Abstract
Effect of gamma irradiation (0.25 to 1.0kGy) on antioxidant properties of ber fruit was studied. Antioxidant properties of ber fruits were determined by Scavenging DPPH radical activity, reducing power assay, super oxide anion radical activity, TBARS, total phenolic content and total flavonoid content. Gamma irradiation treatment up to 1.0kGy elevated the Scavenging DPPH radical activity (9 %), super oxide anion radical activity (26 %) and total flavonoid content (208 %) compared to fresh ber fruit. On the other hand it brought down the reducing power activity (65 %) and total phenolic content (18 %) as compared to raw fruit. The TBARS activity statistically increased upon irradiation of ber fruit. It indicated that total antioxidant activity decreased as TBARS value increased. Therefore 0.25 to 0.5kGy is better dose to retain the natural antioxidant in fruit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ber (Zizyphus mauritiana) is a tropical and subtropical fruit native to the northern hemisphere. It belongs to the genus Ziziphus of the family Rhamnaceae and order Rhamnales. There are two major domesticated jujubes, Z. mauritiana Lam. (Indian jujube or ber) and Z. jujuba Mill (Chinese or common jujube) (Azam Azam-Ali et al. 2001; Zhang et al. 2009). It is one of the most ancient and underutilized fruits of India. It is popularly called the king of arid zone fruits (Yamadagni 1985; Shoba and Bharati 2007). The area under cultivation with this fruit is 8.7 lakh ha with an annual production of 8.9 lakh tones in India (Bolada et al. 2012). About 125 varieties of ber are available in India. A few of these varieties are known for taste, size, amount of pulp and higher yields. The cultivars Umran, Kathapal and Gola are the most promising varieties of ber in North India (Azam-Ali et al. 2001).
Ber fruits are highly nutritious, rich in ascorbic acid and contain fairly good amount of vitamin A and B, minerals like calcium, phosphorus and iron. (Yamadagni 1985; Shoba and Bharati 2007). It is also rich in carotenes and phenolics including caffeic acid, p-hydroxybenzoic acid, ferulic acid and p-coumaric acid. (Tanmay et al. 2011). Indian Ber contain good source of ascorbic acid and total phenolics ranging from 19.54 to 99.49 mg/100 g and 172 to 328.6 mg GAE/100 g respectively (Koley et al. 2011). The average antioxidant activities were 1.6–6.33 and 1.22–5.49 μ mol TE/g as the CUPRAC and FRAP assays, respectively (Krishna and Parashar 2012).
The storage life of ber fruits is extremely short. At ambient temperature a shelf-life of 2–4 days is common. Due to the surplus of fruits in the local markets during peak season, a substantial quantity goes to waste, resulting in heavy postharvest losses. Therefore proper postharvest management of ber fruits is required (Sunil et al. 2011).
Radiation preservation of ber fruits is one post-harvest strategy that may help to delay post-harvest ripening and senescence and thereby reduce losses and extend the shelf life of fruits (Azam-Ali, et al. 2001). Irradiation at 0.25 kGy and 0.75 kGy caused a significant decrease in ascorbic acid content at the end of storage (30 days at 12 ºC). Radiation along with hot water blanching and calcium chloride treatments were suitable for extending the shelf-life and quality of ber fruits (Srijaya et al. 2012). Physiological loss in weight (PLW) and rotting were lowest in fruits treated with UV radiation for 6 h + PBZ 5 ppm during 12 days of storage (Amit et al. 2003).
Ber fruit is rich in antioxidants, requires minimum crop management skills and also available at low cost. Despite being rich in nutrients, it is underutilized and seasonal fruit. So far only nutrient composition (Azam-Ali et al. 2001; Rathore 2009; Koley et al. 2011), antioxidant activity have been studied by some researchers (Koley et al. 2011; Krishna and Parashar 2012; Sunil et al. 2011) and many studies have focused on the effect of irradiation on post-harvest ripening and senescence of ber fruits but not on the antioxidant properties in ber. If data is available on the effect of irradiation on natural antioxidants in the fruit, it can be utilized to promote processing, thereby increasing the utilization, shelf life and consumption. Therefore, our aim of research work is to evaluate the effect of gamma irradiation on antioxidant properties of Ber fruit.
