Abstract
This article addresses the technical and cost competitiveness of microfiltration and centrifugation to produce organic clarified bottle gourd juice. The microfiltration (MF) process was conducted for fresh and centrifuged juices and for transmembrane pressures ranging from 103.4 to 172.4 kPa. Pertinent flux decline was extensively analyzed to account for the extent of irreversible membrane fouling using resistances in series model, flux decline, and recovery ratios. Feed, centrifuged, and permeate juice samples were evaluated for nutritional content and physico-chemical parameters to affirm that MF of fresh juice is the best among all investigated cases. Compared with the centrifugation process, the MF process performed comparatively better in terms of cost competitiveness, juice clarity, microbial, and protein rejection. For a variation in juice production capacity of 110–10,000 L, the MF-based juice processing cost varied from 1.19 to 0.37 $/L, which accounts to 31.27–26.32% of fresh juice cost to infer upon promising performance of the low-cost ceramic membranes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lagenaria siceraria (bottle gourd), popular Asian vegetable in the cucurbitaceae family (Mashilo et al. 2017), is well known to have excellent ingredients to treat diseases such as diabetes mellitus, flatulence, diarrhea and liver diseases, cardiovascular diseases, and hypertension (Ghule et al. 2006). The shelf life of fresh bottle gourd juice could be significantly enhanced through food processing techniques such as enzymatic treatment, straining or screening, centrifugation, freezing or solidification, thermal processing, and membrane separation (Grampp et al. 1978). Among these, membrane-based clarification of bottle gourd juice is highly promising due to lower energy consumption; room temperature operation; elimination of external chemical usage; ease of scale-up and higher productivity (Urošević et al. 2017). Microfiltration/ultrafiltration facilitates significant reduction in undesirable constituents such as haze and colloidal matter (such as pectin) and retention of desired constituents such as flavonoids, phenolic compounds, etc., and is therefore usually recommended for the clarification of bottle gourd juice (Laorko et al. 2013). While both ultrafiltration (UF) and microfiltration (MF) can be used for bottle gourd juice clarification, compared with the UF, MF has several advantages. These are reduced fouling due to wider membrane morphology, low pressure operation and hence lower cost, better product quality due to retention of nutritional constituents, and higher reversible resistances that can be handled through effective cleaning procedures.
Despite indicating the relevance of membrane technology to target effective clarification of various fruit and vegetable juices (such as, Indian blackberry, bitter gourd, apple, beet, black currant, bottle gourd, watermelon, etc.), a mature analysis of the process towards commercial application has not been addressed (Ghosh et al. 2018; Jain et al. 2018; Kujawa et al. 2015; Pap et al. 2012; Rai et al. 2010; Mondal et al. 2016). Most researchers ignored issues associated to conceptual material; fixed and operating cost analysis of the process with respect to centrifugation which can be regarded as the most competent technology for bottle gourd clarified juice production. Also, other relevant characteristics that affirm promising commercial application have been also ignored in these academic investigations. These membrane fouling characteristics, cleaning studies, transport resistances, and flux recovery/delay ratios during microfiltration have not been considered. On the other hand, while analysis of relevant nutritional characteristics of fruit and vegetable juices have been considered, it is important to observe that polymeric materials have been adopted for more complicated processes such as ultrafiltration, hybrid ultra-nano filtration, and osmotic membrane distillation processes. Thus, compared with the simpler microfiltration process, these processes are expensive due to involvement of complex process schemes, equipment, and stream interactions. Hence, the development of a simple yet efficient process needs to be addressed to target a technology that is as effective as the low-cost centrifugation technology for the clarification of vegetable juices such as bottle gourd juice.
Till date, microfiltration studies for bottle gourd juice clarification has been investigated by only one research group (Mondal et al. 2016) that focused towards technical but not economic competitiveness. The authors conducted ultrafiltration of bottle gourd juice using hollow fiber polymeric membranes (Mondal et al. 2016). For comparison, the authors also conducted microfiltration using polymeric membranes. At 104 kPa, the membrane provided 86% flux recovery ratio, 41% flux decline ratio, 60% protein rejection, and 46 L/m2 h steady-state permeate flux. The filtered juice possessed 4.3 mg GAE/mL of polyphenol and 1.8 °Brix total dissolved solids. The economic competence of low-cost ceramic membranes was addressed for the treatment of oil–water emulsions but not for juice processing systems (Suresh et al. 2016). In this work, low-cost green membranes have been targeted for their applicability using inexpensive precursors (such as clay and saw dust) and lower sintering temperature (about 900 °C).
Considering the technical and economic evaluation of low-cost green ceramic membranes and centrifugation as central objectives, this work addresses the microfiltration of bottle gourd juice using kaolin-based low-cost ceramic membranes. Three trans-membrane pressure differential values (103.4, 137.9, and 172.4 kPa) have been chosen to evaluate membrane performance in terms of pertinent flux decline, irreversible and reversible fouling, and permeate quality. The pressure differential range was based on the ceramic membrane morphological characteristics. Fresh and centrifuged juices were considered as feed systems to evaluate membrane performance. The permeate quality of the bottle gourd juice was evaluated in terms of carbohydrate content, protein content, vitamin C content, total flavonoids, total phenol content, pH, salt content, total dissolved solids (TDS), color, and clarity. The pertinent flux decline has been evaluated in terms of several alternate modeling approaches to obtain insights into the extent of irreversible fouling. Thereby, the ultimate objective of this work is to evaluate the techno-economic competitiveness of low-cost green ceramic membranes with respect to polymeric membranes and centrifugation technology.
