Abstract
The present study investigates the effects of guar gum (GGM) based edible coatings containing citric acid (CA) as antibrowning agents and 0, 3, 5, and 10% of mint leaves extract (ME). The fabricated active film was characterized by measuring its physical and mechanical properties, barrier, antioxidant and antimicrobial activities. Storage tests at 23 °C for 15 days were performed for uncoated ber fruit, neat guar gum-citric acid, and guar gum-citric acid with mint leaves extract. Physicochemical and sensory parameters of coated ber fruit were measured. FTIR confirmed physical interactions between GGM and CA. A film with 10% of ME showed 1.81 ± 0.87 antimicrobial activities and 91.22 ± 024% antioxidant capacity. It displayed reduced respiration rate, TSS, weight loss, and was firmer than the non-coated ber at the storage end. The results designated that the film with 10% ME coating indicated a high perspective to prolong the storability of ber, and sensory aspects of 5 and 10% ME containing film increased, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, it is quite important to globalize the food market or industry to ensure the food quality and the extension of the shelf life, which plays a significant role in the field of the food industry. For the past few years, biodegradable material as an additive for antimicrobial and antioxidant properties and the edible packaging matrix has been the center of attraction for the food packaging industry due to concern on the hazardous waste problem and the depletion of natural resources by non-biodegradable products [1,2,3]. The photochemical properties, ecofriendly nature, safety concerns, and consumer acceptance make the plant extracts suitable as active agents for packaging applications [3,4,5]. Ber, a tropical and subtropical fruit, belongs to the family Rhamnaceae. Ber is also familiar as Zizyphus mauritiana vernacular to the land (Northern) hemisphere. Ber fruit is loaded richly with minerals like iron, calcium, and phosphorous and holds vitamin A and B in a reasonable amount [6, 7]. The nutritional value of ber is high compared to other fruits; it also contains carotenes and phenolic compounds such as p-hydroxybenzoic acid, p-coumaric acid, caffeic acid, and ferulic acid, making ber rich in antioxidants [8].
Due to the perishable nature of the ber fruit, it is unable to be stored for a long time, and the rotting of the fruit starts after 2–4 days at ambient temperature as the enzyme activity results in softening of fruit [9]. Along with short shelf life, chilling injury and postharvest rotting are primary concerns during storage time [10]. During the high season, a considerable quantity of ber fruits gets wasted, resulting in substantial postharvest losses leading to the requirement of proper postharvest management [6]. Irradiation preservation, dipping treatment, i.e., cold and hot water dipping, dipping in fungicides, potassium permanganate dipping, etc., growth regulators, zero energy cool chamber, treatment with 1-Methylcyclopropene, and low-temperature storage were some of the postharvest technology that slows down the respiration and ethylene gas production, control the growth of microorganisms and extends the shelf life of ber fruit [11,12,13].
Edible films and coating prolong the shelf life of fresh produce. It is used to improve the appearance of food products and deliver safety to the food products through its eco-friendly nature. One of the main advantages of using edible films, as compared to synthetic polymeric packaging, is that they are an integral part of the food product; they can be consumed without the requirement of unpacking, and the packaging waste disposable issues can be ruled out, as edible coating directly applied on the food and consumed along with the food. Furthermore, edible films are derived from edible components, offering the significant advantage of being eco-friendly. Among all the postharvest techniques, the edible coating has taken much attention in recent years due to its numerous benefits, i.e., it provides a barrier to moisture and oxygen, enhances textural features, retards the growth of microorganisms, and eliminates the problem of food decay. Control of the internal atmosphere of the coated food by actively incorporating substances was the motivation for the success of the edible coatings [14, 15]. Past studies showed the effectiveness of edible coatings in enhancing the shelf life of strawberries [16], cut apple [17], mango [18], grape [19], and plum [14]. The manufacturing cost for edible films and coatings is slightly high as the packaging desires to meet the hygienic requirements while in transit.
Guar gum (GGM) is water-soluble and edible film-forming polysaccharides extracted from the endosperm part of Cyamopsis tetragolonoba. It is a galactomannan with a side group of galactose associated with (1–6) α-d-galactopyranose and a backbone of mannose associated with (1–4) β-d-mannopyranose [20]. The absence of toxicity, emulsification activities, economical thickener, excellent stabilizer, and film-forming properties of guar gum enable its utilization in biomedical, pharmaceutical, textile, and food industries [21]. Recent studies showed the use of hydroxyl citric acid cross-linked with chitosan/guar gum/poly(vinyl alcohol) films [22]. Plant extract such as mint leaves extract (ME) was incorporated into the guar gum and citric acid (GGM/CA) films as an antioxidant, antimicrobial, and flavorings agent. ME comprises phenols and polyphenols with multiple structures like citronellol, α-pinene, and methyl eugenol, which impart functional attributes to the edible film. Mint extract is a rich source of polyphenolic compounds, and these compounds have antioxidant and antimicrobial activities. The edible coating incorporated with mint extract reduces the rate of oxidant and inhibits the growth of microorganisms [23]. To the best of our knowledge, the use of guar gum/citric acid/mint extract as an active edible food coating for the storage of ber fruit has not yet been reported.
The present research aims to develop the active edible coatings on ber fruit, which uses guar gum as a polymer matrix and mint leaves to extract as an active compound for functional attributes by dipping coating forming process. The effect of adding citric acid to the guar gum was also investigated. The consequence of incorporation of different concentrations of mint leaves extract on water vapor barrier properties of the resulting guar gum, citric acid incorporated with mint extract (GGM/CA/ME) film was evaluated. Also, the film was fabricated by the solution casting method, and after that, physical and chemical properties were assessed. The antimicrobial activity of the coating film was also examined. Finally, the influence of ME incorporated into GGM/CA films on ‘Ber’ fruit quality during storage was inspected.
