Abstract
Cashew apple juice is one of the highly nutritious and perishable fruit juice. In this study, the shelf life extension of pre-treated cashew apple juice was undertaken using a non-thermal ultrafiltration technology. A total of 16 experiments were carried out at four different molecular weight cut-offs (5 kDa, 10 kDa, 30 kDa, and 50 kDa) and transmembrane pressures (35 kPa, 69 kPa, 103 kPa, and 138 kPa) to get the optimum process conditions. After considering the highest productivity and superior quality of the filtered juice, 30 kDa molecular weight cut-off and 138 kPa transmembrane pressure were found to be the optimum processing condition for the ultrafiltration of cashew apple juice. At the optimum condition, the ultrafiltered juice had 8.22°Brix TSS, 4.36 pH, 1.16 cP viscosity, 99.88%T clarity, 1.22 NTU turbidity, 0.064 abs color intensity, 196.00 mg/100 mL ascorbic acid content, 13.49 mg tannic acid/100 mL total tannin content and 110.77 mg GAE/100 mL total polyphenol content. Further, a resistance-in-series model was developed to quantify and study the limiting flux behaviour of the ultrafiltration membrane. The developed model has a huge significance (p < 0.05) during the commercialization of the system since it derived a mathematical relationship between the process parameters and the limiting flux of the membrane. Finally, a storage study was carried out and it was observed that the ultrafiltered juice was successfully stored for 12 weeks at 4 °C without any chemical and heat treatment (as compared to the normal shelf life of 1–2 days). This study helps to efficiently design and scaling-up of an ultrafiltration system for the clarification and cold sterilization of cashew apple juice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cashew (Anacardium occidentale L.) belongs to the tropical category of fruits and is originated from South America (specifically from the Northern and North-eastern regions of Brazil). Currently, the world production of cashew is about 4.18 million tons from nearly 7.10 million hectares of the cultivated area [1]. Cashew apples (CA), the underutilized pseudo fruit of cashew, are slightly acidic (with pH 4.4) and highly nutritious with rich amounts of sugars (10.6%), especially sucrose and fructose, vitamins, organic acids, phenolic compounds (365 mg/100 g GAE), including tannins, anacardic acids, carotenoids, and flavonoids, and other antioxidants. They also have a significant amount of ascorbic acid content (219 mg/100 g vitamin C), which is considerably higher than commonly available citrus fruits like pineapples and oranges [2, 3].
CA juice is a value-added product with a high medicinal and nutritional profile. However, the presence of some insoluble macromolecules such as pectin, cellulose and starch, escalates the turbidity and limits the shelf life of the juice [4]. The existence of microorganisms also reduces the shelf life of the juice. In addition, CA juice also contains a major phenolic compound named tannin, which limits the consumer acceptability of the juice by developing an astringent sensation [5]. Therefore processing the CA juice is essential to address these issues.
Appropriate physical (including modified atmospheric packing, pressurized inert gases, electron beam irradiation, pulsed light, ultraviolet light and cold plasma), chemical (such as acidic electrolyzed water, nano-based preservatives, ozone, and so on.) or biopreservation technologies (such as using bacteriophages, bacteriocins and bioprotective microorganism) must be applied for the processing and preservation of fruits and vegetables[6, 7]. Several efforts have been made to process and preserve the CA juice using chemical preservatives, high-pressure processing, osmotic dehydration, irradiation, hot filling and aseptic filling methods [3]. However, these processing technologies cannot be commercialized since it accelerates the loss of sensory characteristics and heat-sensitive compounds such as ascorbic acid [8]. Hence an alternative non-thermal treatment is necessary. Membrane processing technologies (MPT) such as microfiltration, ultrafiltration (UF) and reverse osmosis have recently gained popularity among the food industries due to their non-thermal nature, easy-scalability, simplicity of operation and energy efficiency [9]. Moreover, these technologies are used in fruit juice processing to clarify the juices to obtain a final product with extended shelf life and maximum retention of its natural qualities [10]. Among different MPT, UF is the most popular technique for the clarification of juices since it can reject higher molecular weight compounds such as suspended colloids, microorganisms, polysaccharides and proteins. While, it allows smaller-sized particles like vitamins, salts and sugars to pass through the membrane [8]. This mechanism of restricting higher molecular weight compounds and microorganisms from the clarified juice is the primary reason behind the shelf life extension of the juice.
Numerous studies have been carried out on the clarification of various fruit juices using the UF technique [8, 11,12,13,14]. Ghosh et al., [14] compared the performance of UF on the clarification of Jamun juice with other clarifying methods such as enzymatic treatment, centrifugation, and microfiltration. The study found that the UF using a 50 kDa molecular weight cut-off (MWCO) membrane and 20 psi transmembrane pressure (TMP) was the best UF condition with refers to the nutritional quality and storage stability of the juice. Similarly, another research on the UF of apple juice reported that the juice treated at an optimum condition of 10 kDa MWCO and 0.75 MPa TMP delivered clarified juice with higher nutritional qualities. In the same way, Panigrahi et al., [15] studied the shelflife extension of sugarcane juice using polysulphone-based hollow fiber UF membranes and found 30 kDa MWCO membrane at 104 kPa TMP as the optimum condition to extend the shelf life of juice up to 9 weeks when stored at 4 ºC. All these investigations demonstrated that UF of fruit juices can result in superior quality, microbial-free, clarified juices with higher storage stability.