Materials and Methods
Materials
Sodium metasilicate nonahydrate (95% pure) and kaolin powder (99% pure) were procured from Central Drug House (P) Ltd., New Delhi, India; feldspar was acquired from National Chemicals, Gujarat, India; sodium hydroxide pellets, sodium hypochlorite solution, oxalic acid dehydrate, sodium carbonate, aluminum chloride anhydrous sublimed, sucrose, sodium nitrite sulfuric acid (98%), and boric acid (99.5% pure) were purchased from Merck India; Anthrone extrapure and 2, 6-dichlorophenol indophenol sodium salt (DCPIP) were procured from SRL Pvt. Ltd., India; sodium bicarbonate was procured from Rankem, India; l-Ascorbic acid, gallic acid monohydrate, and Folin-Ciocalteu phenol reagent were obtained from Sigma-Aldrich, USA. Nutrient agar was procured from HiMedia Laboratories, India. Quercetin dihydrate (extrapure) and bovine serum albumin (BSA) were procured from SRL, India, and Bradford Reagent was obtained from Abcam, India. Saw dust was prepared from wood flakes purchased from local furniture shops, and fresh bottle gourds were purchased from regional vegetable vendors.
Membrane Fabrication
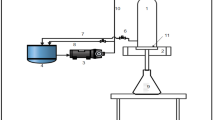
Low-cost ceramic membranes were fabricated using low-cost membrane precursors, namely, kaolin, feldspar, saw dust, sodium metasilicate, and boric acid in 48 wt.%, 28.62 wt.%, 8.19 wt.%, 7.5 wt.%, and 7.5 wt.%, respectively and uniaxial dry compaction method (Chakraborty et al. 2018). The membranes possessed an average pore size of 1 μm and porosity of 28.47%. Other details with respect to fabrication process and membrane characterization parameters have been reported elsewhere (Chakraborty et al. 2018). Indigenous laboratory scale dead-end microfiltration setup (shown in Fig. 1) has been deployed for the experimental investigations. The setup consists of two sections namely the bottom section that holds the membrane and the top section that holds the fluid. Nuts and bolts have been used to achieve a gas-tight assembly of both sections. To do so, silicon gaskets were used. Higher pressure has been facilitated in the setup by using an air compressor (Make: Crompton Greaves, Model: 190TC1). To facilitate cross-flow microfiltration, the suggested precursor composition can be utilized to achieve a hollow-fiber membrane module using procedures reported in the literature (Aziz et al. 2019) Thereby, modules compatible for medium scale continuous process operations can be realized. For such circumstances, the total permeation resistance is anticipated to reduce by 30 to 40% due to the greater role of shear effect in reducing irreversible membrane fouling. Thus, in situ membrane fouling using cross-flow operation is very likely to have greater role of reversible fouling, which can be easily overcome through standard cleaning procedures. Thereby, the sustainability of the membrane module shelf life can be enhanced to 5 to 7 years.
Bottle Gourd Juice Preparation
Firstly, the bottle gourd juice preparation process involves washing and peeling of bottle gourd. Thereby, the peeled bottle gourd was sliced into small pieces and fed to a mixer grinder. The paste was then filtered using cloth filter, and the filtrate was used for clarification purpose. For the study involving centrifuged juice, the filtrate was centrifuged (Centrifuge Model No. 2-16P, Make: Sigma, Germany) at 10000 rpm for 10 min.
Microfiltration of Bottle Gourd Juice
Dead-end microfiltration study of bottle gourd juice was carried out using ceramic membranes at various applied pressures (103.4, 137.9, and 172.4 kPa) using both fresh and centrifuged juices. Before initiating microfiltration experiments, the membrane was compacted and a pure water (Mili-Q water; Model No.: Elix-3, Milli-Q; Make: M/s Millipore, USA) run was taken to note pure water flux at various pressures. The permeation setup was then filled with adequate amount of juice. Desired trans-membrane pressure differential was applied, and samples were collected at time intervals of 1 min. Pure water and permeate flux were evaluated with the measured data using following expressions (Nandi et al. 2009):
where,
- Jw:
is the pure water flux of the membrane, m3/m2sec
- Vs:
is the volume of the setup, m3
- Am:
is the effective permeable area of the membrane, m2
- t:
is the time required for the entire volume of water to flow out of the setup, sec
- Jp, t:
is the permeate Flux at time t, m3/m2sec
- Vt:
is the volume of the permeate collected at time t, m3
- tt:
is the instantaneous time, sec.