Materials and methods
Materials
Mint leaves; Minthostachys mollis (lamiaceae) were collected from the Indian Institute of Technology (IIT) Roorkee, Uttarakhand, India. Guar gum powder E-230 (GGM, Brookfield viscosity-4400 cps) was provided by Nuevo Polymers Private Limited (Gurgaon, India). Citric acid was purchased from the Subhash Chemical Agencies (Noida, India). Glycerol as a plasticizing agent and methanol as a solvent for the extraction process was procured from Baga Chemicals Pvt Ltd, India (Moga, Panjab, India). The distilled water utilized in the film formation and all procured chemicals were of analytical grade.
Microwave-assisted extraction of mint leaves
The mint leaves extract was extracted using the microwave-assisted extraction technique as it was the edge over the other extraction techniques, i.e., less time consumption, high yield, and less solvent used. A domestic microwave oven was used for this extraction purpose. The collected mint leaves were washed with water to remove the dirt. Primarily, 20 g of mint leaves were added to the 500 mL flask, and then 200 mL methanol was added as a solvent into the flask. The mixture was placed inside the microwave oven and exposed to irradiation for a period of 40 s at 320 W irradiation power. After the extraction, the heated mixture was cooled down to room temperature and filtered through a Whatman 42 filter paper immediately after the cooling. The obtained supernatant was collected in a reagent bottle and stored in a cool atmosphere [24, 25].
Preparation of edible GGM/CA/ME coating film
Guar gum (GGM) and citric acid (CA) based functional film incorporated with mint leaves extract (ME) for edible coating applications were fabricated using solution casting described by [26] with slight modifications. The schematic illustration of the fabrication of edible coating/coating for ber fruits application is shown in Fig. 1. The film-forming solution for Neat GGM/CA film was formed by adding 0.75% (w/v) of guar gum and 0.5% (w/v) of citric acid in 100 mL of deionized water into the 200 mL volumetric flask. The prepared solution was placed onto the hot plate magnetic stirrer and stirred continuously for 2 h at 90 °C and at 500 rpm [27, 28]. Then, 40 wt% glycerol (on a guar gum basis) was added to the forming solution as a plasticizer. For GGM/CA/ME films, the mint extract was incorporated in 3, 5, and 10% (v/v) into the film-forming solution before the addition of glycerol. The formed solution was thoroughly mixed at 50 °C for around 30 min to get a homogeneous gel-like solution. The gel-like solution was poured into the 15 cm diameter petri plate and dried in a hot air oven at 50 °C for 24 h. The dried film was peeled off from the Petri plate, and the neat film was labeled GGM/CA. The films incorporated with mint extract were labeled as a GGM/CA/ME-3, 5, and 10%, respectively. All the developed films were stored in a controlled atmosphere at 24 °C and 50% RH before further characterization. Further, three sets of samples for each composition were taken for physical and mechanical properties [22].
Characterization of the developed GGM/CA/ME films
ATR FTIR analysis
An attenuated Total Reflectance Fourier Transform Infrared (ATR FT-IR) spectrophotometer (Perkin Elmer FTIR C91158, Chicago, USA) was used to draw the spectra of GGM/CA/ME films. ATR FTIR analysis was carried out to investigate the structural interactions of guar gum films incorporated with citric acid and mint extract. The analysis was carried out from wavenumber 4000 cm−1 to 400 cm−1 at a resolution of 4 cm−1 by using an FTIR spectrophotometer [2].
Morphology of films
The microstructure of the GGM/CA/ME films was visualized with the help of microphotographs of surfaces of the developed GGM/CA/ME films by utilizing a field emission scanning electron microscopy (FESEM) (MIRA3 LMH TESCAN USA) at an accelerating voltage of 5 kV. For clear microphotographs of the developed films, each film sample was sputter-coated with a thin gold layer utilizing gold discharge plasma which improves the conductivity of the sample mounted on aluminum stubs [5].
Water vapor permeability (WVP)
ASTM E-96 determined the WVP of fabricated GGM/CA/ME films with slight modifications. The cup method was utilized to determine the value of WVP of the developed GGM/CA/ME films with three replicates of each film. Each film sample was conditioned at 55% RH for 24 h. First, the films with a 25 cm2 area were sealed between the O-ring and the aluminum (al) cups, which were initially packed with 15 g of amorphous and porous silica gel. The cups sealed with films were correctly positioned in the desiccators maintaining a 75% RH by saturated sodium chloride (NaCl) solution. Finally, the desiccators with cups were kept in a controlled atmosphere maintained at 25 °C temperature. The weight gain by cups was measured every 24 h for 8 days [29]. The WVP of each sample was evaluated as per Eq. 1.
where Δp is the change in water vapor pressure inner and other side of the test sample (kPa), and X is the mean of the thickness of the test sample (mm).
Water vapor transmission rate (WVTR) was analyzed from the fluctuations in the test specimen weight (Δw) having a contact area A at a stated time period (Δt), as designated by Eq. 2.
X-ray diffraction
X-ray patterns were utilized to determine the crystalline behavior of the developed GGM/CA/ME films. X-ray patterns were recorded by using an X-ray diffractometer (Rigaku Ultima IV Japan) utilizing Cu Kα radiation at a voltage of 40 kV and a current of 40 mA. X-ray patterns were recorded between a range of 2θ = 3–80° with a scanning speed of 4° min−1. Each sample was dried and stored in a controlled atmosphere before testing [29].
Mechanical properties
The mechanical properties of the edible film, such as tensile strength (Ts) and elongation at break (EAB), were determined using the approach described by [26] with slight alterations. Rectangular film samples (1 cm × 10 cm) were conditioned at 50% RH for 48 h. These samples were clamped in the universal testing machine (INSTRON 5566, USA) with a 50 mm−1 min−1 cross-head speed, and the distance between the grips was 90 mm. Three samples were tested for each film composition, and a mean value was calculated for each composition.
where Ts = Tensile strength (MPa), F = Maximum tensile force at break (N), S = Area of cross-section of film (m2), EAB = Elongation at break (%), L0 = Original length of film (mm), L = Length of the film at break (mm).