Since the UF process involves no heat, preservatives or additives and is carried out in a scalable system, this technology has a great chance for commercialization. However, to the best of our knowledge, no research has been conducted on the UF of cashew apple juice. Hence, this study aims to develop a process with an optimum condition for the UF of cashew apple juice using a hollow fiber membrane. For this purpose, the pre-treated (centrifuged followed by microfiltered) cashew apple juice was ultrafiltered and various physicochemical analyses such as total soluble solids, pH, viscosity, clarity, turbidity, color, ascorbic acid content, total tannin content and total polyphenol content of the UF juice was investigated. Moreover, the permeate flux decline of the process with change in the processing conditions was examined using a resistance-in-series model. Finally, a 12 week storage study of the juice filtered at the optimum process condition was also conducted.
Materials and methods
Raw material
The research was conducted at the Department of Food Process Engineering, National Institute of Technology, Rourkela, India. The CA juice was extracted following the method reported by Abdullah et al., [2] from the fresh CAs brought to the department from Bamra (22.0562ºN latitude, 84.2912ºE longitudes), Odisha India. The juice was then shifted to the deep freezer (at− 20 ± 2 °C) and was stored at the same temperature till further study.
Primary clarification
The commercial ultrafiltration of juice requires an appropriate primary clarification process to ensure the removal of higher molecular weight compounds and suspended particles from the juice [15, 16]. Cashew apple juice was initially centrifuged at 7532 rpm rotational speed (Fixed-angle rotor: 12181; Angle: 90º; Max. RCF, 2-16P / 2-16KL: 10621/15294; Max. Speed, 2-16P / 2-16KL: 10000/12000; Max. capacity: 6 × 50 ml) for 52.6 min, followed by microfiltration using a 0.2 μm polysulfone- based hollow fiber membrane at 138 kPa TMP [4]. The primary clarification removed maximum suspended solids and higher molecular weight compounds, including tannin, from the cashew apple juice.
Ultrafiltration
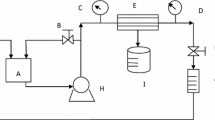
A laboratory scale membrane filtration system and the polysulfone-based hollow fiber membranes (Explorer 12, m-PES, Mumbai, India) with dimensions of 1.27 cm × 30.48 cm and a surface area of 51.80 cm2 bought from CleanSep (Mumbai, India) were used to conduct the ultrafiltration experiments. The details regarding the membrane system are explained by Abdullah et al., [4]. Also, a schematic representation of the UF system is displayed in Fig. 1.
Ultrafiltration trials were conducted at four different TMPs (35 kPa, 69 kPa, 103 kPa, and 138 kPa) and four different MWCOs (5 kDa, 10 kDa, 30 kDa, and 50 kDa) using 500 mL primarily clarified juice. A total recycle mode was followed to conduct all the experiments since it recycles both the permeate and retentate back to the feed tank and thus regulates the feed concentration. The permeate flux profile was calculated for each combination of TMP and MWCO. Each experiment was conducted for 1 h at room temperature (25 ± 1 °C), and the clarified cashew apple juice was collected to determine its physicochemical properties.
Before the beginning of each trial, the membranes were thoroughly washed and the steady-state permeate flux of the distilled water was measured at various TMPs to determine its permeability. The procedure for membrane washing and the determination of membrane permeability is explained by Abdullah et al., [4].
Mathematical modelling of permeate flux decline
The decline in flux during the UF of juices is mainly attributable to the formation of a fouling layer on the surface of the membrane by the rejected solutes. Hence, membrane fouling resistance (MFR) and hydraulic resistance (MHR) are the two main resistances encountered by the permeates during UF [13]. Similarly, MHR0 and MHR1 are the fouling resistance for the membrane before and after the first run, respectively. Also, MRIRR is the membrane resistance after washing the membrane at the end of the first run. The MHR1 is determined by passing distilled water at the end of the first experiment, where MHR1 = MHR0 + \({MR}_{IRR}^{1}\) [17]. Thus the membrane resistance corresponds to the Nth experimental run is calculated as:
where, \({\mu }_{PW}\) represents the viscosity of pure water (at 30 ± 1 °C), \(\Delta P\) represents the TMP drop and \({V}_{PW}^{0}\) is the pure waste flux after the Nth experiment through the membrane. On the other hand, for the estimation of membrane resistance at the Nth experimental run, irreversible membrane resistance is also included. In addition, the decline in experimental flux can be exploited to estimate the resistance to fouling at any period. Which is represented as:
where, VP (t) and \({\mu }_{P}\) is the flux and viscosity of the clarified juice after UF at 30 °C.