Flux Decline Study
The variation of the permeate flux with time was noted. The measured data was represented through a flux decay plot that summarizes time dependent flux decline. The flux decline plot is usually dependent on the degrees of freedom which for the considered case study refer to trans-membrane pressure differential and type of juice. Pure water flux of the membrane (before and after fouling) was measured so as to evaluate flux decline coefficient of the membrane (FDC). The FDC is a measure of the extent of membrane fouling after microfiltration and is expressed as (Mondal et al. 2016):
where,
- J0:
is the initial water flux of the membrane, m3/m2sec
- Jp:
is the water flux of the fouled membrane, m3/m2sec.
Membrane Cleaning
After each experiment, membranes were cleaned using a standard cleaning process adopted for membrane technology (Li et al. 2018). The cleaned membranes were subjected to pure water flux evaluation test to determine flux recovery ratio (FRR) (Mondal et al. 2016). FRR enables evaluation of flux recovery of the membrane after cleaning and hence indicates extent reversible fouling. The FRR is determined using the expression:
where,
- Jc:
Pure water flux of the cleaned membrane, m3/m2sec
- Jw:
Pure water flux of the original membrane, m3/m2sec.
Membrane Fouling Mechanisms
The following expressions sequentially represent complete, standard and intermediate, and cake filtration pore blocking models under constant pressure filtration conditions (Hermia 1982):
Among the above four alternate pore-blocking/filtration models, the most competent model would be chosen based on fitness of measured data (R2 values), positive slope, and positive intercept of the models.
Fouling Resistances
The total fouling resistance (Rt) can be regarded to be the sum of hydraulic resistance (Rm) (offered by the membrane material), reversible resistance (Rr) (caused primarily due to formation of cake layer and concentration polarization), and irreversible resistance (Ri) (irreversible blocking of membrane pores). The total fouling resistance is represented with the expression (Emani et al. 2014):
The total fouling resistance is evaluated using the expression:
The hydraulic resistance or the membrane resistance is determined using the equation:
The pure water flux after membrane cleaning is used to determine irreversible resistance of the membrane as:
Role of Fouling on Membrane Morphology
During microfiltration, the pores of the membranes get fouled and eventually reduce the flow area. This variation in the membrane morphology may be attributed to the reduction in the pore diameter and porosity of the membrane. Ideally, membrane pure water flux can be expressed as (Nandi et al. 2011):
The variant membrane permeate flux can be represented using the expression:
The variation of the pore length may be considered to be negligible in comparison with the variation in cake layer thickness. Hence, the variation in the morphological parameters with time may be obtained as follows:
In the above expressions, terms \( {\varepsilon}_{m,t}{d}_t^2 \) and εmd2 may be called as the effective permeability factor of the membrane at time t and of the original membrane, respectively. Rearranging Eq. 7, the final expression becomes:
Feed and Permeate Analysis
The feed and the permeate samples were analyzed in terms of nutrient contents such as protein, carbohydrate, vitamin C and total flavonoids content, and physico-chemical properties such as pH, TDS, and salt concentration, color, and clarity. The protein content of the samples were determined using standard Bradford’s method (Bradford 1976), carbohydrate content using Anthrone’s method (Agbaire 2011), the vitamin C content using the DCPIP method (Anjali et al. 2012), total flavonoid content using aluminum chloride calorimetric method (Tharasena and Lawan 2014), and total phenol content was determined using Folin-Ciocalteu phenol reagent method (Tharasena and Lawan 2014). The pH, TDS, and salt concentration of the samples were determined using a digital meter (Model: AM-AL-01; Make: Aquasol, Mumbai, India). The color of the juice is expressed in terms of optical absorbance (A) measured using UV visible spectrophotometer at a wavelength of 420 nm, and the clarity of juice is expressed as percentage transmission (%T) that was evaluated using absorbance measured at a wavelength of 660 nm with the following expression (Mondal et al. 2016):
where, A is the absorbance at 660 nm.
Microbial Analysis
To conduct microbial analysis of the juice samples, the feed and permeate samples were subjected to several fold dilution, and the bacterial colonies were determined using the spread plate method. Nutrient agar was used as the culturing media. The concentration of the solutions was represented in terms of colony-forming units (CFU)/mL, and nutrient agar was used as the growth media. Visible colonies in the plates were counted and were converted to CFU/mL using the following expression (Khandpur and Gogate 2016):
The microbial separation efficiency of the membranes was evaluated using the expression (Kaniganti et al. 2015):
where,
- Cp:
is the CFU/mL of the permeate sample
- Cf:
is the CFU/mL of the feed sample.