Antioxidant activity of the GGM/CA/ME films
Diphenyl picryl hydrazine (DPPH) radical has been widely applied to test the free radical scavenging activity of compounds or potential hydrogen donors to evaluate the antioxidant activity. The scavenging activity of 2,2 diphenyl-1-picrylhydrazyl (DPPH) radicals were evaluated by edible films of GGM/CA/ME using a UV–Vis spectrophotometer. DPPH solution as reagent prepared by dissolving the 4 mg of DPPH in 100 mL of methanol [30]. Subsequently, 20 mg of each film sample were dissolved in vials in 5 mL of prepared DPPH solution. These vials were incubated in the dark at ambient temperature conditions for 12 h. Then UV–vis spectrophotometer was used to record the absorbance of the extracted solution at 517 nm. Pure methanol (99.99% purity) was used as a blank solution for spectroscopy. For each film composition, three samples were tested. The antioxidant activity of each active edible film was calculated according to the following Eq. 5.
where \({A}_{DPPH solution}\) and \({A}_{ sample extract}\) are absorbance value of DPPH solution and sample extract at 517 nm after 12 h storage.
Antimicrobial properties of GGM/CA/ME films
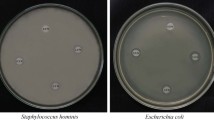
An antimicrobial analysis was carried out to study the % reduction of the growth of Escherichia coli (E. coli) using JIS Z 2801:2000, a technique for target microorganisms present on the film sample (40 × 40 mm). All film samples were kept under 20-W Ultraviolet (UV) light of wavelengths between 200 and 600 nm for 2 h to sterilize before the antimicrobial analysis. The microbes for the test is prepared, commonly by growing in a medium of culture. The suspension of the test microbes is regulated by dilution in a nutritive broth. Neat and surface test area were inoculated with 0.5 mL E. coli containing solution having 106 CFUs was poured on the surface of polyolefin film (40 × 40 mm), and the polyolefin film was then permitted to shield the test sample, avoiding it from vaporizing and confirming adjacent with the antimicrobial surface [29]. The growth of microbes is assessed on day 0 by elution, subsequently dilution, as well as plating. A neat sample was analyzed to confirm the elution method successfully deactivates the antimicrobial compound on the tested surface. Inoculated, protected, neat, and antimicrobial test surfaces are permitted to incubate uninterrupted in a moist environment for around 24 h. Furthermore, after incubation, the concentration of microbes is calculated. The reduction of microorganisms relative to initial concentrations and the control surface is calculated using Eq. 6.
B = Mean microorganism number in control film sample after 24 h, C = Mean microorganism number in edible films sample after 24 h.
Edible coating application on ber fruits
Freshly harvested ‘ber’ were procured from a local farm in Saharanpur, Uttar Pradesh, India. The ber fruits are taken to be identical in size, shape, and visual appearance, free from physical injuries during post-harvest operations and any possible disease symptoms. Previous studies have shown that active edible coating by dipping method on strawberries (Garcia et al. 2012), cut apple (Perez-Gago et al. 2006), and cut kiwi (Manzoor et al. 2021) has improved the physicochemical properties and shelf life of fruits. The ber fruits were randomly ordered into four coating formulations and one uncoated. All four coating formulations included ber fruits that were dipped in one of four coating formulations, namely GGM/CA, GGM/CA/ME-3%, GGM/CA/ME-5%, GGM/CA/ME-10% for 2 min, and further dried in a hot air oven at 35 °C temperature for 23 h presented in Fig. 2. Each ber fruit was prepared as uncoated and coated samples. After drying, the coated and non-coated ber fruits were further packaged in polyethylene bags and used for the storage test stored at 23 ± 2 °C and 75% RH for 15 days.
Quality attributes of ber fruits
The influence of GGM/CA/ME edible coating on the process of ripening of ber fruits was studied as a role of the storage period. The quality parameters, including respiration percentage, weight loss, firmness, TSS, and sensory evaluation, were considered for the storage test.
Respiration rate analysis
Three gas analyzer (Model F-950, M/s Felix Instruments Inc., USA) was used to analyze headspace gas (CO2) concentration in the package headspace. Ber fruits produce carbon dioxide during the respiration process. Each uncoated and GGM/CA/ME edible solution coated ber sample was packed in pouches, and they were used once for testing and discarded after assessment. The gas analysis was followed by the unpacking of ber fruits from pouches for further evaluation.
Total soluble solids (TSS)
TSS concentrations in fruits and vegetables are indexed as TSS. Blended ber pulp was squeezed via muslin cloth to extract the juice. PARISA BRIX_2014 digital refractometer (Parisa technology, Dahisar East, Mumbai, India) was then used to determine the TSS of the extracted sample. Results were expressed as TSS%.
Weight loss
Weight loss during 15 days of storage period was analyzed by weighing 10 ber fruit pieces of every coating treatment. The weight loss of ber was expressed as the percentage loss (%) of the initial weight.
Firmness of fruit
The uncoated and coated ber fruit firmness was determined with a Fruit texture analyzer (Mohr Digi-Test, MDT-1 Richland, WA, USA) equipped with an 11 mm MDT2-AC-P11M probe with a force that reached a 3% deformation of the ber diameter. Obtained results were expressed as the force–deformation (N mm−1).
Sensory analysis
The Sensory analysis of uncoated and coated ber fruit was performed to detect whether the GGM/CA/ME edible coatings did undesirably impact buyers' opinions. The members who participated in the sensory panel included 30 persons with ages group 20 to 40 years. All sensory panelists were earlier asked for any food ingredient allergies issues present in the developed coatings. Randomly, coded ber fruits were offered to the sensory panel and requested to score based on appearance, flavor, and overall acceptability of ber fruits through a 9-point hedonic scale ranging from “extreme dislike, score 9” to “extremely like, score 1”. Panel members also requested to confirm whether the application of the edible coating and the addition of mint leaves extract in the edible coating would impact their ber buying perception.