During the UF of the juice through a cross-flow system, its fouling resistance initially increases gradually and then as a result of the forced convection, it reaches a steady state. This phenomenon can be effectively explained by the following first-order growth law [17].
where, \({MFR}^{SN}\) represents the steady-state fouling resistance. The integration of Eq. 3 at t = 0 and \({MFR}^{N}\)=0 (at the initial condition), provides [4],
where k generally represents the fouling layer’s growth rate, however, in Eq. 4, k denotes the constant of proportionality. Therefore, a graph representing \(\mathrm{ln}\left[\left({MFR}^{SN}\right)/\left({MFR}^{SN}-{MFR}^{N}\right)\right]\) against ‘t’ contributes a line having a slope’k’, which passes through the origin.
Furthermore, the graph denoting the pure water flux at various TMP provides a line with a slope LPM (membrane permeability). The membrane hydraulic resistance in terms of permeability can be denoted as:
where,\({\mu }_{PW}\) is in Pa.s, \({MHR}^{N}\) is in m−1 and \({L}^{PM}\) is in m/Pa.s.
Physico-chemical analysis
The CA juice after UF was analysed in terms of total soluble solids (TSS), pH, viscosity, clarity, turbidity, color, ascorbic acid content, total tannin content and total polyphenol content. The TSS (in °Brix), pH, viscosity (in centipoise, cP) and turbidity (in Nephelometric Turbidity Units, NTU) were determined using a digital refractometer (RFM 700, Bellingham and Stanley, UK), pH meter (Eutech, India), viscometer (DV2TLV, Brookfield, USA), and digital turbidity meter (Model 335, Deluxe Company, Bengaluru, India), respectively according to Benam et al. [18].
The colour and clarity of the juice were measured by analysing its absorbance at 420 nm and transmittance at 660 nm, respectively, in a UV–visible spectrophotometer (AU2701, Systronics India Ltd., Bengaluru, India) [19]. The total phenolic content was analysed using the Folin – Ciocaltaeu method and was expressed in mg Gallic acid equivalent (GAE)/100 ml [20,21,22]. The total tannin content was determined using the Folin-Denis method and was expressed in mg tannic acid/100 ml [23, 24]. The gallic acid and tannic acid standards for the study were procured from Otto Chemie Pvt. Ltd. (Mumbai, India) and Sigma-Aldrich Corporation (St. Louis, USA) respectively. The ascorbic acid content was measured by the 2,6- dichlorophenol indophenol method and was expressed in mg/100 ml [2, 23, 24]. The standard ascorbic acid and 2, 6-dichloroindophenol were purchased from HiMedia Laboratories Pvt. Ltd. (Mumbai, India).,
Microbial examination
The microbial stability of the ultrafiltered cashew apple juice, represented as colony-forming unit per mL (CFU/mL), was evaluated by determining the yeast and mould count (YMC), and total plate count (TPC). For this purpose, the clarified juice from the ultrafiltration unit was directly taken to the laminar airflow chamber aseptically using a sterilized pipe [14]. 0.1 mL of the juice was then serially diluted from 101 to 105, and then 0.01 mL of each was then spread to potato dextrose agar (for YMC) plates and nutrient agar (for TPC) plates. Finally, the plates were incubated for 72 h at 25 °C for YMC and 24 h at 37 °C for TPC. After incubation, the plates were observed for colony growth and were manually enumerated. The colony-forming units were calculated using the following equation.
Storage study
The storage study of the ultrafiltered CA juices was conducted for 12 weeks using colourless glass bottles made of borosilicate. All the glass bottles, pipelines, valves, and pumps were dried at 80 °C for 15 min after washing with 30% H2O2 to kill microorganisms [17]. The samples were collected in the bottles to the brim, without any headspace. It was then stored at 4 °C (refrigerated temperature) after wax sealing (airtight), and various quality attributes such as pH, TSS, viscosity, clarity, total solid content, turbidity, color, ascorbic acid content, antioxidant activity, total tannin content, and total polyphenol content were determined every week. Furthermore, the microbial parameters (YMC and TPC) were also conducted on the 0th and 12th weeks, as mentioned above.
Statistical analysis
A completely randomized design with MWCO (5 kDa, 10 kDa, 30 kDa, and 50 kDa) and TMP (35 kPa, 69 kPa, 103 kPa, and 138 kPa) as two variables was utilized for this study. A total of 16 experiments were investigated at different combinations of MWCO and TMP. All the experiments were repeated thrice and the data were expressed, except otherwise specified, as mean± standard deviation (SD). Correspondingly, the Duncan homogeneity test using SPSS software (version 23.0, IBM Corporation, Armonk) was utilized to measure the significant differences (confidence level: 95%) [25].