Cost and Economic Evaluation
The economic competence of centrifugation and low-cost ceramic membrane process were considered based on capital and operating costs of various equipment and membrane fabrication costs. The membrane fabrication cost was evaluated as the sum of membrane raw material cost, equipment time handling costs for fabrication, electricity, manpower, and miscellaneous costs with membrane shelf life assumed to be 1 year. The fixed costs associated to microfiltration sub-process include costs of compressor, permeation setup and housing cost, and annual depreciation. The operating cost of the microfiltration sub-process was evaluated as a sum of membrane fabrication, cleaning, and associated manpower costs. For the centrifugation process, the fixed cost corresponds to the cost of the centrifuge, and the operating cost corresponds to the sum of electricity and manpower costs. The economic evaluation was first considered for an annual bottle gourd juice production capacity of 110 L, which was based on the membrane flux obtained after 80 min during dead end MF using the laboratory scale experimental setup. Thereafter, the economic competence of processes was evaluated for a higher throughput and juice production capacity up to 10,000 L per year. Further, industrial systems may utilize booster pumps and for such cases, the MF process economics are likely to be improvised by 20–30% due to lower costs of the booster pump (by 30 to 40%) in comparison with the expensive compressor cost. Therefore, the reported economic indices in this article could serve as useful benchmark for the cost competitive commercial processing of bottle gourd juice. Relevant cost parameters for economic evaluation have been summarized in Table 1 and relevant models associated to the cost and economic evaluation are summarized in Supplementary Material.
Results and Discussions
Flux Decline Analysis
Figure 2a presents the flux decline profiles for the fresh juice at 103.4, 137.9, and 172.4 kPa pressure differentials. The flux decline severity was maximum for 103.4 kPa followed with flux data obtained at 172.4 and 137.9 kPa. Usually, owing to significance or reduction in pore blocking, corresponding severity in flux decline is expected to increase or decrease respectively with applied pressure differential across the membrane. At lower applied pressure of 103.4 kPa, severe flux decline is accounted to pore blocking, which need not be the case at 172.4 kPa at which cake filtration is likely to control the flux decline profile. The intermediate pressure (137.9 kPa) indicated lowest severity in flux decline, and this is possibly due to the contribution of both pore blocking and cake filtration towards pertinent flux decline.
Similar investigations conducted for centrifuged juice as the feed indicated totally different trends in the flux decline profiles (Fig. 2b). For the case, the flux decline severity was maximum for 137.9 kPa but not 172.4 kPa and 103.4 kPa. Thus, juice pretreatment prior to membrane permeation can be analyzed to have strongly influenced pore blocking characteristics under varied pressure, given the fact that some critical heavier and complex biological macromolecules may have been omitted during centrifugation. The flux data for both fresh and centrifuged juices were taken thrice and were found to lie within an error of ± 3%.
Table 2 summarizes FDC and FRR values for various cases. For the fresh juice, pressure variation did not have a significant effect on both FDC (91.57 ± 0.65–94.13 ± 0.86) and FRR (91.89 ± 0.49–94.54 ± 0.72). On the other hand, significant variations in FDC and FRR do exist for the centrifuged juice with respect to applied pressure. Lowest value of FRR was obtained for the centrifuged juice at 137.9 kPa, which accounts to irreversible fouling that occurred due to significant pore blocking. For the same case, lowest value of FDC was obtained at 103.4 kPa, thus indicating that lower pressure was favorable for centrifuged juice. However, this needs to be further verified from the permeate quality, given that the fact that higher flux need not confirm upon good permeate quality. On the other hand, for the fresh juice, FRR values are significantly higher and indicated lower reversible fouling (about 6–7%).
Competent Fouling Mechanisms
Figures 3 and S1 respectively present the fitness plots for various flux decline models summarized in the “Membrane Fouling Mechanisms” section for fresh and centrifuged bottle gourd juice respectively. Table 3 summarizes corresponding R2 values. For the fresh juice case, highest R2 values have been obtained for cake filtration model (Table 3) and Fig. 3d also indicates the same. However, negative intercept is affirming that cake filtration model is invalid. The next model with maximum R2 values is the intermediate pore blocking model, which fortunately also indicates positive slope and intercept. Overall, Fig. 3 and Table 3 affirm that any pore blocking model is unable to represent pertinent flux decline. Hence, short-term flux decline of the fresh juice appears to have a significant non-fitness with the standard pore blocking models. The fitness of intermediate pore blocking model at a later stage of microfiltration for the case refers to the membrane pore sizes to be comparable with the particle sizes of the solutes in the feed juice constitution. Thereby, significant in situ flux decline occurred.
For the centrifuged juice case (Fig. S1), at 103.4 kPa pressure, complete and standard pore blocking models had similar competence in terms of model fitness (R2 values are very close to one another and close to 1). However, at higher pressure, intermediate pore blocking model can be analyzed to be most competent. The lack of fitness of cake filtration model conveys that the membrane morphology needs to be further engineered to achieve minimal fouling, maximum flux, and appropriate permeate quality.
Further insights into the onset of various fouling mechanisms with time of microfiltration operation can be obtained by considering the flux decline profiles as a combination of short-term and long-term flux declines. The flux decline data has been considered as two separate sets corresponding to two time intervals (1–15 min and 15 min onwards). For each time duration, all four fouling mechanism models have been considered for both fresh and centrifuged juice. Table 4 summarizes the R2 values obtained for the four alternate models for fresh juice. It has been analyzed that standard and intermediate pore blocking have best fitness to represent initial (short term) and final (long term) flux decline profiles. Similar observations were made for centrifuged juice (Table S1).