Statistical analysis
Statistical analysis for all the experimental data was performed using SPSS ver.25 (IBM Corporation, Germany). A one-way analysis of variance was calculated for the triplicate data, and the results were shown as a mean ± standard deviation value. Tukey post hoc test (p < 0.05) was used for testing significant differences.
Results and discussion
ATR FTIR analysis
The qualitative approach includes ATR FTIR analysis to study the functional groups present in the GGM/CA and GGM/CA/ME films. ATR FTIR analysis was pre-eminent to realize the change in chemical structure and to know the interactions among the various components of the GGM/CA and GGM/CA/ME films. Figure 3 depicts the alteration of ATR FTIR spectra for GGM/CA and GGM/CA/ME films with different concentrations of ME. The –OH stretching vibration due to the presence of GGM/CA films and H2O molecules involved in hydrogen bonding was confirmed by the broad peak at 3291 cm−1 [31]. The apparent change in the position of a peak at 3291 cm−1 to 3333 cm−1 was perceived on the addition of mint extract in GGM/CA/ME films. This was plausible due to the formation of new inter-molecular, and intra-molecular hydrogen bonding interactions among the structure of guar gum, citric acid, and mint leaves extract [32] observed the shift in peak due to the addition of grapefruit seed extract in carrageenan films. All the films exhibited an absorption peak at 2928 cm−1 was accredited to the C–H stretching of the CH2 group. The existence of C=O stretching vibration corresponds to an intense peak at 1715 cm−1 found in carboxyl groups, confirming the presence of citric acid (CA) in all the developed films [33]. A peak at 1199 cm−1 corresponds to the C–O–C stretching vibrations of ester groups observed in GGM/CA films which shifts to 1210 cm−1 on the addition of ME due to some interaction that takes place between the GGM, CA, and ME. Our results were in good concurrence with [31], who stated that only physical interactions took place between the polylactide and nanoparticles due to a slight shift in wavenumber and observed no new peaks. A small absorption peak at 1147 cm−1 corresponds to the C–O–C asymmetric stretching vibration observed in all the developed films. In addition, a strong absorption peak at 1023 cm−1 appeared in all the spectra, depicting the stretching vibration of C–O–H [31]. Formation of glycosidic linkages ascribed to β-d-mannopyranose units and α- d-galactopyranose units and presence of anomeric configurations (α and β conformers) validated by the peaks of galactomannan shown in ATR FTIR spectra at 870 cm−1 and 811 cm−1, respectively [27]. So, no new peak is observed, which results in no chemical interactions and only physical interactions.
Morphology of GGM/CA/ME coating films
The microphotographs taken by FESEM qualitatively visualized the distribution of constituents of the developed films. Figure 4 demonstrates the FESEM microphotographs of the surfaces of developed GGM/CA and GGM/CA/ME films with 3%, 5%, and 10% ME. It was visualized that the GGM/CA film microstructure exhibits a smooth and homogeneous surface without pores. The compact and dense structure of the GGM/CA film was observed, indicating a no separation occurred. This was probably due to the use of citric acid in guar gum film, which acts as a cross-linking agent, and the surface topography of the developed film was found to be dense without any pores [34] reported that the film's dense homogeneous nature was enhanced by adding glycerol and citric acid in starch/polyvinyl alcohol/citric acid antimicrobial functional food packaging film. The binding characteristics of starch and polyvinyl alcohol films were improved due to the cross-linking of starch/polyvinyl alcohol/citric acid film. However, some white dots appear in GGM/CA film. This may be attributed to the guar gum, which remains insoluble in water and, after drying, appears as a dot. On increased ME concentration in GGM/CA/ME films, the morphology of the developed films changed significantly. The uneven surface was seen with the addition of mint leaves extract. A comparatively rough surface without any pores and cracks was observed in GGM/CA/ME films. This was plausibly due to the incorporation of mint leaves extract in the films. Some biopolymeric aggregation was seen in GGM/CA/ME-10% film. Our results were in good resemblance with [35], who reported the increase in roughness on the incorporation of essential oils in sago starch/guar gum matrix, and roughness increased at higher concentrations.
Water vapor permeability (WVP) of the GGM/CA/ME coating films
Edible films and fruit coatings were very sensible to the moisture as they deteriorate the food and reduce shelf life. It was important to prevent or reduce the amount of moisture from the outside environment to the food matrix. So, WVP depicts the value of the mass of moisture that penetrates through one side to the other side of the film (that depends upon the RH or pressure difference) per unit time [36]. Figure 5 presented the WVP of GGM/CA and GGM/CA/ME films incorporated with 3%, 5%, and 10% mint leaves extract. It was observed from the results that the WVP values of all the films do not differ significantly (P > 0.05). The WVP value for GGM/CA film was 1.44 g mm−1 m−2 day kPa, which slightly increased to 1.62 g mm−1 m−2 day kPa for GGM/CA/ME-10% but did not change significantly. The hydrophilic nature of guar gum was the main reason for clustering water molecules in the developed film. On incorporating mint leaves extract in GGM/CA/ME films, the WVP value changed due to the agglomeration ME, which forms water clustering as shown in the SEM microphotographs. Also, the hydrophilicity of the film creates free space and increased mobility as mint leaves extract make bonding with the active sites available in guar gum. Increased mobility leads to a less dense structure and easily permeates the water molecules through them, which in turn increases the WVP of the films. Also, all films containing glycerol as a plasticizer interacts with water molecules through hydrogen bonding due to the availability of a free hydroxyl group [37, 38] found similar results on incorporating mango kernel into starch-gum composite films. Their results depict that both guar gum and xanthan gum, due to their hydrophilic nature, attract the water molecule and create micro-cavities responsible for the poor water barrier properties of the developed films.
