Abstract
This study evaluated the effects of Kurdi gum (KG) and Farsi gum (FG) based coatings with and without ethanolic Prosopis farcta extract (PFE; 0, 0.25 and 0.5%) on microbial, physicochemical, and sensory properties as well as respiration and ethylene production rates of banana fruits during storage (13 °C, 80% relative humidity (RH)) for 21 days and afterward 7 days at simulated market conditions (25 °C, 60% RH). The treatment of fruits with KG + PFE 0.5% resulted in the best bacterial, chemical, and sensory properties at the end of the storage period. It can be concluded that the application of KG and FG coatings enriched with PFE can be applied to increase the commercialization of bananas during prolonged storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Banana belongs to the family of Musaceae, which is widely grown throughout the tropical and subtropical regions in Africa, Latin America, and Asia (Pratiwi et al. 2015). It is rich in carbohydrates, minerals (potassium and calcium), vitamins (B6 and C), phenolics, and carotenoid compounds (Huang et al. 2014). Banana has a relatively short shelf life (6–8 days) at room temperature and is highly sensitive to low temperature (below 10 ± 1 °C), due to its richness of nutrients and primary changes during inappropriate postharvest handling and storage, including oxidation damage, peel spotting, and microbial spoilage (Al-Qurashi et al. 2017; Huang et al. 2014). Several post-harvest treatments have been evaluated to enhance sensory quality, microbiological safety, and nutritional value of bananas, such as modified atmosphere packaging (Siriwardana et al. 2017), edible coating (Andrade et al. 2015; Maqbool et al. 2010; Soradech et al. 2017), and essential oils (EOs) (Siriwardana et al. 2017; Vilaplana et al. 2018).
Biopolymer-based films and coatings have been used to protect various food products from mechanical damage, sensory quality, physicochemical and microbiological activities (Rezaei and Shahbazi 2018). Pistacia atlantica subsp. kurdica belongs to the Anacardiaceae family, which mainly grow in the western part of Iran especially Kermanshah and Kurdestan provinces (Minaiyan et al. 2015). Hardened gum obtained from the stems and branches of mountain Pistacia trees is used in Iranian traditional medicine for the treatment of gastrointestinal disorders including peptic and duodenal ulcers, as a liver and kidney tonic, for gastritis and diarrhea (Taran et al. 2010). Pistacia gum (also called Kurdi gum (KG)) consists of α- and β-masticinic acids, masticolic acid, α- and β-masticonic acids, and α- and β-masticoresene (Bozorgi et al. 2013; Morkhade 2017). KG produces a transparent dispersion in water with desirable adhesiveness, making it an appropriate candidate for coatings, emulsification, and stabilization. Farsi gum (FG) is obtained from the trunk and leaves of mountain almond tree/shrub (Amygdalus scoparia Spach), found in the central parts of Iran especially Shiraz and Kerman provinces (Joukar et al. 2017).
Incorporation of EOs/extracts with antimicrobial and antioxidant properties into coating solutions can extend the shelf life of coated foods (Shahbazi 2018). The active compounds in the EOs/extracts of these natural products can exert antimicrobial activities through various mechanisms, by affecting cell membranes, cellular energy generation, and inhibition of cell division (Rezaei and Shahbazi 2018). Prosopis farcta is a member of Fabaceae, sub-family Mimosoideae that is widely distributed in the dry and semi-dry tropical and sub-tropical areas of America, southwestern Asia, and northern Africa (Asadollahi et al. 2014). Prosopis genera exhibit a high degree of tolerance to heat, drought, alkalinity and salinity, and therefore thrive in central, eastern, and southwestern parts of Iran (Hajinezhad et al. 2015). The application of edible coatings with natural antimicrobials and antioxidants is one of the most promising approaches to extending the shelf life of fruits and vegetables (Al-Qurashi et al. 2017; Andrade et al. 2015; Siriwardana et al. 2017; Soradech et al. 2017). However, there are no published data about the effects of KG- and FG-based coatings on shelf life extension of banana fruits. Therefore, the aim of the present study was to evaluate the effects of KG and FG coatings containing ethanolic P. farcta extract (PFE) on the microbial, physicochemical and sensory properties of banana fruits during storage plus simulated market conditions.