Results and discussion
Effect of process parameters on permeate flux
The effect of MWCO and TMP on the permeate flux profiles is presented in Fig. 2. The figure reveals that for all the combinations of process parameters, the permeate flux quickly declined during the beginning stage of the filtration. This phenomenon is due to the blockage of membrane pores and the growth in concentration polarization. Later, the permeate flux slowly and smoothly declined and reached a steady state after 10 min of the process. This can be attributed to the increase in solute accumulation over the surface of the membrane resulting in an acute concentration polarization and the formation of a cake layer on the surface of the membrane [13, 26, 27]. Cai et al. [11] also observed a similar trend in permeate flux while working on the UF of apple juice.
Both MWCO and TMP have an evident influence on the flux profile. The degree of flux decline for every operating condition is also evident in this figure. At a constant MWCO, the maximum flux decline was observed at the highest TMP (138 kPa). The Higher TMP carries more solutes towards the surface of the membrane, thereby increasing the fouling resistance as well as the flux decline [17]. The flux decline was relatively minutest at 34 kPa TMP for all the MWCO membranes. By the same token, the MWCO also provides a similar impact on the permeate flux. The highest flux decline was observed at the highest MWCO, at the same TMP. For example, at 138 kPa TMP and 50 kDa MWCO, the decline in flux is 29% (from 28 L/m2h to 20 L/m2h). However, at the same TMP, the flux decline corresponds to 5 kDa MWCO is 8% (7.4 L/m2h to 6.8 L/m2h). This occurrence is because the higher pore-sized membrane has more chance of blockage by the particle. The results were harmonized with Laorko et al. [27].
On the other hand, the highest steady-state permeate flux was observed at the highest TMP and for the membrane with the maximum MWCO (i.e., at 50 kDa MWCO and 138 kPa TMP), and the lowest was observed at the lowest TMP and for the membrane with minimum MWCO (i.e., at 5 kDa MWCO and 34 kPa TMP). However, at the higher TMPs of 138 kPa and 103 kPa, the difference in the steady-state flux between 30 and 50 kDa membrane is only 17%.
Physico-chemical analysis of permeate
The feed (microfiltered CA juice) was characterized by a TSS of 11.67°Brix, pH of 4.37, a viscosity of 1.36 cP, clarity of 99.51%T, turbidity of 1.89 NTU, color of 0.050 abs, ascorbic acid content of 227 mg/100 mL, total tannin content of 15.31 mg tannic acid/100 mL and total polyphenol content of 117.82 mg GAE/100 mL (Table 1). The effect of ultrafiltration process parameters such as TMP and MWCO on the quality of cashew apple juice is also given in Table 1. The table displays that the pH, clarity, color, and total tannin content of the feed and UF CA juice do not show any significant differences (at a 95% confidence level). However, significant differences (p < 0.05) were observed in the TSS, viscosity, turbidity, ascorbic acid content, and total polyphenolic content, at various combinations of MWCO and TMP. Gulec et al. [25] and Perreault et al. [28] also observed an insignificant difference (p < 0.05) in the pH value after UF of lemon and cranberry juice, respectively. Similarly, Panigrahi et al. [15] studied the UF of microfiltered sugarcane juice and reported that the UF process parameters do not significantly influence (p < 0.05) the juice's color and clarity. The reason might be because of the low molecular size of the feed compounds, which might have helped these compounds to pass through the UF membrane, irrespective of the process parameters.
Effect on TSS, viscosity, and turbidity
The TSS and viscosity of the feed and the UF juice are given in Table 1. There was a significant reduction (p < 0.05) in TSS (17–65%) and viscosity (4–21%) after UF. The influence of MWCO and TMP on the TSS and viscosity of the juice followed a similar trend. The highest TSS and viscosity (minimum reduction) were obtained at the maximum TMP (138 kPa) and MWCO (50 kDa). However, the minimum TSS and viscosity (maximum reduction) were observed at the lowest TMP (34 kPa) and MWCO (5 kDa). In the case of viscosity, the effect of TMP on it was insignificant, and the effect of MWCO was significant (p < 0.05). A minimum viscosity is the desirable characteristic of a good juice [29]. Though the reduction in viscosity for the 50 kDa membrane was only 4%, the 30 kDa, 10 kDa, and 5 kDa membranes drastically reduced the viscosity of the juice by 15%, 18%, and 21%, respectively.
On the other hand, the TSS content showed an increasing trend with the increase in TMP. However, this trend is insignificant (p < 0.05) for all the MWCO membranes except 5 kDa. Karmakar and De [17] also observed a similar relationship between TMP and TSS content during the ultrafiltration of tender coconut water. Nevertheless, the effect of MWCO on the TSS content was significant (p < 0.05). The reduction in TSS content for 50 kDa, 30 kDa, 10 kDa, and 5 kDa was about 18%, 30%, 32%, and 60%. This shows that the TSS reduction is maximum for the membrane with a larger pore size and minimum for a smaller pore size. In addition, the TSS reduction between the 30 kDa and 10 kDa is comparable. This reduction in TSS and viscosity is mainly due to the retention of suspended and dissolved solutes by the membranes [30].