X-ray diffraction
Diffractograms originating with the help of X-ray diffraction were used to inspect the crystalline and amorphous domains of GGM/CA and GGM/CA/ME films. X-ray diffractograms of GGM/CA and GGM/CA/ME with 3%, 5%, and 10% concentrations of ME were shown in Fig. 6 [39] conducted a study that proved that pure guar gum films show a very small crystallinity. Carboxymethyl groups take the place of hydroxyl groups due to which carboxymethyl guar gum shows lower crystallinity or amorphous nature. The guar gum films reported similar outcomes on the incorporation of citric acid. The GGM/CA films show a broad peak at 2θ = 19.74° with high intensity, as depicted from the diffractograms. This was evident from the diffractograms that the GGM/CA films exhibited amorphous behavior. A peak at 2θ = 11.09° also depicted the amorphous domain of the film. A shoulder peak at 17.62° and 20.55° were reported in GGM/CA film. On incorporation of the mint leaves extract, the amorphous domains increased as proved by the diffractograms at 2θ = 20.07°, 20.04°, and 20.08° for GGM/CA/ME-3%, 5%, 10%, respectively. This consequence was drawn due to low intensity, and broader peaks appear compared to GGM/CA film observed at 2θ ~ 20°. Also a peak was observed at 2θ = 10.63°, 10.58°, 10.96° for GGM/CA/ME-3%, 5%, 10%, respectively. A new broad peak at 2θ = 16.35°,16.48°, 16.78° for GGM/CA/ME-3%, 5%, and 10%, respectively was also responsible for the high amorphous domains of the GGM/CA/ME films as compared to GGM/CA films. Due to the incorporation of mint leaves, the film structure was become highly disordered and led to the formation of highly amorphous domains. Our results were in accordance with the study conducted by [40], indicating that the crystallinity of polystyrene guar gum was much smaller than the guar gum, and highly amorphous domains were seen in polystyrene guar gum. The increased amorphous domains might affect the barrier properties of the edible films.
Mechanical properties
Mechanical properties, including tensile strength and elongation at break of polysaccharide-based edible films and coatings, are key properties as they are the sign of toughness, interrelation, and determine the structural integrity of the edible film. The mechanical properties of edible films rely on the structural interrelation to be valuable for preserving and enhancing the mechanical safety of food products during production and distribution. Tensile strength (TS) was calculated as the maximum force per area that the edible films could tolerate before breaking, and elongation at break (EB) was determined as the ratio between extension and initial length of the films before breaking. As presented in Table 1, the EB (%) of the fabricated film improved significantly (p < 0.05). On the other hand, TS (MPa) reduced significantly (p < 0.05) after the incorporation of the ME in the guar gum matrix. The TS value of film decreases with the addition of mint extract (ME). The ME contains polyphenols; these polyphenols may enter the guar gum matrix and disturb the existing inter-molecular and intra-molecular interactions. The addition of ME into the guar gum matrix can form new weak interaction between polyphenols and guar gum matrix, replacing strong intermolecular interactions. A similar TS pattern was observed when rich polyphenol extract was incorporated into the polymer matrix [4]. Our results are in good agreement with [5], who reported that when pineapple peel extract is added to polyvinyl alcohol and corn starch film, the film's tensile strength is reduced. Furthermore, with the incorporation of ME, EB increased due to disruption of hydrogen bonding between guar gum and ME, leading to the improved mobility of the polymeric chains in the guar gum matrix. It was reported that the addition of coconut shell extract to PVA film enhanced the elongation break property of the resulting film [30]. We also found these comparable outcomes in the present study.
Antioxidant properties of coating films
Antioxidant packaging with plant or food waste extract is a major emerging area of sustainable active packaging and a promising way to improve the food product shelf-life. Employing biodegradable films with natural antioxidant components allows nutritional and aesthetic quality to be preserved for a longer time [41]. Oxidation is a significant cause of fruit spoilage, and this results in flavor change, color change, and downgrading the product quality [42]. In the present study, the antioxidant activity of GGM/CA/ME films was determined using the DPPH free radical scavenging assay. As shown in Fig. 7, the results demonstrated that the film GGM/CA containing 3% ME has higher (93.38 ± 025%) antioxidant activity than the other GGM/CA/ME films. The edible film with the absence of ME displayed 44.16% of RSA is due to the combined influence of the base matrix of GGM and the addition of citric acid, which has previously been reported where conjugation of GGM improves the antioxidant activity and due to chelating action citric acid provided antioxidant effect in the film [43]. The high antioxidant activity possessed by the ME is due to the presence of phenolic compounds such as caffeoylquinic acid 3.3, salvianic acid, rosmarinic acid, salvigenin, chrysoeriol, thymonin, carnosol [44]. These polyphenols and phenolic compounds scavenge the reactive oxygen species, which are generated during the oxidative metabolism reaction of fruits [45]. Past studies conducted on the antioxidant properties of fruit peel extract have concluded that the plant extract has significant antioxidant activity, which may be contributed to the development of the packaging film to reduce the lipid oxidation of high lipid or oxygen-sensitive foods [46, 47]. Moreover, this extract has numerous pharmacological, antibacterial, anti-mutagenic, anti-carcinogenic, and anti-genotype properties, which can be further explored to exploit the full potential of natural resources.
