyzer (Tanita BC-418, Tanita Corp., Tokyo, Japan)), sarcopenic obesity (defined by an FM/FFM ratio > 0.8) [13], comorbidities (diabetes mellitus, hypertension, hypothyroidism, joint disease, sleep apnea, hyperlipidemia, and psychiatric disease, fatty liver), length of biliopancreatic limb (LBL) (more/less than 200 cm), and weight reduction after 10 days and at 1 month after surgery.
Statistical Analysis
Frequencies and descriptive statistics were calculated for all variables, using mean and standard deviation (SD) or frequencies and percentages for continuous or categorical variables. The unadjusted associations of each variable with poor weight loss were assessed by using a t test for continuous variables, and chi-square (χ2) and Fisher exact tests, as appropriate, for categorical variables.
Binary logistic regression was used in multivariate analysis to identify independent perioperative variables associated with unsuccessful weight loss. The final model was developed using a forward LR method with an entry criterion of p < 0.2 for variables according to univariate analysis. The overall goodness of fit was assessed using the Hosmer-Lemeshow χ2 statistic. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS statistical software ver. 22.0 (Armonk, NY: IBM Corp).
Results
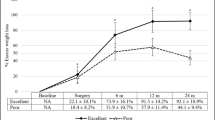
In total, 1701 patients underwent the OAGB-MGB procedure. Follow-up data were available in 89% and 77% of cases at 6 and 12 months, respectively. In the assessment of missing data, it was found that patients with older age, those in employment, and males were the most likely not to attend follow-up sessions. Based on BMI, the highest amount of missing data was found in super-obese patients. However, none of the factors were associated with EWL. So, there was no loss to follow-up bias in the present study. Demographic and perioperative clinical features of all patients and those with successful vs unsuccessful EWL are presented in Table 1. The female population was almost more than three times as large as the male population (77.4% vs 22.6%). Most patients (62.4%) were morbidly obese with BMI = 40–50 kg/m2, and 26.8% of them were super obese (BMI > 50 kg/m2). Initial weight and BMI were significantly higher in patients with unsuccessful EWL (p = 0.034) and (p = 0.005). Also, LBL was associated with EWL 1-year post-surgery (p = 0.006). Anthropometric results showed all patients with visceral fat level ≤ 12 had successful EWL (p = 0.026). Patients in unsuccessful EWL after 1 year had a significantly higher pre-operative waist-to-height ratio (p = 0.008). Weight reduction analysis has demonstrated that greater weight loss in the early phase, 10 and 30 days after surgery, led to a significant difference in long-term EWL (p < 0.001).
Outcomes related to weight loss are shown in Table 2. The mean %EWL was over 80% during follow-up until 1 year post-operatively, and 48 patients (3.7%) had unsuccessful weight loss after 1 year. Greater %EWL was observed in severely obese patients (96.88 ± 23.40); however, the lowest losses were seen in super-obese patients (70.09 ± 12.41). The trend of weight loss outcomes was significant at different time points in the follow-up (p < 0.001).
Binary logistic regression revealed perioperative factors including higher BMI (OR,1.34; 95% CI, 1.14, 1.59), the presence of type 2 diabetes (OR, 4.26; 95% CI, 1.08, 16.85), and longer LBL (OR, 1.05; 95% CI, 1.01, 1.09) being associated with unsuccessful weight loss (EWL < 50%) at 1-year follow-up; however, being volume eater (OR, 8.62; 95% CI, 2.05, 37.04), and greater weight reduction values in the first month after surgery (OR, 1.25; 95% CI, 1.09, 1.45) significantly predicted successful weight loss (Table 3).
Discussion
The main findings of the present study are that 96.33% of all patients whose data was available at 1 year after surgery had successful EWL. Different lines of evidence have also reported a significant post-OAGB-MGB excess weight loss after 1 year or more (80–93%) [14,15,16].