There was a 98% reduction in turbidity after the microfiltration [4] of cashew apple juice. However, after UF, the turbidity of the juice further reduced from 1.89 NTU to 0. NTU. Yet, the effect of TMP on turbidity was mostly insignificant (p < 0.05). The reduction in turbidity is primarily due to the blocking of turbidity-causing juice components by the membrane [14]. Hence, due to the higher membrane pore size, the slightest reduction in turbidity was observed for the 50 kDa MWCO membrane, and the highest reduction was obtained for the 5 kDa MWCO membrane at 34 kPa TMP. The 30 kDa and 10 kDa have a significantly similar (p < 0.05) effect on turbidity reduction, and it was 32% higher than that of 50 kDa.
Effect on the ascorbic acid content
A significant (p < 0.05) ascorbic acid reduction of 8–34% was observed after UF. The reduction in ascorbic acid can be attributed to the exposure of cashew apple juice to oxygen and light during the filtration process [31]. Hence, the highest ascorbic acid was observed in the process with maximum treatment time (at 5 kDa MWCO and 34 kPa TMP), and the least ascorbic acid was achieved for the process with minimum treatment time (at 50 kDa MWCO and 34 kPa TMP). The effect of TMP and MWCO on ascorbic acid reduction was also similar. Since a higher MWCO and TMP requires less treatment time, the ascorbic acid reduction decreases with an increase in MWCO and TMP. However, at 138 kPa TMP, the reduction in ascorbic acid between the 50 kDa and 30 kDa MWCO membranes was only 4%. This result was in agreement with the observations of Laorko et al. [27] after studying on pineapple juice.
Effect on total phenolic content
The total phenolic content between the feed and the juice filtered at 50 kDa membrane was significantly the same (p < 0.05). However, there was a reduction in phenolic content at smaller MWCO membranes such as 30 kDa, 10 kDa, and 5 kDa. This reduction in phenolic content can be due to the elimination of high molecular weight compounds such as phenols bound to other juice components and self-conjugated phenolic compounds by the UF membrane [31]. Nevertheless, at these MWCOs, the TMP does not show any significant effect on phenolic content (p < 0.05). Conidi et al. [32] also reported a similar effect of TMP on phenolic content.
Process optimization
The productivity (steady-state permeate flux) and quality of the filtered juice were the basic parameters considered for optimizing UF process parameters (TMP and MWCO). Despite the fact that the highest steady-state flux of 19.85 L/m2h was achieved at 50 kDa MWCO and 138 kPa TMP, the physico-chemical characteristics of the juice at this condition were comparatively lower. However, at 30 kDa MWCO and 138 kPa, a steady-state permeate flux of 16.55 L/m2h were attained, only 16% lesser than the flux at 50 kDa MWCO and 138 kPa TMP, which is comparable. In addition, this condition provides a comparatively higher reduction in turbidity, viscosity, color, total tannin, and total phenolic contents, with comparable retention of ascorbic acid content. Hence, 30 kDa MWCO and 138 kPa were selected as the optimum condition for the UF of cashew apple juice.
At this optimum condition, the juice ultrafiltered has 8.22°Brix TSS, 4.36 pH, 1.16 cP viscosity, 99.88%T clarity, 1.22 NTU turbidity, 0.064 abs color intensity, 196.00 mg/100 mL ascorbic acid content, 13.49 mg tannic acid/100 mL total tannin content and 110.77 mg GAE/100 mL total polyphenol content.
Microbial examination
The TPC and YMC were determined to check the presence of microbial population in the CA juice after UF at its optimum process conditions. The microfiltered cashew apple juice showed a TPC count of 9 × 102 CFU/mL and a YMC of 14 × 101 CFU/mL. However, the TPC and YMC after the UF process were zero, indicating that the 30 kDa MWCO and 138 kPa TMP UF treatment completely removed the aerobic microbial population from the CA juice. Various researchers also reported the complete elimination of microorganisms after the UF of different fruit juices such as sugarcane juice [15], jamun juice [14], and pineapple juice [27].
Modelling of flux decline
A resistance-in-series model as described by Abdullah et al., [4] was used to examine the permeate flux decline of the 30 kDa membrane. For this, after each experimental run, its membrane permeability was measured and the hydraulic resistance (\({MHR}^{N})\) was determined using Eq. 5. The variation of \({MHR}^{N}\) with regard to TMP ranges from 2.5 × 1013 m−1 to 2.6 × 1013 m−1 and the average \({MHR}^{N}\) (\({MHR}_{AVG}^{N})\) attained was 2.5 × 1013 m−1. Additionally, the irreversible resistance of the membrane, after cleaning, was measured using Eq. 1 and observed insignificant (p < 0.05) as compared to membrane resistance.