Material and methods
Procurement of raw materials
Mature ripe (yellowish-green color) ber fruits were procured from the local market and the cultivar was identified by the Taxonomy experts of the College of Horticulture, Rajendranagar, Hyderabad. They identified the cultivar as Gola. Ber fruits were packed into polyethylene bags and subjected to irradiation treatments at dose of 0.25 kGy for 15 min, 0.50 kGy for 30 min, and 0.75 kGy for 45 min and 1.00 kGy for 60 min at room temperature 32 to 35 º C in gamma irradiation unit ANGRAU, Rajendranagar, Hyderabad. After irradiation of ber fruits were used to antioxidant analysis.
Sample preparation
Extract was prepared from 50 g irradiated fruit and fresh fruit separately by using mixture of equal quantity of acetone methanoland water (250 ml). The extract was centrifuged at 10,000 rpm for 15 min. The resulting supernatant was collected and the pellet re-extracted and the supernatants were pooled together. The filtered extract was used for analysis of Scavenging DPPH radicals, Reducing power, Superoxide anion radical scavenging activity, total phenolic compounds and total flavonoid content.
Scavenging DPPH radicals (Dorman et al. 2004)
The free radical scavenging capacity of the extracts was determined using 1,1-diphenyl-2-picryl-hydrazil (DPPH). 2 ml of methanol solution of DPPH radical in the concentration of 0.05 mg/ml and 1 ml of extract were placed in cuvettes. The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. Then the absorbance was measured at 517 nm against methanol as blank in spectrophotometer. The DPPH free radical concentration was calculated using the following equation:
Where, A0 was the absorbance of the negative control or blank and A 1 was the absorbance of reaction mixture or standards.
Reducing power (Oyaizu 1986)
One ml of extract were mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 ml, 1 %). The mixtures were incubated at 50 °C for 20 min. Then trichloroacetic acid (10 %, 2.5 ml) was added to the mixture and centrifuged. Finally, the upper layer (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml; 0.1 %). The absorbance of solution was measured at 700 nm in spectrophotometer. Blank was prepared with all the reaction mixture, indicated that the reducing power is increased.
Superoxide anion radical scavenging activity (Nishimiki et al. 1972)
0.1 ml of extracts was mixed with 1 ml nitro blue tetrazolium (NBT) solution (156 μM in 0.1 M phosphate buffer, pH 7.4) and 1 ml NADH (Nicotinamide Adenine Dinucleotide) solution (468 μM in 0.1 M phosphate buffer, pH 7.4). The reaction was started by adding 100 μL of phenazine methosulphate (PMS) solution (60 μM in 0.1 M phosphate buffer, pH 7.4). The mixture was incubated at room temperature for 5 min and the absorbance was measured at 560 nm in spectrophotometer against blank samples.
The following formula was used to calculate the percentage inhibition of superoxide anion generation
Where A0 is the absorbance of the negative control consisting of all the reaction agents except the extract); A1 is the absorbance of reaction mixture or standards.
Total phenolic compounds
Total soluble phenolic compounds in the ber fruit extract were determined with Folin-Ciocalteu reagent according to the method of Slinkard (Slinkard and Singleton 1997) using pyrocatechol as a standard phenolic compound. Briefly, 1 ml of the extract was diluted with 46 ml of distilled water. Then, one milliliter of Folin Ciocalteu reagent was added and the mixture was stirred vigorously. 3 ml of Na2CO3 (2 %) was added after 3 min and then was allowed to stand for 2 h with intermittent shaking. After that, absorbance was measured at 760 nm in spectrophotometer against blank consisting of all the reaction agents except the extract. The total phenol content in the extract was determined as microgram of PE according to the equation that was obtained from standard pyrocatechol graph as
Total flavonoid content
The total flavonoid content was determined using the Dowd method (Meda et al. 2005). Two ml of the extract solution was mixed with 2 ml of 2 % aluminiumtrichloride (AlCl3) in methanol. The mixture was incubated for 10 min at room temperature and the absorbance was measured at 415 nm in spectrophotometer against blank samples. The total concentration of flavonoids in the extracts was determined as microgram of RE (rutin equivalent) according to the formula that was obtained from standard rutin graph as
TBARS (Thiobarbituric acid reactive substances) method (Nikols et al. 1994)
This method selected for calculating the total antioxidant activity of ber fruit.