Antimicrobial activity of coating films
Antimicrobial activity of GGM/CA edible films combined with 3, 5, and 10% of ME against E. coli the % of reduction rate of viable bacterial cells (% R) are presented in Table 1. The number of viable microbial cells in the pure GGM/CA sample was 8.65 ± 0.03 Log CFU g−1. On the other hand, the number of viable microbial cells of GGM/CA films containing 3, 5, and 10% ME were 6.81 ± 0.76, 4.33 ± 0.91, 1.81 ± 0.87 Log CFU g−1 respectively, which denoted significant decreases in the number of viable microbial cells compared to pure GGM/CA film. The % reduction rates of E. coli for each 3, 5, and 10% ME containing GGM/CA films were 30.6, 46.6, and 83.2%, respectively. These outcomes designated that E. coli cell counts declined ME amount in the film increased. These results could be described by the antimicrobial nature of the polyphenolic compounds (naringin, limonin) present in ME [48, 49]. The antimicrobial nature of ME which are believed to exhibit antimicrobial activity as they penetrate the cell membrane and bind to cellular proteins, thus deactivating their function [14]. Our results are in good agreement with [50], who reported antimicrobial in polyvinyl alcohol-based functional films when incorporated with grapefruit seed extract (GSE). A GGM/CA films combined with 10% of ME have the highest antimicrobial property compared to other concentration against tested strains. The microbial activity of the mint extract was influenced by the amount of phenolic compounds and associated with the classification of mint plants utilized, harvesting season, processing, and storage conditions, including temperature and RH [51].
Quality attributes of the coated ber fruits
Respiration rate analysis
Respiration rates in fresh produce are reflected in good indexes for evaluating the storability of fresh produce. As displayed in Fig. 8a, the respiration rate of uncoated ber samples initiated to rise on day 1 when stored at 23 ± 2 °C. The highest respiration rates of the uncoated ber occurred at 15 days when stored at 23 °C, respectively. At 23 °C, the respiration rates of the uncoated ber, neat GGM/CA, and GGM/CA/ME-10% coated fruits were 47.26, 36.27, and 22.77 mL CO2 kg−1 h−1, respectively, on day 15. This result presented a significant difference among uncoated, neat GGM/CA and GGM/CA/ME coated ber fruits after day 3. After coating treatment, a decrease in the respiration process in ber fruits might be associated with delayed ripening and reduced susceptibility to decay in ber fruits during 15 days of storage [42, 43]. The trends of respiration rate in the present study were in agreement with the findings of [52], who reported that the respiration rate of tomatoes decreased when coated with active (antioxidant) gum arabic by altering the internal atmosphere of the fruit. Several scientists reported a lower respiration rate due to edible coating on the fruit's surface in different fruits, such as plum, tomato, strawberries, and guava [13].
Total soluble solids (TSS)
The variations of TSS for the uncoated and GGM/CA/ME coated ber fruits during 15 days storage as presented in Fig. 8b. The TSS values of the uncoated ber regularly increased significantly (P < 0.05) during storage compared to coated samples. On day 15, the values of TSS of the uncoated, neat GGM/CA, GGM/CA/ME-3%, GGM/CA/ME-5%, and GGM/CA/ME-10% coated ber were 8.54, 6.87, 6.11, 5.99, 5.87°Brix, respectively, at 23 °C. The rise in TSS may result from moisture loss and synthesis of sugars from carbohydrates, except starch [14, 53]. Compared with the uncoated ber fruits, the neat GGM/CA and GGM/CA/ME coating reduced moisture evaporation from the ber surface, mainly related to weight loss, hence, retaining the TSS of ber fruits at the end of the storage period. Similar to our results, [54] also observed that the functional edible coating based on alginate and chitosan and ZnO nano-particles maintained the TSS content in guava fruits during storage.
Weight loss in coated ber fruits
Ber fruits coated with GGM/CA/ME-3% and GGM/CA/ME-5% mint extract had less weight loss during 15 days storage than uncoated GGM/CA sample as presented in Fig. 8c, a percentage of weight loss increasingly throughout the storage. The primary reason for weight reduction from fresh produce is the transpiration process calculated by the descent of water vapor pressure among the fresh produce and the atmosphere air [10]. This drop-in weight loss is attributed to the effects of the GGM/CA/ME coating, which acts as a barrier against oxygen, carbon dioxide, and water vapor, decreasing the respiration rate of ber fruits, and subsequently less weight loss. These results are supported by the water vapor transmission rate data, which confirm the GGM/CA/ME-3% and GGM/CA/ME-5% have the lowest water vapor transmission rate. Our results are in good agreement with [14], who reported Hydroxypropyl methylcellulose edible coating containing oregano and bergamot essential oils helped in maintaining the weight of Formosa plum during the storage period. Weight loss decreased in 10 and 15% gum arabic coated tomato during storage period compared to uncoated tomato [52]. A significantly (p ≤ 0.05) greater weight loss in GGM/CA/ME10% coating could be related to the thickness of coatings. The 10% ME containing guar gum coating was not so thick; hence the water vapor transmission rate was high, and thus moisture loss was increased, while the 3% guar gum coating was so thick that it completely covered the surface of the ber fruit and acted as a water vapor barrier. The key reason for the higher weight loss of densely coated fruits is associated with the production of heat by-products from the anaerobic fermentation process during the storage period [55].
Firmness of coated ber fruit
Firmness of the fruits is a critical quality parameter responsible for the ripening of the fruits. It is also crucial in determining the resistance of fruits to mechanical injury. Measurement of firmness was conducted using a ber fruits sample non-coated and coated with GGM/CA/ME at storage conditions of 23 °C as presented in Fig. 9. The firmness of ber fruits at 23 °C was nearly 8.3 kg−1 cm−2 for the uncoated, 8.82 kg−1 cm−2 for neat GGM/CA coating, 7.91 kg−1 cm−2 for GGM/CA/ME-3%, 8.76 kg−1 cm−2 for GGM/CA/ME-5%, and 8.81 kg−1 cm−2 for GGM/CA/ME-10% coating on the day 0. The effect of the edible coating on firmness was significant. The uncoated and GGM/CA/ME coated ber fruits indicated continuously reducing firmness till the 15 days of storage. The firmness value of uncoated ber fruits speedily declined to 5.91 kg−1 cm−2 on day 15 compared to the firmness in the GGM/CA/ME-10% coated fruit, which yielded a value of 7.83 kg−1 cm−2 on the same day at 23 °C. The mint leaves extract containing GGM/CA-coated ber fruits exhibited a relatively stable firmness to the uncoated and neat GGM/CA-coated ber. These results exhibited that inclusion of ME in coated material GGM/CA delivered a more positive effect in preserving the firmness of stored ber fruits than the GGM/CA coating, which has been studied well as an edible fruits coating material. This difference in firmness values could be accredited to GGM/CA/ME treatment, which effectively delays the ripening process in the ber fruits during storage. Our results are in good agreement with [47], who reported the firmness value of the kiwifruits coated with cellulose nanofibers chitosan and curcumin were lower compared to non-coated (control) samples. The texture of fresh fruits in terms of firmness can describe the eating quality of the ber fruits.