Moreover, the fouling resistance of the Nth experiment was determined using Eq. 2. Also, \({MFR}^{SN}\) and k represents the fouling resistance (steady-state) and the rate constant for the developed fouling layer, respectively. The \({MFR}^{SN}\) for every process variables are interrelated with TMP as:
where \({MHR}_{AVG}^{N}\) is in m−1 and \(\Delta P\) is in kPa.
The R2 of Eq. 7 is 0.99, hence, the effect of process variables on \({MFR}^{SN}\) can be efficiently expressed by this equation. It also shows that the fouling resistance has a negative correlation with the TMP. This might be due to the penetration of solutes through the membrane with the increase in TMP, which results in a reduction in fouling resistance.
Correspondingly, to determine the growth rate of fouling resistance, the slope of the straight-line between \(\mathrm{ln}\left[\frac{{MFR}^{SN}}{{MFR}^{SN}-{MFR}^{N}}\right]\) with time (t) was investigated for each process parameter. The rate constant (k) for every condition differs in a range from 0.0026 to 0.0636 s−1. Therefore, the average rate constant value, k = 0.0195 s−1 is used for every process parameter.
For the Nth experimental run, the flux profile at any point of time can be calculated using the following equation (Eq. 8).
Merging Eqs. (4), (7) and (8), the flux of the permeate can be calculated using the given equation,
where ∆P, \({\mu }_{P}\) and \({V}_{p}\left(t\right)\) is in kPa, Pa.s and L/m2.h, respectively.
Furthermore, the experimental and calculated permeate flux at different TMP is illustrated in Fig. 3. The experimental and calculated values are represented by symbols and solid lines, respectively. The closeness of the experimental and calculated data in the figure clearly indicates that the developed model represents the filtration behaviour accurately.
Storage study
The results of the storage study of the ultrafiltered CA juice for 12 weeks are shown in Fig. 4. All the parameters changed slightly during the 12-week storage period. The variation in the viscosity of the juice was observed after 5th week of storage. The viscosity of the juice was reduced by 4.31% (from 1.16 to 1.11 cP) by the end of storage. The pH of the juice was reduced gradually every week. A 7.57% (from pH 4.36 to 4.03) reduction was observed on the final day of storage. The reduction in pH of juice was minor compared to the 23% reported by Karmakar and De [17] after studying the effect of storage on ultrafiltered tender coconut water.
The turbidity of the juice increased (4.92%), and the clarity decreased (4.68%) marginally after storage. Besides, a two-fold increase in colour was also observed at the end of storage. The turbidity increased after the second week, the clarity reduced after the third week, and the colour changed after the fourth week of storage. Ghosh et al. [14] and Panigrahi et al. [15] also reported similar changes while studying jamun and sugarcane juice, respectively. Nevertheless, these changes were least noticeable, and in the 12th week, turbidity of 1.28 NTU, clarity of 95.21%, and colour intensity of 0.14 abs could be achieved, which were acceptable.
Correspondingly, there was a steady reduction in TSS (10.98%), ascorbic acid (23.68%), and total phenolic content (14.36%) after storage. According to Lu et al. [33], the reduction in TSS was mainly due to browning reactions and the degradation of sugars such as sucrose, fructose, and glucose. Similarly, the reduction of phenolic content might be due to polymerization and polyphenolic oxidation reaction, decreasing the number of free hydroxyl groups. The primary factors responsible for the loss of ascorbic acid during storage are light, oxygen, and temperature. However, during low-temperature storage, the ascorbic acid degradation is mainly because of the oxygen present in the bottle headspace.
Half-life or 50% reduction in ascorbic acid is used to determine the shelf life of the juices [27]. Here, an ascorbic acid reduction of only 23.68% was observed after 12 weeks of storage, indicating the superior quality of the product. It can also be witnessed that the total tannin content of the stored juice increased from 13.49 to 15.03 mg tannic acid/100 mL, only. Therefore, the ultrafiltered CA juice can be successfully stored for 12 weeks without significantly altering the physicochemical properties.
Microbial examination
The TPC and YMC of the ultrafiltered CA juice were determined at the beginning (0th week) and the final day (12th week) of the storage study. The TPC was done to investigate the presence of bacterial populations, whereas the YMC was done to evaluate the presence of yeast and mold. In the 0th week and 12th weeks, the number of colonies detected for both microbial examinations (TPC and YMC) was zero. It indicates that the low temperature (4 ± 1 °C) storage can ensure the microbial stability of juice for a minimum period of 12 weeks. Similar results have been reported by Ghosh et al. [14] and Panigrahi et al. [15] while studying the storage stability of jamun and sugarcane juice, respectively.