Sample preparation
One gram irradiated ber fruit was homogenized in a motor and pestle with 10 ml of 0.1 M phosphate buffer (pH 7.8) and one percent of 0.05 M EDTA and centrifuged at 4,000 rpm for 15 min at 5 ºC. The clear supernatant extract was used for analysis. The reaction mixture contained 2.3 g aliquot of sample, coconut oil (0.24 ml) in phosphate buffer (0.26) ml, 0.1 M, pH 7.8), ferrous sulphate (0.05 mM), ascorbic acid (0.4 mM), potassium hydrogenpthalate 100 mM, pH 6.0) BHT (Butylatedhydroxytoulene) (25 mM in 5 ml hexane) in a final volume of 2.4 ml. Content of the tube were incubated for 30 min at 37 ºC. TCA (Trichloro acetic acid) (0.75 ml, 20 %) was added and centrifuged at 10,000 rpm for 30 min at 4 ºC, followed by addition of TBA (Thiobarbituric acid) (0.5 % in 0.1 N NaOH). Distilled water was added to equalize the final volume to 3.24 ml. This was heated at 95 ºC in water bath for 30 min followed by immediate cooling in ice pack for 5 min. Finally the reaction mixture was submitted to read the absorbance at 532 nm against TBA.
Statistical analysis
The results obtained were subjected to statistical analysis with the window STAT programme. To determine the statistical significance of antioxidant activity, Analysis of Variance (ANOVA) technique was used. Pearson’s bivariate correlation test was used to calculate correlation coefficients between the content of total phenolic, total flavonoid content, Scavenging DPPH radical activity, reducing power and superoxide anion radical scavenging activity.
Results and discussion
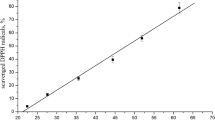
The results of scavenging DPPH radicals of studied ber fruits are summarized in the table 1. Ber fruits were subjected to irradiation at various dosages 0.25, 0.5, 0.75 and 1.0 kGy. It was observed that at higher dose (0.50, 0.75, 1.0 kGy) the free radical activity significantly (p > 0.05) increased (80.88 ± 0.49, 82.15 ± 0.56 to 85.77 ± 0.34 % inhibition) where as at low dose (0.25 kGy) of irradiation there was no significant (p > 0.05) increase in the free radical activity (78.57 ± 0.42 to 78.97 1 ± 0.35 % inhibition) as compared to raw fruit. Radiation treatments have been shown to either increase or decrease the antioxidant content of fresh plant produce, which is dependent on the dose delivered, exposure time and the raw material used. The enhanced antioxidant capacity/activity of a plant after irradiation is mainly attributed either to increased enzyme activity (e.g., phenylalanine ammonialyase and peroxidase activity) or to the increased extractability from the tissues (Althoman et al. 2009). An increase in free radical content was also observed in nine aromatic spices (basil, bird pepper, black pepper, cinnamon, nutmeg, oregano, parsley, rosemary, and sage) at 10 kGy dose (Calucci et al. 2003).
Reducing power assay
Reducing power assay measures the electron-donating capacity of an antioxidant. The reduction of the ferric ion (Fe3+) to ferrous ion (Fe2+) is measured by the intensity of the resultant blue-green solution which absorbs at 700 nm and an increased absorbance is indicative of higher reducing power (Nagendran et al. 2005). The results of reducing power activity of studied ber fruits are depicted in the table 1. The reducing power activity of irradiated ber fruits showed significance (p > 0.05) declined from 0.25, 0.50, 0.75 and 1.0 kGy (1.82 ± 0.07, 1.68 ± 0.01, 1.50 ± 0.00 and 1.20 ± 0.06 absorbance) respectively as compared to raw fruit. Gamma irradiation at 5 and 30 kGy cause a decrease in reducing power activity of black pepper (Suhaj et al. 2006) whereas the same dose of irradiation did not affect the reducing power activity in Zinger and Clove (Suhaj and Horváthová 2007). It indicates that irradiation either increase reducing power activity or remains the same. The decrease of reducing power activity may be due to decrease in reductones (ascorbic acid). A study by Srijaya et al. (2012) reported irradiation at 0.25 kGy and 0.75 kGy caused a significant decrease in ascorbic acid content by the end of storage (30 days at 12 ºC) of ber fruits.