Sensory evaluation
The Uncoated, neat GGM/CA, and GGM/CA/ME coating of ber fruits significantly improved its aspect value by showing a glossy look, as presented in Table 2. The overall aspects score for GGM/CA/ME ber fruits was much more excellent than uncoated ber samples, as displayed in Table 1. The GGM/CA/ME ber samples had a stronger flavor and overall acceptance rate than uncoated ber samples. Mint leaves extract has a very strong flavor at the time of coating. Nevertheless, on the day of the sensorial evaluation test, the sensory properties of the ber fruit were not affected; this might be associated with the solvent in the mint extract being evaporated during the drying and storage period. In general, the sensory test revealed that the GGM/CA/ME coating did not adversely impact the buyers' opinion. Our results were in good agreement with [14], who stated the enhanced overall appearance of Formosa plum, has a significant effect on the oregano and bergamot-based essential oil coated products since buyers are inclined to purchase fruits having an excellent visual appearance. The sensory analysis confirmed that the GGM/CA/ME coated ber fruits have a higher acceptance rate than the uncoated ber fruits.
Conclusion
The present study results indicated the possible application of GGM/CA edible coating with mint leaves extract to prolong the storability and maintain the quality of ber fruits stored at 23 °C and 75% RH for 15 days. The developed coatings decreased weight loss, slower the respiration rate, and maintained the firmness, which delayed the ripening phenomenon, as designated by the firmness of the fruit. However, increased mint leaves extract concentration in the GGM/CA had not significantly affected the film's water vapor permeability. A GGM/CA/ME-10% edible coating for ber fruits proved promising in delaying respiration, weight loss, and fruit softening. Hence, using a GGM/CA film incorporated with mint leaves extract may be beneficial as an edible coating for fresh ber fruits to maintain their quality attributes during the storage period. The present study proves that an edible coating using GGM/CA/ME is worth extending produce freshness during the storage period.
Data availability
The data supporting the results of this study are available from the corresponding author, Kirtiraj K. Gaikwad (kirtiraj.gaikwad@pt.iir.ac.in).
References
S. Singh, K.K. Gaikwad, Y.S. Lee, Int. J. Biol. Macromol. 107, 1879 (2018)
R.K. Deshmukh, K.K. Gaikwad, Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-02623-w
A.A. Kadam, S. Singh, K.K. Gaikwad, Food Control 124, 107877 (2021)
S.A. Mir, B.N. Dar, A.A. Wani, M.A. Shah, Trends Food Sci. Technol. 80, 141 (2018)
P. Kumar, R. Tanwar, V. Gupta, A. Upadhyay, A. Kumar, K.K. Gaikwad, Int. J. Biol. Macromol. 187, 223 (2021)
C. Kavitha, A. Kuna, T. Supraja, S.B. Sagar, T.V. Padmavathi, N. Prabhakar, J. Food Sci. Technol. 52, 3123 (2014)
P. Nallathambi, C. Umamaheswari, B.B.L. Thakore, T.A. More, Crop Prot. 28, 525 (2009)
T.K. Koley, S. Walia, P. Nath, O.P. Awasthi, C. Kaur, Int. J. Food Sci. Nutr. 62, 276 (2010)
T. Defraeye, P. Cronjé, T. Berry, U.L. Opara, A. East, M. Hertog, P. Verboven, B. Nicolai, Trends Food Sci. Technol. 44, 201 (2015)
K.K. Gaikwad, S. Singh, Y.S. Negi, Environ. Chem. Lett. 18, 269 (2019)
A. Kumar, R.K. Deshmukh, K.K. Gaikwad, Biomass Convers. Biorefin. 2022, 1–12 (2022)
A. Upadhyay, P. Kumar, S.K. Kardam, K.K. Gaikwad, Food Biosci. 46, 101556 (2022)
A. Kumar, V. Gupta, S. Singh, S. Saini, K.K. Gaikwad, Ind. Crops Prod. 170, 113752 (2021)
W.S. Choi, S. Singh, Y.S. Lee, LWT - Food Sci. Technol. 70, 213 (2016)
L. Kumar, D. Ramakanth, K. Akhila, K.K. Gaikwad, Environ. Chem. Lett. 20, 875 (2022)
C. Ribeiro, A.A. Vicente, J.A. Teixeira, C. Miranda, Postharvest Biol. Technol. 44, 63 (2007)
M.V. Alvarez, M.F. Bambace, G. Quintana, A. Gomez-Zavaglia, M.R. del Moreira, LWT 137, 110483 (2021)
J. Ma, Z. Zhou, K. Li, K. Li, L. Liu, W. Zhang, J. Xu, X. Tu, L. Du, H. Zhang, Food Chem. 354, 129510 (2021)
J.M. Valverde, D. Valero, D. Martínez-Romero, F. Guillén, S. Castillo, M. Serrano, J. Agric. Food Chem. 53, 7807 (2005)