One interesting finding of the present study was that 100% of patients with pre-operative visceral fat levels of ≤ 12 cm2 had successful EWL; however, 4.8% of those with levels of > 12 cm2 failed to succeed. The effect of pre-surgical visceral fat on the success of weight loss afterward has not been reported in any previous studies. However, the correlation between weight changes and visceral fat changes in a time interval of pre- and post-bariatric surgery has been investigated [17]. Visceral fat accumulation is associated with a range of metabolic disorders [18], which probably due to its role in the production of some bioactive molecules like leptin [19] which causes excessive weight gain [20]. This result was supported by the current findings with regard to the waist-to-height ratio (WHtR), another central obesity index [21] which was significantly different between groups.
Higher pre-operative weight and BMI were inversely associated with %EWL after 1 year. Similar to earlier studies, these factors are associated with suboptimal weight loss after RYGB [22]. Likewise, Palmisano et al. reported that baseline BMI < 50 kg/m2 was a predictor factor of successful weight loss after RYGB. On the contrary, we did not find an association between parameters such as dyslipidemia and age with weight loss after OAGB-MGB [23]. Categorized baseline BMI revealed that patients with severe obesity (35–40 kg/m2) had the highest %EWL, which is opposed to %TWL considerations, which point out that individuals who are pre-operatively more obese have better total weight loss percent after bariatric surgery. On the other hand, the relatively short length of follow-up (12 months) is possibly not an adequate time period for patients with higher BMI to lose sufficient extra weight.
Prior experience with OAGB-MGB has shown that a range of 150–200 cm for LBL was suitable to prevent malnutrition [24]. Additionally, some surgeons prefer the LBL of 200 cm [25, 26]. In line with existing studies, the mean of LBL in most of the current participants (97.7%) was ≤ 200 cm, which seems to have a beneficial effect on successful EWL. It was indicated 18.2% of those whose LBL was > 200 cm had an unsuccessful EWL, while this value was only 3.2% for LBL ≤ 200 cm. The association between LBL and post-RYGB weight loss was evaluated before, which displayed weight loss 2 years post-surgery was significantly higher for LBL of 150 cm than 75 or for 200 than 60 cm [27, 28]. One possible explanation could be that LBL greater than 200 cm has generally been used for patients with higher BMI who do not sufficiently respond to weight loss within 1 year. Future studies will need to be implemented with more number of participants and further classification into groups with less variation in length of BL. Furthermore, the present study found that greater weight loss during the first month after surgery (9.29–18.85 kg), and being a pre-operative volume eater, decreased the odds of unsuccessful EWL by 20.3% and 88.4%, respectively. However, the unsuccessful EWL risk in people with diabetes was 4.2 times higher than in non-diabetic patients. Similarly, the weight loss rate at 1-month post-RYGB surgery was identified as a significant predictor and had a significant correlation with EWL (r = 0.36, p = 0.0001) at 12 months [29]. Additionally, it has been shown that patients who had inappropriate pre-surgical eating patterns may be at higher post-surgery risk of this [30]. However, it seems that a pre-operative high-volume eater may not continue this consumption pattern following OAGB-MGB, due to the reduced capacity of the stomach. These results were partly consistent with those of the study by Morseth et al. [31], which demonstrated that pre-operative objective bulimic episodes can be predictive of weight loss outcome post-RYGB and biliopancreatic diversion with duodenal switch.
The post-operative weight loss amount, as affected by diabetes, remains controversial in the literature. Similar to the present study, some have found that patients with diabetes were less likely to lose weight after RYGB surgery than others [8, 32]. However, in this area, no data was available to challenge post-OAGB-MGB patients. One suggested reason is the consumption of insulin or a glycemic control drug by most diabetic patients that might stimulate lipogenesis, synthesis of triglycerides, and adipocyte differentiation, perhaps consequently diminishing the amount of weight reduction after weight loss surgery. It has been proposed that hypoglycemia episodes may cause an increase in calorie intake which is related to weight gain in patients with diabetes [6].