Super oxide anion radical scavenging activity
Numerous biological reactions generate superoxide radical scavenging activity which is a highly toxic species although they cannot directly initiate lipid peroxidation, super oxide anion radical are potential precursor of damaging oxygen species and thus the study of superoxide anion radical is important. In the PMS/NADH-NBT system, superoxide anion derived from dissolved oxygen by PMS/NADH coupling reaction reduces NBT (Anand et al. 2007). The decrease of absorbance at 560 nm with antioxidants thus indicates the consumption of superoxide anion in the reaction mixture (Gulcin 2004). Increased dose of gamma irradiation from 0.25, 0.50, 0.75 and 1.0 kGy of ber fruits reported significant (p > 0.05) increment in super oxide anion radical scavenging activity as given in the table 1. A study by Helaly and Hanan (2011) reported freshly isolated protoplast of salinized and non salinized lemon shoots were subjected to gamma irradiation. The activities of SOD (Super Oxide Dismutase), CAT (Catalase), POX (Peroxidase) and GR (Glutothione Reductase) were increased in irradiated shoots compared with non-irradiated under salinized and non-salinized condition. It was found that, irradiated shoots had a higher hereditary and induced capability under salt stress which provide to it a better protection from oxidative and cellular damage caused by NaCl salinity. Ber is also known for its ability to withstand adverse conditions, such as salinity, drought and water logging (Bolada et al. 2012).
Thio barbituric acid reactive substances
MDA (Malondialdehyde) and other aldehydes have been identified as products of lipid peroxidation that reacts with TBA to give a pink colored species that absorbs at 532 nm. TBARS was originally developed in 1958 for testing rancidity due to oxidized lipids in food materials (Devasagayam et al. 2003). The per cent TBARS and MDA are inversely proportional to antioxidant activity i.e., with increase of TBARS content, there will be decrease in antioxidant activity of fruit. The results of TBARS of studied ber fruits are represented in the table 1. Irradiation with 0.25 to 1.0 kGy of ber fruits showed a significant (p > 0.05) increase (381.86 ± 4.72 % to 620.59 ± 6.40 %) in TBARS activity as compared to raw fruit. It indicates that, total antioxidant activity of fruit has decreased as increased dose of irradiation. Study by Beltagi et al. (2011) reported high dose irradiation cause oxidative burst (MDA, H2O2, O− 2) in rosemary callus cells.
Total phenolic content
The antioxidant activity of phenolic compounds is mainly attributed to their redox actions, neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides.
The results of total phenolic content and flaonoid content of investigated ber fruits are summarized in table 1. The total phenolic content of fresh fruit was found to be 94.7 ± 0.27 (μg of PE). Irradiation significantly (p > 0.05) brought down the phenolic content (58.51 ± 1.2 to 77.08 ± 1.37) in ber fruits as compared to control. Ber is rich in carotenes and phenolics including caffeic acid, p-hydroxybenzoic acid, ferulic acid and p-coumaric acid (Tanmay et al. 2011) and predominate flavonoid is naringenin (Ayaz et al. 2011). Gamma irradiation cause a very strong decrease of ferulic acid, p-coumaric acid, rutin and naringenin concentration. A study by Michaela et al. (2005) repored gamma-irradiation reduced the concentration of the phenolic compounds markedly in tomato samples. Higher doses (0 to 20Gy) of gamma irradiation positively enhanced secondary products accumulation of total phenols and total flavonoids in rosemary callus culture (Beltagi et al. 2011). As dose of irradiation increases, the total phenolic content also increases due to alterations in cellular compounds at high temperatures or due to the decomposition of some insoluble phenolic compounds. This can explain the increase of total phenolic compounds at high dose of irradiation (Villavicencio et al. 2000).
Total flavonoids content
The protective effect of flavonoids is due to several mechanisms such as free radicals trapping, enzymes inhibition and metallic ions chelation. These properties depend on the structure of the flavonoids and the degree of substitution and saturation. Fruits and vegetables are rich source of flavonoids (Ioannou and Ghoul 2012).
The results of total flavonoid content of ber fruits has increased upon irradiation at 0.25 to 1.0 kGy as compared to raw fruit as shown in the table 1. Gamma radiation can interacts with atoms and molecules to create free radicals in cells that are able to modify important components of plant cells. These radicals have been demonstrated to affect the morphology, anatomy, biochemistry and physiology of plants, depending on the irradiation dosage. The effects consist of changes in the plant cellular structure and metabolism, e.g., dilation of thylakoid membranes, change in photosynthesis, modulation of the antioxidative system and enhancement of phenolic compounds (Sina et al., 2011). A study by Khatun et al. (2012) revealed that the total flavonoids content increased significantly with increasing dose of radiation in bitter gourd as compared to control sample. The increase in total flavonoid can be attributed to the phenylalanine ammonialyase activity, which is one of the key enzymes in the synthesis of phenolic compounds in plant tissue.