Membrane Resistances
The variation of total permeation resistances to the flow of the permeate for both fresh juice and centrifuged juice with variation on applied pressure (Fig. 4 a and b) indicates that with increasing time, the total resistance increases gradually from 5.39 × 1012 to 6.39 × 1013 m2/m3, 4.60 × 1012 to 7.84 × 1013 m2/m3, and 8.38 × 1012 to 1.05 × 1014 m2/m3 respectively at 103.4 kPa, 137.9 kPa, and 172.4 kPa applied pressures for fresh juice and from 6.37 × 1012 to 1.75 × 1012 m2/m3, 4.45 × 1012 to 1.03 × 1013 m2/m3, and 6.83 × 1012 to 1.01 × 1013 m2/m3 respectively at 103.44 kPa, 137.9 kPa, and 172.4 kPa applied pressures for centrifuged juice. The membrane permeation resistance for pure water was evaluated to be 1.98 × 1011 ± 0.43 × 1011 m2/m3. With an increase in pressure, the total permeation resistance also increases for fresh juice. For centrifuged juice, total permeation resistance was minimal for 103.4 kPa case. However, the permeation resistance at 137.9 kPa and 172.4 kPa are quite similar to each other. Fresh juice offered greater permeation resistance due to the presence of significant amount of large solute particles that are removed during centrifugation for centrifuged juice.
The contributions of different resistances viz., support resistance, irreversible resistance, and reversible resistance on the total resistance were evaluated (Table S2). The reversible resistance (due to concentration polarization and cake layer formation on the support) varied from 95 to 99.7%. However, for centrifuged juice and 103.4 kPa case, the contribution of irreversible resistance is 85.70%, and the contribution of support resistance to the total resistance was marginally higher. This is due to significant irreversible pore blocking of the membrane with time.
Morphological Changes During Microfiltration
The time dependent variation of effective permeability factor (\( {\upvarepsilon}_{\mathrm{m},\mathrm{t}}{\mathrm{d}}_{\mathrm{t}}^2 \)), as a function of the original membrane effective permeability (εmd2), has been evaluated for fresh and centrifuged juice cases at various applied transmembrane pressure differentials (Figs. S2 a and b). For both cases and for all pressure values, the effective permeability factor decreases sharply for the first 20–30 min followed with gradual reduction in the membrane morphology.
Comparing the fresh juice and centrifuged juice, the membrane treated with centrifuged juice was found to be less prone to pore blocking as compared with the membranes treated with fresh juice, since, the centrifugation removes larger particles from the juice. At reduced applied pressure, the pore blocking and internal fouling of the membranes were lesser as compared with higher pressures, in case of centrifuged juice. A reverse trend was observed for the microfiltration carried out using fresh juice due to formation of cake layer on the membrane surface at lower applied pressure. Hence, the plots obtained in Fig. 2a and b were found similar to the plots (Fig. S2 a and b) obtained here.
Permeate Quality Analysis
After conducting microfiltration experiments, the permeate and feed samples were tested for carbohydrate content, protein content, vitamin C content, total flavonoids, total phenol content, pH, salt content, TDS, color, and clarity (Table 5 a and b). Irrespective of pressure differentials, the vitamin C content remained fairly constant (28.16 ± 0.93 mg/g) for both feed and permeate samples. For the fresh juice, best permeate quality was obtained at 103.4 kPa in terms of highest total phenols and flavonoids content and minimal carbohydrate content in the permeate. Incidentally, the membrane performance at 103.4 kPa indicated severe fouling and hence the in situ fouling is regarded to have a strong role in influencing the superior permeate quality. Similar inference can be deduced for the permeate quality obtained for 137.9 kPa and fresh juice case, for which marginally inferior total phenols content and lower in situ fouling were obtained than those obtained at 103.4 kPa. However, the protein content was found to be comparatively lower (192.8 ± 28.23) for 137.9 kPa applied pressure. The retention of pH is very important factor for the ceramic membranes which was highly sensitive towards carbonate precursor–based membranes (Kaniganti et al. 2015). Since pH retention can be observed for all permeate samples, saw dust can be concluded to an appropriate pore forming agent to fabricate low-cost ceramic membranes. On the other hand, for the centrifuged juice and all pressure differentials, the protein content in the permeate is significantly higher with minimal separation. While flavonoids and total phenols are significantly retained in the permeate, the presence of protein in the permeate is detrimental, as it enables significant reduction in the shelf life of the product.
For all cases, the pH and salt content remained similar in both feed and permeate samples. Significant reduction in color (0.15 ± 0.09 and 0.45 ± 0.18) and enhancement in juice clarity (92.46% ± 0.59 and 85.31% ± 1.81) were observed for fresh juice and permeate samples obtained at 137.9 kPa and 172.4 kPa. Among all cases, 137.9 kPa can be regarded to be the best due to optimal combinations of flux (highest among all cases) and appropriate permeate quality.