Materials and methods
Materials
FG and KG were obtained from Freerco (Shiraz, Iran) and Khomali (Sanandaj, Iran) companies, respectively. Fruit of P. farcta was obtained from Ilam province, Iran. Bananas were purchased in Kermanshah, Iran. All media and solvents were purchased from Merck, Darmstadt, Germany.
Extraction procedure of Prosopis farcta
The fruits of P. farcta were air-dried for 2 weeks at ambient temperature (24 ± 1 °C) and then ground to obtain a fine powder using a Moulinex food processor. 1 g of the ground fruit was initially extracted with 10 ml pure ethanol using a magnetic stirrer (IKA, Germany) for 24 h at room temperature in darkness. The extract was passed through a Whatman No. 4 filter paper, and the residue was extracted again, evaporated under pressure at 40 °C to remove the solvent, and then stored at 4 ± 1 °C for further use.
Proximate analysis of Farsi gum and Kurdi gum
The moisture, protein, fat, and ash of FG and KG were determined based on the standard method of AOAC (1995). The pH, total carbohydrate content, and monosaccharide composition of KG and FG were quantified using the methods indicated by Bhushette and Annapure (2018).
Preparation of Farsi and Kurdi coatings and treatments
Farsi and Kurdi gum coatings (2.5% w/v) were separately prepared by dissolving the required amounts of powder in distilled water using a magnetic stirrer (IKA, Germany) at room temperature until the powder was completely dispersed. Glycerol (0.75 ml/100 ml) was added to the mixtures to plasticize the solutions. Ethanolic PFE (0, 0.25 and 0.5%) was added to portions of each solution. The mixtures were stirred at room temperature for 30 min to form emulsions for coating of the bananas (Hadian et al. 2017; Shahbazi 2018).
The fruits were selected for uniformity in size and visual quality, mature all green-index 1, 11–12 weeks after bloom without physical damage, immersed in 0.1% sodium hypochlorite for 1 min, rinsed with distilled water, and dried at room temperature. These bananas were randomly divided into seven groups (ten bananas per group) to receive the following treatments: (1) control (uncoated), (2) coated with pure FG, (3) coated with FG + PFE 0.25%, (4) coated with FG + PFE 0.5%, (5) coated with pure KG, (6) coated with KG + PFE 0.25%, and (7) coated with KG + PFE 0.5%. The fruits were dipped in designated coating solutions for 5 min, drained of the excess solution, and dried at room temperature for 30 min (Soradech et al. 2017). Treated and untreated (control) bananas were kept at 13 °C, 80% RH for up to 21 days. Initially and at weekly intervals, samples were held for 7 days at simulated market conditions (25 °C, 60% RH) and the physicochemical, microbial, and sensory properties of the bananas were evaluated at 0, 7, 14, 21 and 28 days. All experiments were conducted in three replicates.
In vitro antimicrobial activity of Farsi and Kurdi coatings
The in vitro antimicrobial property of FG and KG based-coatings were evaluated against Staphylococcus aureus (ATCC 6538), Bacillus cereus (ATCC 11774), Escherichia coli O157:H7 (ATCC 10536), and Salmonella typhimurium (ATCC 14028). Briefly, 0.1 ml of each culture strain containing 8 log CFU/ml was spread on the Brain Heart Infusion (BHI) agar (diameter = 90 mm) using a sterile swab. Blank sterile disks (diameter = 7 mm) soaked with 40 µl of each prepared coating were placed onto the inoculated plate surface. The plates were incubated at 37 °C overnight, and the diameter of the inhibition zone was determined using a caliper (Parsmadar Asia, Tehran, Iran) to the nearest 0.02 mm (Shahbazi 2017).