To the researchers’ knowledge, this is the first study to describe the perioperative predictors of unsuccessful EWL following the OAGB-MGB surgery. One possible source of bias in the present study was the 392 patients who did not provide 12-month follow-up data. As has been shown, generally older, super obese, employed males attended post-operative visits less often. However, despite 23% of patients missing in the final analysis, different features associated with lost to follow-up did not relate to EWL and decreased concerns about bias to introduce. This study had a large sample size, although the participants were people who had been referred to this specific obesity clinic, which might reduce its generalizability. On the other hand, since patients had been referred from all over the country, the findings can to some degree be generalized to the entire community. Another limitation was the unavailability of physical activity level data, which may have an effect on weight loss after surgery. However, the body composition values were recorded, which can be used to evaluate muscle or fat mass associations with EWL. The researchers suggest that this variable should be given more emphasis in future studies.
Conclusion
Although many factors may contribute to successful post-surgery weight loss, overall, OAGB-MGB is an effective weight loss method in patients with morbid obesity, controlled diabetes, and volume eating habits. Specifically, this method of surgery can show desirable outcomes in patients who lose more weight in the early phases after surgery. The early identification of predictive factors might help to select the right patients or advance interventions directed towards specific patient requirements. Further studies are needed to explore whether exact pre- or perioperative factors can predict a clinically meaningful difference in long-term weight loss after the OAGB-MGB surgery.
References
Noria SF, Grantcharov T. Biological effects of bariatric surgery on obesity-related comorbidities. Can J Surg. 2013;56(1):47–57.
Chaim EA, Ramos AC, Cazzo E. Mini-gastric bypass: description of the technique and preliminary results. Arq Bras Cir Dig (São Paulo). 2017;30:264–6.
Mahawar KK, Carr WR, Balupuri S, et al. Controversy surrounding ‘mini’ gastric bypass. Obes Surg. 2014;24(2):324–33.
Mahawar KK, Jennings N, Brown J, et al. “Mini” gastric bypass: systematic review of a controversial procedure. Obes Surg. 2013;23(11):1890–8.
Georgiadou D, Sergentanis TN, Nixon A, et al. Efficacy and safety of laparoscopic mini gastric bypass. A systematic review. Surg Obes Relat Dis. 2014;10(5):984–91.
Campos GM, Rabl C, Mulligan K, et al. Factors associated with weight loss after gastric bypass. Arch Surg. 2008;143(9):877–83. discussion 84
Jambhekar A, Maselli A, Robinson S, et al. Demographics and socioeconomic status as predictors of weight loss after laparoscopic sleeve gastrectomy: a prospective cohort study. Int J Surg. 2018;54(Pt A):163–9.
Melton GB, Steele KE, Schweitzer MA, et al. Suboptimal weight loss after gastric bypass surgery: correlation of demographics, comorbidities, and insurance status with outcomes. J Gastrointest Surg. 2008;12(2):250–5.
Bloomston M, Zervos EE, Camps MA, et al. Outcome following bariatric surgery in super versus morbidly obese patients: does weight matter? Obes Surg. 1997;7(5):414–9.
Still CD, Wood GC, Chu X, et al. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring). 2014;22(3):888–94.
Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992;55(2 Suppl):615s-9s.
van de Laar AW, van Rijswijk AS, Kakar H, et al. Sensitivity and specificity of 50% excess weight loss (50%EWL) and twelve other bariatric criteria for weight loss success. Obes Surg. 2018;28(8):2297–304.
Gomez-Peralta F, Abreu C, Cruz-Bravo M, et al. Relationship between “a body shape index (ABSI)” and body composition in obese patients with type 2 diabetes. Diabetol Metab Syndr. 2018;10:21.
Peraglie C. Laparoscopic mini-gastric bypass in patients age 60 and older. Surg Endosc. 2016;30(1):38–43.
Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg. 2005;15(9):1304–8.
Pazouki A, Esmaeili SK. Excessive weight loss following laparoscopic gastric mini bypass or Roux-en-Y gastric bypass surgery. Int J Clin Med. 2016;07(07):5.