The results of correlation of antioxidants activity in ber fruits upon irradiation (0.25, 0.50, 0.75 and 1.0 kGy) are presented in table 2. Scavenging DPPH radical activity was strongly correlated with the total favonoid content and super oxide anion radical activity in irradiated ber fruits. It was observed that the reducing power activity of irradiated ber fruit was negatively correlated (r = −0.97) with total flavonoid content. Therefore irradiation dose increased the DPPH activity as well as total flavonoid content in fruit.
Conclusion
Ber fruits are rich in ascorbic acid content, as well as phenolics and flavonoids. Irradiation treatment cause strong increase in flavonoid content and superoxide anion radical activity, DPPH radical activity but slight reduction of total phenolics and reducing power activity. Total antioxidant activity (TBARS) of irradiated fruit has decreased 64 to167% compare to raw fruit at 0.25 to 1.0 kGy. The per cent TBARS and MDA are inversely proportional to antioxidant activity i.e., with increase of TBARS content, there will be decrease in antioxidant activity of fruit. Therefore 0.25 to 0.5 kGy is better dose to retain the natural antioxidant in ber fruit.
References
Althoman M, Rajeev B, Karim AA (2009) Effects of radiation processing on phytochemicals and antioxidants in plant produce. Trends in Food Science and Technology 20(5):201–212
Amit KP, Rawat TS, Arvind K (2003) Shelf life and quality of ber (Zizyphus maurtiana) fruit cv. Umran in response to post harvest application of ultraviolet radiation and paclobutrazol. Plant Foods Hum Nutr 58(3):1–7
Anand PK, Policegoudra RS, Aradhya SM (2007) Chemical composition and antioxidant activity of sapota (Achras sapota Linn.). Fruit J of Food Biochem 31:399–414
Ayaz AM, Najma M, Devan LL, Amanat AP, Muhammad IB (2011) Phenolic Compounds and Seed Oil Composition of Ziziphus mauritiana L. Fruit. Polish Journal of Food Nutrition Science 62 (1):15–21
Azam-Ali S, Bonkoungou E, Bowe C, DeKock C, Godara A, Williams JT (2001) Fruits for the future (revised edition) Ber and other jujubes. International Centre for Underutilised Crops, University of Southampton, Southampton, SO17 1BJ, UK
Beltagi HSE, Osama KA, Desouky WE (2011) Effect of low doses γ-irradiation on oxidative stress and secondary metabolites production of rosemary (Rosmarinus officinalis L.) callus culture. Radiat Phys Chem 80(2011):968–976
Bolada S, Sehrawat SK, Yadav BS, Ahlawat VP, Sulthan S (2012) Present status of ber production and future thrusts in India-a review. Agri Rev 33(3):256–264
Calucci L, Pinzono C, Zandomeneghi M, Capocchi A (2003) Effects of γ-irradiation on the free radical and antioxidant contents in nine aromatic herbs and spices. J Agric Food Chem 51:927–934
Devasagayam TPA, Boloor KK, Ramasarma T (2003) Methods for estimating lipid peroxidation: An analysis of merits and demerits. Indian J Biochem Biophys 40:300–308
Dorman HJ, Bachmayer O, Kosar M, Hiltunen R (2004) Antioxidant properties of aqueous extracts from selected Lamiaceae species grown in Turkey. J Agric Food Chem 52:762–770
Gulcin I, Uguz MT, Oktay M, Beydemirs KOI (2004) Evaluation of antioxidant antimicrobial activities of clary sage (salvia sclarea l.). Turk Journal of Agriculture 28:25–33
Helaly MNM, Hanan AMR (2011) Effectiveness of Gamma Irradiated Protoplast on Improving Salt Tolerance of Lemon(Citrus limon L. Burm.f.). A J Plant Physiol 6(4):190–208
Ioannou I, Ghoul M (2012) Biological Activities and Effects of Food Processing on Flavonoids as Phenolic Antioxidants,.Advances in Applied Biotechnology, Prof. Marian Petre (Ed.), ISBN: 978-953-307-820-5
Khatun A, Hossain A, Islam M, Hossain A, Munshi K, Huque R (2012) Effect of gamma radiation on antioxidant marker and microbial safety of fresh bitter gourd (Momordica charantia L.). Int. J. Biosci. 2(11): 43–49. http://www.innspub.net
Koley TK, Charanjit K, Shweta N, Shweta W, Seema J, Sarika (2011) Antioxidant Activity and phenolic content in genotypes of Indian jujube (Zyziphus mauritiana l.). Arabian Journal of Chemistry
Krishna H, Parashar A (2012) Phytochemical Constituents and Antioxidant Activities of Some Indian Jujube (Ziziphus mauritiana l.) Cultivars. Journal of Food Biochemistry. 1–6. ISSN 1745–4514
Meda A, Lamicn CE, Romito M, Millogo J, Nacoulma OG (2005) Determination of the total phenolic, flavonoid and proline contents in Burkina fasan honey, as well as their radical scavenging activity. Food Chem 91:571–577
Michaela S, Sonja S, Gerhard S (2005) Phenolic compounds in tomatoes. Natural variations and effect of gamma-irradiation. Springer-Verlag.