For the centrifuged juice, the juice quality was marginally higher (total phenols 20.62 ± 0.62 GAE/100 g and flavonoids 153.13 ± 11.64 to 148.2 ± 1.58 mg Quarcetin/g) than the permeate obtained with microfiltration. However, enhancement in clarity (92.46 ± 0.59%) and reduction in color (0.15 ± 0.09 A) and protein content (192.8 ± 28.23 mg/100 mL) were pertinent in the MF-clarified juice with respect to centrifuged juice. Hence, centrifugation and associated costs of energy and process can be omitted by replacing centrifugation with inexpensive ceramic membrane technology for bottle gourd juice clarification.
Microbial Analysis
The microbial analysis was conducted for fresh feed and microfiltered permeate samples (at 137.9 kPa), 100 times dilution, 24 h, and 37 °C. The microbial feed concentration was evaluated as 1.76 × 106 CFU/mL for fresh feed samples (Fig. S3a). The centrifuged juice sample was found to have a microbial concentration of 3.50 × 105 CFU/mL thus indicating 80.11% microbial separation via centrifugation process. The permeate samples did not exhibit visible growth after 24 h. However, after 48 h, small colonies (Fig. S3 b) were observed, using which permeate microbial concentration was evaluated as 1.10 × 104 CFU/mL. Using these values, the membrane rejection efficiency was evaluated as 99.37%. The obtained separation efficiency is comparable to that reported in the literature for microfiltered pineapple juice with 0.2-μm hollow fiber membranes (100% removal efficiency) (Laorko et al. 2013). Thus, it can be inferred that the low-cost ceramic membranes are suitable to retain microbes prevalent in the fresh juice samples.
Literature Comparison
The permeate quality in terms of various physico-chemical properties and nutrient content of the permeate obtained with microfiltration at 137.9 kPa pressure was compared (Table 6) with the literature data obtained with cross flow microfiltration and microfiltration–ultrafiltration hybrid process (Mondal et al. 2016). The permeate quality was found to be better with the dead-end microfiltration process investigated in this research work (676.6 ± 25.32 protein, 7.93 ± 2.12 mg GAE/100 g total phenolics, color 0.15 ± 0.09 A, and 92.46 ± 0.59% clarity) than reported in the literature for microfiltration (Mondal et al. 2016). The higher concentration of protein in the permeate obtained with the ceramic membrane is possibly due to wider pore size distribution in the ceramic membranes in comparison with the hybrid system.
Compared to the hybrid or integrated microfiltration–ultrafiltration system reported in the literature, the low-cost ceramic membrane provided better FRR (91.89 ± 0.49% but not 85%) but poorer FDC (94.13 ± 0.86% but not 40%). A lower FDC suggested in literature might be because of the cross flow process deployed by the authors. However, it shall be noted that 7–9% higher FRR is promising and indicative of greater irreversible fouling in the conducted work in comparison with the literature.
Economic Competence
Based on assumed cost parameters, the fabrication based cost for the low-cost ceramic membrane was evaluated to be 440.9 $/m2, which is comparable with the lower value reported in the literature (460–4000 $/m2) (Suresh et al. 2016; Cheryan 1998; Koros and Mahajan 2000; Tennison 2000). For a clarified juice production capacity of 110 L (at 137.9 kPa and 80 min processing time), the total juice processing cost has been evaluated to be 1.19 and 3.99 $/L (87.29 and 292.91 Rs/L) for microfiltration and centrifugation processes respectively. The microfiltration and centrifugation processes incur an additional processing cost of 31.26 and 79.52% respectively with respect to the fresh juice cost. A further introspection of involved costs is summarized in Fig. 5 for the microfiltration process. As shown, the MF-based clarification involved a processing cost of 0.372 $/L in which the fixed and operating costs contribute to 83.13 and 16.84% respectively. The evaluated fixed cost of the MF process (0.31 $/L) involves the contribution of total costs due to compressor, membrane housing assembly and depreciation as 82.82, 8.59 and 8.59% respectively. The evaluated operating cost of the MF process (0.06 $/L) involves the contribution of costs associated to membrane replacement, membrane cleaning and manpower cost during juice processing as 15.19, 42.41, and 42.41% respectively. The membrane replacement cost of 0.0095 $/L involves the costs associated to raw material, manpower, equipment, electricity, and miscellaneous costs as 23.74, 40.73, 6.48, 25.48, and 3.59% respectively. On the other hand, the centrifugation process involved a cost of 3.54 $/L in which the fixed and operating costs contribute to 93.39 and 6.61% respectively. The evaluated fixed cost of the centrifugation process (3.31 $/L) involves the contribution of total costs due to centrifuge, whereas, the operating cost (0.23 $/L) involves manpower cost and electricity cost as 77.7 and 22.9% respectively.
The scale-up related economic competence provided interesting insights (Fig. 6). As shown for the MF process, the juice processing cost reduced from 1.19 to 0.37 $/L for the MF process for an enhancement in the juice production capacity from 110 to 10,000 L/y of which only 31.27–26.32% of the total cost comprise of the process cost. On the other hand, the corresponding juice processing cost reduced from 3.99 to 0.42 $/L for the centrifugation process. Incidentally, the market price of bottle gourd juice is about 2–5 $/L, which is significantly higher than the costs evaluated based on fresh juice and juice processing costs (1.19–0.37 $/L) (Weblink 1 n.d.; Weblink 2 n.d.).