M.S. Rao, S.R. Kanatt, S.P. Chawla, A. Sharma, Carbohydr. Polym. 82, 1243 (2010)
M. Srivastava, V.P. Kapoor, Chem. Biodivers. 2, 295 (2005)
V.G. Bhat, S.S. Narasagoudr, S.P. Masti, R.B. Chougale, Y. Shanbhag, Int. J. Biol. Macromol. 177, 166 (2021)
R. Akhter, F.A. Masoodi, T.A. Wani, S.A. Rather, Int. J. Biol. Macromol. 137, 1245 (2019)
D.M. Pramila, J. Med. Plants Res. 6, 331–335 (2012)
E. Padmini, K. Prema, B. Vijaya Geetha, M.U. Rani, Int. J. Food Sci. Technol. 43, 1887 (2008)
K.K. Gaikwad, J.Y. Lee, Y.S. Lee, J. Food Sci. Technol. 53, 1608 (2016)
N. Reddy, Q. Jiang, Y. Yang, Ind. Crops Prod. 39, 26 (2012)
N. Reddy, Y. Yang, Food Chem. 118, 702 (2010)
L. Kumar, R.K. Deshmukh, K.K. Gaikwad, Int. J. Biol. Micromol. 215, 596 (2022)
R. Tanwar, V. Gupta, P. Kumar, A. Kumar, S. Singh, K.K. Gaikwad, Int. J. Biol. Macromol. 185, 451 (2021)
Y.A. Arfat, M. Ejaz, H. Jacob, J. Ahmed, Carbohydr. Polym. 157, 65 (2017)
P. Kanmani, J.W. Rhim, Carbohydr. Polym. 102, 708 (2014)
R. Shi, Z. Zhang, Q. Liu, Y. Han, L. Zhang, D. Chen, W. Tian, Carbohydr. Polym. 69, 748 (2007)
W. Z, W. J, P. T, L. Y, L. D, X. B, L. C, Y. Y, Y. L, Z. L, M. R, W. W, L. X, D. J, H. G. Polymers (Basel) 9, (2017)
C.V. Dhumal, J. Ahmed, N. Bandara, P. Sarkar, Food Packag. Shelf Life 21, 100380 (2019)
L. Yavari Maroufi, M. Ghorbani, M. Tabibiazar, Food Bioprocess Technol. 13, 1633 (2020)
S. Nandi, P. Guha, Carbohydr. Polym. 200, 498 (2018)
A. Nawab, F. Alam, M.A. Haq, Z. Lutfi, A. Hasnain, Int. J. Biol. Macromol. 98, 869 (2017)
H. Gong, M. Liu, J. Chen, F. Han, C. Gao, B. Zhang, Carbohydr. Polym. 88, 1015 (2012)
Y. Yang, F. Chen, Q. Chen, J. He, T. Bu, X. He, Carbohydr. Polym. 176, 266 (2017)
S. Guilbert, N. Gontard, L.G.M. Gorris, LWT - Food Sci. Technol. 29, 10 (1996)
A.S. Seddiek, G.M. Hamad, A.A. Zeitoun, M.A.M. Zeitoun, S. Ali, Eur. J. Nutr. Food Saf. 12, 1–12 (2020)
X. He, F. Luzi, X. Hao, W. Yang, L. Torre, Z. Xiao, Y. Xie, D. Puglia, Int. J. Biol. Macromol. 127, 665 (2019)
N. Brown, J.A. John, F. Shahidi, Food Prod. Process. Nutr. 1, 1 (2019)
K. Meitha, Y. Pramesti, S. Suhandono, Int. J. Food Sci. 2020, 1–11 (2020)
X. Zhang, H. Lian, J. Shi, W. Meng, Y. Peng, Int. J. Biol. Macromol. 148, 1242 (2020)
K. Oudjedi, S. Manso, C. Nerin, N. Hassissen, F. Zaidi, Food Control 98, 216 (2019)
W. Ling, T. Dai, J. Zhang, Y. Liang, W. Yin, B. Zhong, J. Zhang, Chem. Biodivers. 18, e2100679 (2021)
S.Ć Zeljković, J. Šišková, K. Komzáková, N. De Diego, K. Kaffková, P. Tarkowski, Plants 10, 550 (2021)
S. Roy, J.W. Rhim, J. Environ. Chem. Eng. 9, 104694 (2021)
B. Pavlić, M. Kaplan, O. Bera, E. Oktem Olgun, O. Canli, N. Milosavljević, B. Antić, Z. Zeković, Food Bioprod. Process. 118, 258 (2019)
A. Ali, M. Maqbool, S. Ramachandran, P.G. Alderson, Postharvest Biol. Technol. 58, 42 (2010)
S. Singh, K.K. Gaikwad, Y.S. Lee, Sci. Hortic. (Amsterdam) 256, 108548 (2019)
B.J. Arroyo, A.C. Bezerra, L.L. Oliveira, S.J. Arroyo, E.A. de Melo, A.M. Santos, Food Chem. 309, 125566 (2020)
J. Weichmann, Postharvest Physiology of Vegetables (M. Dekker, New York, 1987)
Acknowledgements
Author K. K. Gaikwad would like to sincerely thank the Science and Engineering Research Board (SERB), Government of India, for the financial support provided under the Start Up research Grant (SRG) (SRG/2021/001549).
Author information
Authors and Affiliations
Contributions
PK: Investigation, Formal analysis, Visualization, Data curation, Writing—Original Draft; LK: Investigation, Formal analysis, Visualization, Data curation, Revision of manuscript; RT: Experimental; SS: Conceptualization, Methodology, Resources, Writing—Original Draft; KKG: Conceptualization, Methodology, Resources, Writing—Original Draft, Manuscript editing and review, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, P., Kumar, L., Tanwar, R. et al. Active edible coating based on guar gum with mint extract and antibrowning agents for ber (Ziziphus mauritiana) fruits preservation. Food Measure 17, 129–142 (2023). https://doi.org/10.1007/s11694-022-01609-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01609-6