Conclusion
Consumer prefers clarified juices due to its sole taste, flavour and light-transmitting property. In this current work, pre-treated cashew apple juice was clarified using hollow fiber ultrafiltration membrane at different MWCO and TMP to get the optimum process conditions. After considering the quality and productivity of the juice, 30 kDa MWCO membrane at 138 kPa TMP with a steady-state permeate flux of 16.55 L/m2h was selected as the optimum condition. At this condition the ultrafiltered cashew apple juice was found to retain maximum clarity, ascorbic acid content and phenolic content and minimum turbidity, color intensity and total tannin content. Additionally, a microbial examination was conducted and confirmed that the clarified juice is free from TPC and YMC. Furthermore, a storage study of the clarified juice showed that the ultrafiltered juice can be successfully stored in glass bottles for a minimum of 12 weeks at 4 °C. Finally, the behaviour of the ultrafiltration process was adequately explained by the developed resistance-in-series model. The study suggests that the UF process is suitable for developing microbial-free, clarified cashew apple juice and could be scaled up for industrial use.
Data Availability
The datasets generated during the current study are available from the corresponding author upon request.
References
FAOSTAT 2022 Food and Agriculture Data -Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#home
S. Abdullah, R.C. Pradhan, D. Pradhan, S. Mishra, Modeling and optimization of pectinase-assisted low-temperature extraction of cashew apple juice using artificial neural network coupled with genetic algorithm. Food Chem. 339, 127862 (2021). https://doi.org/10.1016/j.foodchem.2020.127862
I. Das, A. Arora, Post-harvest processing technology for cashew apple – a review. J. Food Eng. 194, 87–98 (2017). https://doi.org/10.1016/j.jfoodeng.2016.09.011
S. Abdullah, S. Karmakar, R.C. Pradhan, S. Mishra, Pressure-driven crossflow microfiltration coupled with centrifugation for tannin reduction and clarification of cashew apple juice: modeling of permeate flux decline and optimization of process parameters. J. Food Process Preserv. (2022). https://doi.org/10.1111/jfpp.16497
R. Kaprasob, O. Kerdchoechuen, N. Laohakunjit et al., Changes in physico-chemical, astringency, volatile compounds and antioxidant activity of fresh and concentrated cashew apple juice fermented with Lactobacillus plantarum. J. Food Sci. Technol. 55, 3979–3990 (2018). https://doi.org/10.1007/s13197-018-3323-7
S. Jafarzadeh, A. Mohammadi Nafchi, A. Salehabadi et al., Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid. Interface Sci. 291, 102405 (2021). https://doi.org/10.1016/j.cis.2021.102405
L. Ma, M. Zhang, B. Bhandari, Z. Gao, Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 64, 23–38 (2017). https://doi.org/10.1016/j.tifs.2017.03.005
T.T.H. Le, L.T.K. Vu, N.L. Le, Effects of membrane pore size and transmembrane pressure on ultrafiltration of red-fleshed dragon fruit (Hylocereus polyrhizus) juice. J. Chem. Technol. Biotechnol. 96, 1561–1572 (2021). https://doi.org/10.1002/jctb.6672
T. Vu, J. LeBlanc, C.C. Chou, Clarification of sugarcane juice by ultrafiltration membrane: toward the direct production of refined cane sugar. J. Food Eng. (2020). https://doi.org/10.1016/j.jfoodeng.2019.07.029
T. Urošević, D. Povrenović, P. Vukosavljević et al., Recent developments in microfiltration and ultrafiltration of fruit juices. Food Bioprod. Process. 106, 147–161 (2017). https://doi.org/10.1016/j.fbp.2017.09.009
M. Cai, C. Xie, Y. Lv et al., Changes in physicochemical profiles and quality of apple juice treated by ultrafiltration and during its storage. Food Sci. Nutr. 8, 2913–2919 (2020). https://doi.org/10.1002/fsn3.1593
P. Rai, S. De, Clarification of pectin-containing juice using ultrafiltration. Curr. Sci. 96, 1361–1371 (2009)
A. Roy, S. De, Resistance-in-series model for flux decline and optimal conditions of Stevia extract during ultrafiltration using novel CAP-PAN blend membranes. Food Bioprod. Process. 94, 489–499 (2015). https://doi.org/10.1016/j.fbp.2014.07.006
P. Ghosh, S. Garg, I. Mohanty et al., Comparison and storage study of ultra-filtered clarified jamun (Syzygium cumini) juice. J. Food Sci. Technol. 56, 1877–1889 (2019). https://doi.org/10.1007/s13197-019-03648-2
C. Panigrahi, M. Mondal, S. Karmakar et al., Shelf life extension of sugarcane juice by cross flow hollow fibre ultrafiltration. J. Food Eng. 274, 109880 (2020). https://doi.org/10.1016/j.jfoodeng.2019.109880