Conclusions
During clarification of bottle gourd juice, saw dust based ceramic membranes (1-μm pore size and 28.47% porosity) performed optimally at 137.9 kPa transmembrane pressure and for fresh juice. Thereby, the MF process technology omits the centrifugation process and associated process costs. The membrane exhibited a FDR and FRR of 91.57 ± 0.65–94.13 ± 0.86% and 91.89 ± 0.49–94.54 ± 0.72% respectively, despite undergoing intermediate and standard pore blocking. Compared experimental investigations addressed for centrifugation process, the ceramic membranes exhibited better clarity, microbial, and protein rejection along with similar values for other nutritional parameters but not total phenolic content. The ceramic membrane fabrication cost (conceptual) has been evaluated as 440 $/m2 and thereby, the juice processing conceptual cost have been evaluated to be 0.37–1.19 $/L, which is significantly lower than the centrifugation costs for lower production capacity. In summary, saw dust based ceramic membranes are promising technologies to serve as cost competitive filter aids.
Abbreviations
- J w :
-
pure water flux of the membrane
- J 0 :
-
initial water flux of the membrane
- J p :
-
water flux of the fouled membrane
- J c :
-
pure water flux of the cleaned membrane
- V s :
-
volume of the setup
- A m :
-
effective permeable area of the membrane
- t :
-
time required for the entire volume of water to flow out of the setup
- J p, t :
-
permeate flux at time t
- V t :
-
volume of the permeate collected at time t
- t t :
-
instantaneous time
- ΔP :
-
applied pressure
- η w :
-
viscosity of water
- η p :
-
viscosity of the permeate
- ε m :
-
volumetric porosity of the membrane
- ε m,t :
-
volumetric porosity of the membrane at time t
- d :
-
average membrane pore diameter
- d t :
-
average membrane pore diameter at time t
- l :
-
length of the membrane
- l t :
-
length of the membrane at time t
- FRR:
-
flux recovery ratio
- FDC:
-
flux decline coefficient
- CFU:
-
colony-forming unit
- C p :
-
CFU/mL of the permeate sample
- C f :
-
CFU/mL of the feed sample
- R t :
-
total fouling resistance
- R m :
-
hydraulic resistance or membrane resistance
- R r :
-
reversible resistance
- R i :
-
irreversible resistance
References
Agbaire, P. (2011). Nutritional and anti-nutritional levels of some local vegetables (Vernomia anydalira, Manihot esculenta, Teiferia occidentalis, Talinum triangulare, Amaranthus spinosus) from Delta State, Nigeria. Journal of Applied Sciences and Environmental Management, 15(4), 625–628.
Anjali, K. M., Singh, N., & Pal, K. (2012). Effect of sulphur dioxide on plant biochemicals. International Journal of Pharma Professional’s Research., 3(2), 538–544.
Aziz, M. H. A., Othman, M. H. D., Hashim, N. A., Adam, M. R., & Mustafa, A. (2019). Fabrication and characterization of mullite ceramic hollow fiber membrane from natural occurring ball clay. Applied Clay Science, 177, 51–62.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254.
Chakraborty, S., Uppaluri, R., & Das, C. (2018). Optimal fabrication of carbonate free kaolin based low cost ceramic membranes using mixture model response surface methodology. Applied Clay Science, 162, 101–112.
Cheryan, M. (1998). Ultrafiltration and microfiltration handbook (2nd ed.). Boca Raton: CRC press, LLC.
Emani, S., Uppaluri, R., & Purkait, M. K. (2014). Cross flow microfiltration of oil–water emulsions using kaolin based low cost ceramic membranes. Desalination, 341, 61–71.
Ghosh, P., Pradhan, R. C., Mishra, S., & Rout, P. (2018). Quantification and concentration of anthocyanidin from Indian blackberry (Jamun) by combination of ultra-and nano-filtrations. Food and Bioprocess Technology, 11(12), 2194–2203.
Ghule, B., Ghante, M., Saoji, A., & Yeole, P. (2006). Hypolipidemic and antihyperlipidemic effects of Lagenaria siceraria (Mol.) fruit extracts. Journal of Experimental Biology, 44(11), 905–909.
Grampp, E., Schmitt, R. & Urlaub, R. (1978). Method for clarification of fruit juice. In. P^pp. Google patents.
Hermia, J. (1982). Constant pressure blocking filtration laws: application to power-law non-newtonian fluids. Transactions of the American Institute of Chemical Engineers., 60(3), 183–187.
Jain, A., Sengupta, S., & De, S. (2018). Fundamental understanding of fouling mechanisms during microfiltration of bitter gourd (Momordica charantia) extract and their dependence on operating conditions. Food and Bioprocess Technology, 11(5), 1012–1026.
Kaniganti, C. M., Emani, S., Thorat, P., & Uppaluri, R. (2015). Microfiltration of synthetic bacteria solution using low cost ceramic membranes. Separation Science and Technology, 50(1), 121–135.
Khandpur, P., & Gogate, P. R. (2016). Evaluation of ultrasound based sterilization approaches in terms of shelf life and quality parameters of fruit and vegetable juices. Ultrasonics Sonochemistry, 29, 337–353.