Physicochemical, microbial and sensory properties of bananas
At each sampling day, the physicochemical properties of untreated and treated bananas were evaluated by measuring weight loss (WL), titratable acidity (TA), pH, and firmness based on previously published methods of Bico et al. (2009). For determination of WL, banana pieces were put into the clean petri dish and dried at 80 °C for 48 h. After drying, the petri dish was placed in a desiccator to cool to room temperature. Weight was determined before and after banana drying using an analytical balance (BL-200i, Japan). WL was calculated based on the following formula: WL = 100 − 100 × dry matter% at time 0/dry matter% after drying for 48 h. For pH and TA, 10 g of each sample was mixed in 100 ml doubled distilled water at 10,000 rpm for 2 min, and the pH of the homogenized sample was determined with a pH meter (Metrohm, Switzerland). The mixture was also titrated with 0.1 M NaOH at pH 8.3 to determine TA value. The firmness of each sample was determined using the force required for a 2 mm digital probe (20 N) to penetrate 10 mm into the cut surface. Peel color values of bananas were measured using a Minolta Chroma meter CR-400 (Minolta, Japan). The values of L* (0 = black and 100 = white), h° (color hue angle), a* (− 120 = green and 120 = red) and b* (− 120 = blue and 120 = yellow) were measured in the middle of each of the ten fruits. The color meter was calibrated using a standard white plate (L = 94.24, a = − 0.52 and b = 4.19) before measurements. The h° value was calculated using the following formula: h° = tan−1 b*/a* (Huang et al. 2014). The published method by Shahbazi (2018) was followed for counting total viable count (TVC), total coliform, and total yeasts/molds counts. 10 g of each treated and un-treated sample was aseptically weighed, transferred into a sterile plastic package, homogenized with 90 ml of 0.1% peptone water, and serial dilutions were performed in triplicate. TV counts were enumerated using plate count agar (PCA; 30 °C for 48 h), total coliform using violet red bile glucose agar (VRBGA; 30 °C for 24–48 h), and total yeasts/molds using Dicloran Rose-Bengal chloramphenicol agar (25 °C for 48–72 h). For sensory properties, thirty panelists (18 females and 12 males, 22–40 years old) were trained a total of 5 times, 3 h training session over a period of one week. Each panelist received three banana cubes which had a random three-digit blind code and presented in the individual booths. The panelist scored the appearance, texture, and overall acceptability of the bananas using a 10-point scale (10, extremely desirable; 1, extremely unacceptable; Khorram et al. (2017). Respiration and ethylene production rates of samples were determined as previously reported by Huang et al. (2014).
Statistical analysis
The experiment was used in a completely randomized design in three repetitions. Microbiological data were transformed into logarithms of the number of colony forming units (CFU/g). Mean ± standard deviations of microbiological, physicochemical, and sensory properties were calculated. The two way repeated measures analysis of variance (SPSS 25) containing a within-subject factor (five levels of storage time) and a between-subject factor (seven different treatments) were applied. Significant differences were determined at P < 0.05.
Results and discussion
Proximate composition of Farsi gum and Kurdi gum
In the present study, the moisture, protein, fat, ash, pH, and total carbohydrate content of FG was found to be 8.65% ± 0.34, 0.23% ± 0.03, 2.32% ± 1.67, 3.57% ± 0.03, 4.95 ± 0.09, and 85.23% ± 1.67, respectively. The FG mainly consisted of rhamnose (0.84% ± 0.03), mannose (0.38% ± 0.02), glucose (0.15% ± 0.05), xylose (5.67% ± 0.03), arabinose (63.67% ± 1.11), and galactose (27.12% ± 1.23), indicating an arabinogalactan polysaccharide. In the case of KG, moisture, protein, fat, ash, pH, and total carbohydrate content was recorded to be 7.34% ± 0.76, 0.12% ± 0.01, 25.18% ± 0.54, 3.51% ± 0.22, 5.14 ± 0.01, and 63.85% ± 3.56, respectively. The KG mainly composed of arabinose (51.39% ± 2.56), galactose (40.01% ± 2.56), mannose (3.19% ± 0.09), rhamnose (2.33% ± 0.07), and fucose (1.55% ± 0.01).