Weiss R, Appelbaum L, Schweiger C, et al. Short-term dynamics and metabolic impact of abdominal fat depots after bariatric surgery. Diabetes Care. 2009;32(10):1910–5.
Pontiroli AE, Frige F, Paganelli M, et al. In morbid obesity, metabolic abnormalities and adhesion molecules correlate with visceral fat, not with subcutaneous fat: effect of weight loss through surgery. Obes Surg. 2009;19(6):745–50.
Shuster A, Patlas M, Pinthus JH, et al. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85(1009):1–10.
Scarpace PJ, Zhang Y. Elevated leptin: consequence or cause of obesity? Front Biosci. 2007;12:3531–44.
Langenberg C, Shipley MJ, Batty GD, et al. Adult socioeconomic position and the association between height and coronary heart disease mortality: findings from 33 years of follow-up in the Whitehall Study. Am J Public Health. 2005;95(4):628–32.
Sillen L, Andersson E. Patient factors predicting weight loss after Roux-en-Y gastric bypass. J Obes. 2017;2017:3278751.
Palmisano S, Silvestri M, Giuricin M, et al. Preoperative predictive factors of successful weight loss and glycaemic control 1 year after gastric bypass for morbid obesity. Obes Surg. 2015;25(11):2040–6.
Ugale S, Vennapusa A, Katakwar A, et al. Laparoscopic bariatric surgery-current trends and controversies. Ann Laparosc Endosc Surg. 2017;2(10)
Carbajo M, Garcia-Caballero M, Toledano M, et al. One-anastomosis gastric bypass by laparoscopy: results of the first 209 patients. Obes Surg. 2005;15(3):398–404.
Parmar CD, Mahawar KK, Boyle M, et al. Mini Gastric Bypass: first report of 125 consecutive cases from United Kingdom. Clin Obes. 2016;6(1):61–7.
Boerboom A, Homan J, Aarts E, et al. A long biliopancreatic and short alimentary limb results in more weight loss in revisional RYGB surgery. Outcomes of the randomized controlled ELEGANCE REDO trial. Surg Obes Relat Dis. 2019;15(1):60–9.
Nergaard BJ, Leifsson BG, Hedenbro J, et al. Gastric bypass with long alimentary limb or long pancreato-biliary limb-long-term results on weight loss, resolution of co-morbidities and metabolic parameters. Obes Surg. 2014;24(10):1595–602.
Mor A, Sharp L, Portenier D, et al. Weight loss at first postoperative visit predicts long-term outcome of Roux-en-Y gastric bypass using Duke weight loss surgery chart. Surg Obes Relat Dis. 2012;8(5):556–60.
Colles SL, Dixon JB, O’Brien PE. Grazing and loss of control related to eating: two high-risk factors following bariatric surgery. Obesity (Silver Spring). 2008;16(3):615–22.
Morseth MS, Hanvold SE, Ro O, et al. Self-reported eating disorder symptoms before and after gastric bypass and duodenal switch for super obesity-a 5-year follow-up study. Obes Surg. 2016;26(3):588–94.
Ma Y, Pagoto SL, Olendzki BC, et al. Predictors of weight status following laparoscopic gastric bypass. Obes Surg. 2006;16(9):1227–31.
Acknowledgments
Data were obtained from the National Obesity Surgery Database, Iran. The authors extend their sincere thanks to all participants and the National Obesity Surgery Database team, who prepared us very useful data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by Iran University of Medical Science Ethics Committee (Ethic number: IR.IUMS.REC.13970134).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ansar, H., Zamaninour, N., Pazouki, A. et al. Weight Loss After One Anastomosis Gastric Bypass-Mini Gastric Bypass (OAGB-MGB): Patient-Related Perioperative Predictive Factors. OBES SURG 30, 1316–1323 (2020). https://doi.org/10.1007/s11695-019-04270-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04270-z