H. Susanto, A. Roihatin, I.N. Widiasa, Production of colorless liquid sugar by ultrafiltration coupled with ion exchange. Food Bioprod. Process. 98, 11–20 (2016)
S. Karmakar, S. De, Cold sterilization and process modeling of tender coconut water by hollow fibers. J. Food Eng. 200, 70–80 (2017). https://doi.org/10.1016/j.jfoodeng.2016.12.021
N. Sadeghzadeh Benam, M. Goli, S.M. Seyedain Ardebili, N. Vaezshoushtari, The quality characteristics of dough and toast bread prepared with wheat flour containing different levels of Portulaca oleracea leaf powder. Food Sci. Technol. 42, e60820 (2021). https://doi.org/10.1590/fst.60820
S. Abdullah, R.C. Pradhan, S. Mishra, Effect of cellulase and tannase on yield, ascorbic acid and other physicochemical properties of cashew apple juice. Fruits 76, 51–60 (2021)
A. Patra, S. Abdullah, R.C. Pradhan, Microwave-assisted extraction of bioactive compounds from cashew apple (Anacardium occidenatale L.) bagasse: modeling and optimization of the process using response surface methodology. J. Food Meas. Charact. 15, 4781–4793 (2021)
G. Patel, A. Patra, S. Abdullah, M. Dwivedi, Indian jujube (Ziziphus mauritiana L.) fruit juice extraction using cellulase enzyme: modelling and optimization of approach by ANN-GA. Appl. Food Res. 2(1), 100080 (2022)
A. Patra, S. Abdullah, R.C. Pradhan, Application of artificial neural network-genetic algorithm and response surface methodology for optimization of ultrasound-assisted extraction of phenolic compounds from cashew apple bagasse. J. Food Process. Eng. 44, e13828 (2021)
S. Abdullah, R.C. Pradhan, M. Aflah, S. Mishra, Efficiency of tannase enzyme for degradation of tannin from cashew apple juice : modeling and optimization of process using artificial neural network and response surface methodology. J. Food Process. Eng. (2020). https://doi.org/10.1111/jfpe.13499
A. Patra, S. Abdullah, R.C. Pradhan, Optimization of ultrasound-assisted extraction of ascorbic acid, protein and total antioxidants from cashew apple bagasse using artificial neural network-genetic algorithm and response surface methodology. J. Food Process. Preserv. 46, 1–17 (2022). https://doi.org/10.1111/jfpp.16317
Z. Jafari, M. Goli, M. Toghyani, The effects of phosphorylation and microwave treatment on the functional characteristics of freeze-dried egg white powder. Foods 11(17), 2711 (2022)
H.A. Gulec, P.O. Bagci, U. Bagci, Clarification of apple juice using polymeric ultrafiltration membranes: a comparative evaluation of membrane fouling and juice quality. Food Bioprocess. Technol. 10, 875–885 (2017). https://doi.org/10.1007/s11947-017-1871-x
A. Laorko, Z. Li, S. Tongchitpakdee et al., Effect of membrane property and operating conditions on phytochemical properties and permeate flux during clarification of pineapple juice. J. Food Eng. 100, 514–521 (2010). https://doi.org/10.1016/j.jfoodeng.2010.04.039
V. Perreault, N. Gouin, A. Bérubé et al., Effect of pectinolytic enzyme pretreatment on the clarification of cranberry juice by ultrafiltration. Membranes (Basel) 11, 1–17 (2021). https://doi.org/10.3390/membranes11010055
A. Servent, F.A.P. Abreu, C. Dhuique-Mayer et al., Concentration and purification by crossflow microfiltration with diafiltration of carotenoids from a by-product of cashew apple juice processing. Innov. Food Sci. Emerg. Technol. 66, 102519 (2020). https://doi.org/10.1016/j.ifset.2020.102519
S. Mondal, C. Rai, S. De, Identification of fouling mechanism during ultrafiltration of stevia extract. Food Bioprocess. Technol. 6, 931–940 (2013). https://doi.org/10.1007/s11947-011-0754-9
L. Zhao, Y. Wang, X. Hu et al., Korla pear juice treated by ultrafiltration followed by high pressure processing or high temperature short time. LWT - Food Sci. Technol. 65, 283–289 (2016). https://doi.org/10.1016/j.lwt.2015.08.011
C. Conidi, A. Cassano, F. Caiazzo, E. Drioli, Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. J. Food Eng. 195, 1–13 (2017). https://doi.org/10.1016/j.jfoodeng.2016.09.017
Q. Lu, L. Li, S. Xue et al., Stability of flavonoid, carotenoid, soluble sugar and vitamin C in ‘Cara Cara’ Juice during storage. Foods 8, 1–15 (2019). https://doi.org/10.3390/FOODS8090417
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdullah, S., Karmakar, S., Mishra, S. et al. Ultrafiltration of cashew apple juice using hollow fibers for shelf life extension: process optimization, flux modelling and storage study. Food Measure 17, 2182–2192 (2023). https://doi.org/10.1007/s11694-022-01790-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01790-8