In vitro antimicrobial activity of Farsi and Kurdi coatings
Based on our findings (Table 1), straight FG coating didn’t show any antimicrobial activity against S. aureus, B. cereus, E. coli O157:H7 and S. typhimurium. The order of antibacterial activity of coatings are as follows: KG + PFE 0.5% > FG + PFE 0.5% > KG + PFE 0.25% > FG + PFE 0.25% > KG. Some studies have reported on in vitro antimicrobial effects of extract/EO from KG against E. coli, S. typhimurium, Serratia marscens, B. cereus, S. aureus, Staphylococcus epidermidis, Bacillus subtilis, Pseudomonas fluorescens, and Enterobacter aerogenes (Ghalem and Mohamed 2009; Sharifi and Hazell 2011). These studies found that α- and β-pinene were the major constituents of the EO obtained from KG. It has been demonstrated that phenolic compounds are the main antimicrobial and antioxidant components of herbs (Rezaei and Shahbazi 2018). Our preliminary study of total phenolic compounds and total flavonoid content of PFE found 70.89 ± 0.01 mg gallic acid/g and 21 ± 0.08 mg quercetin/g, respectively. The DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical scavenging activity and β-carotene bleaching ability of the extract were found to be 1.16 μg/ml and 0.09 mg/ml, respectively. It is likely that the phenolic compounds, as the major constituents of the EOs and extracts, inhibited the protective enzymes which play important roles in regulation of energy and synthesis of structural components, increased the permeabilization of the bacterial membrane, and disrupted the layers of polysaccharides, fatty acids and phospholipids, which resulted in the death of the microorganisms (Hassoun and Emir Çoban 2017).
Physicochemical quality changes of bananas
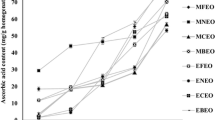
Our findings (Fig. 1a) indicated that the WL percentages of all bananas increased as a function of storage time (P < 0.05), reaching a value of 32.57% for uncoated samples after storage at 13 °C, 80% RH for 21 days and 7 days at simulated market conditions (25 °C, 60% RH). WL during prolonged storage at ambient and refrigerated temperatures was also reported for fresh strawberries (Shahbazi 2018), bananas (Kittur et al. 2001; Maqbool et al. 2010), cut pineapple (Azarakhsh et al. 2014), and mango (Kittur et al. 2001). By the end of the storage duration, the WLs of bananas coated with straight FG and KG were recorded to be 26.63% and 24.71%, respectively (Fig. 1a). This finding is consistent with previous studies indicating a decrease in WLs of fruits coated with biopolymer packaging materials including shellac-gelatin (Soradech et al. 2017), FG (Khorram et al. 2017), carrageenan (Bico et al. 2009), carboxymethyl cellulose and chitosan (Shahbazi 2018). It was also observed that all samples coated with FG and KG fortified with PFE (0.25 and 0.5%) presented significant lower WLs than uncoated samples (P < 0.05). The lower WL% probably was a result of the semipermeable barrier property of the tested coating materials against migration of water from fruits to the environment and reduced respiration rates which minimize drastic WL of coated bananas (Maqbool et al. 2011). Azarakhsh et al. (2014) found significant differences in WL of fresh-cut pineapple coated with different concentrations of lemongrass EO.
Changes in weight loss (a), titratable acidity (b), pH (c), firmness (d), color values (L* (e) and h° (f)), respiration (g) and ethylene production (h) rates of bananas coated with Farsi gum (FG) and Kurdi gum (KG) based-coatings with or without Prosopis farcta extract (PFE) during storage. Each bar represents the mean of three samples taken from different experiments. Each sample was analyzed in triplicate. For each sampling day, different lowercase letters indicate significant differences among treatments (P < 0.05)
The effects of different coating treatments on TA and pH values of bananas are presented in Fig. 1b, c, respectively. Results showed that pH increased as TA reduced in either uncoated or coated fruits throughout the storage period (P < 0.05). The decreasing of the most important organic acids present in the banana fruit including malic acid and citric acid, which act as substrates for the enzymatic reactions of respiration is the most likely reason for TA reduction and the accompanying pH increase (Maqbool et al. 2010; Siriwardana et al. 2017). The corresponding values in the coating treatments, especially KG + 0.5% PFE and FG + 0.5% PFE, were significantly lower than the untreated control samples (P < 0.05). Soradech et al. (2017) also found that TA values of untreated bananas decreased from 110.06 mg malic acid/100 g sample to 45.63 mg malic acid/100 g sample. In contrast with this finding, Vilaplana et al. (2018) reported that TA of non-treated bananas slowly increased from 0.2 g citric acid/100 g sample (day 0) to 0.6 g citric acid/100 g sample (day 21).