Koros, W. J., & Mahajan, R. (2000). Pushing the limits on possibilities for large scale gas separation: which strategies? Journal of Membrane Science, 175(2), 181–196.
Kujawa, J., Guillen-Burrieza, E., Arafat, H. A., Kurzawa, M., Wolan, A., & Kujawski, W. (2015). Raw juice concentration by osmotic membrane distillation process with hydrophobic polymeric membranes. Food and Bioprocess Technology, 8(10), 2146–2158.
Laorko, A., Tongchitpakdee, S., & Youravong, W. (2013). Storage quality of pineapple juice non-thermally pasteurized and clarified by microfiltration. Journal of Food Engineering, 116(2), 554–561.
Li, W., Ling, G., Lei, F., Li, N., Peng, W., Li, K., Lu, H., Hang, F., & Zhang, Y. (2018). Ceramic membrane fouling and cleaning during ultrafiltration of limed sugarcane juice. Separation and Purification Technology, 190, 9–24.
Mashilo, J., Shimelis, H., & Odindo, A. (2017). Phenotypic and genotypic characterization of bottle gourd [Lagenaria siceraria (Molina) Standl.] and implications for breeding: a review. Scientia Horticulturae, 222, 136–144.
Mondal, M., Biswas, P. P., & De, S. (2016). Clarification and storage study of bottle gourd (Lagenaria siceraria) juice by hollow fiber ultrafiltration. Food and Bioproducts Processing, 100, 1–15.
Nandi, B., Das, B., Uppaluri, R., & Purkait, M. (2009). Microfiltration of mosambi juice using low cost ceramic membrane. Journal of Food Engineering, 95(4), 597–605.
Nandi, B., Uppaluri, R., & Purkait, M. (2011). Identification of optimal membrane morphological parameters during microfiltration of mosambi juice using low cost ceramic membranes. LWT- Food Science and Technology, 44(1), 214–223.
Pap, N., Mahosenaho, M., Pongrácz, E., Mikkonen, H., Jaakkola, M., Virtanen, V., Myllykoski, L., Horváth-Hovorka, Z., Hodúr, C., & Vatai, G. (2012). Effect of ultrafiltration on anthocyanin and flavonol content of black currant juice (Ribes nigrum L.). Food and Bioprocess Technology, 5(3), 921–928.
Rai, C., Rai, P., Majumdar, G., De, S., & DasGupta, S. (2010). Mechanism of permeate flux decline during microfiltration of watermelon (Citrullus lanatus) juice. Food and Bioprocess Technology, 3(4), 545–553.
Suresh, K., Pugazhenthi, G., & Uppaluri, R. (2016). Fly ash based ceramic microfiltration membranes for oil-water emulsion treatment: parametric optimization using response surface methodology. Journal of Water Process Engineering., 13, 27–43.
Tennison, S. (2000). Current hurdles in the commercial development of inorganic membrane reactors. Membrane Technology, 2000(128), 4–9.
Tharasena, B., & Lawan, S. (2014). Phenolics, flavonoids and antioxidant activity of vegetables as Thai side dish. APCBEE Procedia, 8, 99–104.
Urošević, T., Povrenović, D., Vukosavljević, P., Urošević, I., & Stevanović, S. (2017). Recent developments in microfiltration and ultrafiltration of fruit juices. Food and Bioproducts Processing, 106, 147–161.
Weblink 1 (n.d.) Retrieved on January 09, 2019 from https://www.pinhealth.com/products/axiom-lauki-swaras-500-ml?gclid=EAIaIQobChMI6r65iYnE3gIVyzUrCh1MKgpbEAQYAyABEgKCx_D_BwE&utm_source=google&utm_medium=cpc&utm_campaign=1598644213&ef_id=W864ZgAAAevz8PP%40%3A20181108060836%3As&fbclid=IwAR36KnVAitF9ZSWYyqHDDqBmIdTD8Ii-jkq5EMlpNrb-NTdYaUtoZ8fvXW8/.
Weblink 2 (n.d.) Retrieved on May 02, 2020 from https://www.shoponn.in/bottle_gourd_lauki_juice_bg336/167868?gclid=EAIaIQobChMI6r65iYnE3gIVyzUrCh1MKgpbEAQYByABEgJv4PD_BwE&fbclid=IwAR1orgFer2ox9dNkh8xJnBDBX6bXVFuZX-fZYUDK2lEan_gW8mIISyEzNCQ
Acknowledgements
The authors thankfully acknowledge the Department of Chemical Engineering, and Centre for Instrument Facility, Indian Institute of Technology Guwahati for providing necessary facilities for carrying out this research.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2816 kb)
Rights and permissions
About this article
Cite this article
Chakraborty, S., Das, C. & Uppaluri, R. Feasibility of Low-Cost Kaolin–Based Ceramic Membranes for Organic Lagernaria siceraria Juice Production. Food Bioprocess Technol 13, 1009–1023 (2020). https://doi.org/10.1007/s11947-020-02455-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-020-02455-4