Nagendran B, Tan YA, Ravigadevi S, Kalyana S, Samir S (2005) Antioxidant properties of palm fruit extracts. Asia Pac J Clin Nutr 4(4):319–324
Nikols AB, Dimitrios JF, Georgios EP, Vassilious NV, Antonios JM, Antonios GT (1994) Rapid, sensitive and specific thiobartiburic acid method for measuring Lipid peroxidation in Animal Tissue, food, and Feedstuff samples. J of Agric Food Chem 42:1931–1937
Nishimiki M, Rao NA, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine metho-sulfate and molecular oxygen. Biochem biophys res commun 46:849–853
Oyaizu M (1986) Studies on products of browning reaction prepared from glucoseamine. Jpn J Nutr 44:307–314
Rathore M (2009) Nutrient content of important fruit trees from arid zone of Rajasthan. Journal of Horticulture and Forestry 1(7):103–108.
Shoba D, Bharati P (2007) Value addition to Ber (Zyziphus mauritiana Lamk.) through preparation of pickle. Karnataka J Agric Sci 20(2):353–355
Sina SM, Hawa J, Rusli I, Asmah R, Maheran AA, Elizabeth P (2011) Effects of Acute Gamma Irradiation on Physiological Traits and Flavonoid Accumulation of Centella asiatica. Molecules 16:4994–5007. doi:10.3390/molecules16064994
Slinkard K, Singleton VL (1997) Total phenol analysis: Automation and Comparison with Manual Methods. Am J Enol Vitic 28:49–55
Srijaya M, Parvathi R, Kusuma D (2012) Impact of gamma irradiation combined with hot water blanching and calcium chloride treatment on shelf-life and quality of Ber (Zizyphus Mauritian). indian f packer 66(6):88–96
Suhaj M, Horváthová J (2007) Changes in antioxidant activity induced by irradiationof clove (Syzygium aromaticum) and ginger (Zingiber officinale). J of Food and Nutri Res 46(3):112–122
Suhaj M, Rácová J, Polovka M, Brezová V (2006) Effect of gamma-irradiation on antioxidant activity of black pepper (Piper nigrum L.). Food Chem 97:696–704
Sunil K, Praduman Y, Veena J, Sarla PM (2011) Evaluation of oxidative stress and antioxidative System in ber (Ziziphus mauritiana l.) Fruits during storage. Journal of Food Biochemistry ISSN. 1745–4514
Tanmay KK, Shweta W, Prerna N, Awasthi OP, Charanjit K (2011) Nutraceutical composition of Zizyphus mauritiana Lamk (Indian ber): effect of enzyme-assisted processing. Int J Food Sci Nutr 62(3):276–279
Villavicencio ALCH, Mancini-Filho J, Delincee H, Greiner R (2000) Effect of irradiation on anti-nutrients (total phenolics, tannins and phytate) in Brazilian beans. Radiat Phys Chem 57:289–293
Yamadagni R (1985) Ber in:Fruits of India, Tropical and subtropical. Naya Prakash, Calcutta
Zhang W-M, Lin Han BL, Zhang HD (2009) Antioxidant Activities of Extracts from Areca (Areca catectu L.) flower, husk and seed. Electron J of environ agric and food chem 8(9):740–748
Acknowledgments
Authors thank the department of Foods & Nutrition, PGRC, ANGRAU, Rajendranagar, Hyderabad for providing the facility to carry out whole research work successfully and also thanks to Quality control Laboratory, ANGRAU, permitting me to utilize their irradiation unit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kavitha, C., Kuna, A., Supraja, T. et al. Effect of gamma irradiation on antioxidant properties of ber (Zizyphus mauritiana) fruit. J Food Sci Technol 52, 3123–3128 (2015). https://doi.org/10.1007/s13197-014-1359-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1359-x