Firmness is a very important indicator in the palatability and ripeness of banana (Siriwardana et al. 2017). In this study, firmness values of the fresh banana were in the range of 98.9–99.3 N (Fig. 1d). Firmness values of all samples declined by 31.96–67.78 N from the initial value, indicating softening of the fruits. This decreasing trend of fruit firmness may result from degradation of pectin, hemicelluloses, polysaccharides, and starch, which imparts cellular rigidity; these compounds are broken down to sugars during ripening (Siriwardana et al. 2017). Based on our findings, bananas when coated with pure coating solutions or coatings containing PFE (0.25 and 0.5%) showed significantly greater firmness than the control group (P < 0.05; Fig. 1d). It was reported that fresh fruits such as banana (Lo’ay and Dawood 2017; Maqbool et al. 2011; Maqbool et al. 2010), mango (Kittur et al. 2001), and strawberries (Azarakhsh et al. 2014; Shahbazi 2018) treated with edible biopolymer coatings had better firmness quality than untreated ones.
Surface banana color, is one of the major visual marketable attributes which changes during ripening from green to yellow. It is obviously related to the degradation of the green pigment chlorophyll and its replacement by new pigments such as carotenoids (Ahmed and Palta 2016; Lo’ay and Dawood 2017). Ripened banana fruit goes from marketable (yellow with very few scattered brown spots) to unmarketable (yellow with many scattered brown spots) in 1 to 3 days after cold storage (Ahmed and Palta 2016; Huang et al. 2014). The present study (Fig. 1e) found that the L* value of untreated sample was 55.2 at the beginning of the study, continuously increased during the storage, and reached 74.5 after 21 days of cool storage plus 7 days at simulated market conditions. Nunes et al. (2013) and Chen and Ramaswamy (2002) also reported that banana color changed from green to yellow, as L* value increased from 54–55 to 67–75, which is similar to our findings. In the treated groups, peel color indices also gradually increased with storage time but were significantly lower than the control group (P < 0.05) at all evaluation dates (Fig. 1e). The peel color retardation in bananas could be attributed to the slower respiration rate, decreased ethylene synthesis and less enzymatic process lead to loss of quality (Maqbool et al. 2011).
As shown in Fig. 1f, the hue angle values (h°) exhibited opposite tendencies with L* value. In this study, the h° values of the control group, and the samples coated with FG and KG solutions were 158.8, 159.1, and 159, respectively, on day 0, and decreased to 76.4, 90, and 98.8, respectively, at the end of the study period (day 28). Similar results were reported by Huang et al. (2014), Ahmed and Palta (2016), and Lo’ay and Dawood (2017) who studied the color changes of bananas during storage under refrigerated and ambient condition. Based on the results of the present study, the treated bananas retained significantly higher h° values at the end of storage period compared to control fruits (P < 0.05). Several post-harvest treatments have been evaluated to extend the shelf life of bananas in terms of retardation of color changes (Pratiwi et al. 2015; Vilaplana et al. 2018). For example, Vilaplana et al. (2018) also found few changes in h° and L* values and saturation of the color of bananas treated with different concentrations of thyme EO during the cold storage period. Pratiwi et al. (2015) reported the effectiveness of chitosan coating + bamboo extract in the retardation of maturation, finding relatively slower color changes in comparison with control bananas. According to the results of Lo’ay and Dawood (2017), the h° value of untreated banana was approximately 51–65 lower than samples coated with active chitosan/polyvinyl alcohol containing oxalic acid. Maqbool et al. (2011) found that a composite coating based on gum Arabic and chitosan showed produced good results in maintaining color better results in maintaining banana fruit color.
The respiration and ethylene production rates in fresh fruits are important indices for the determination of storage life (Maqbool et al. 2011). The effects of FG and KG based coatings enriched with PFE on respiration and ethylene production rates of bananas are exhibited in Fig. 1g, h, respectively. The control group had significantly higher respiration and ethylene production rates throughout the study than the other treatments (P < 0.05). The initial respiration and ethylene production rates were 33.21 mg/kg h and 7.37 µl/kg h, respectively. In this study, the edible KG and FG based coatings successfully moderated increases in the respiration and ethylene production rates compared to the controls. These results are consistent with previous studies (Naeem et al. 2018; Shahbazi 2018), indicating edible coatings can play an important role in retarding the rate of internal respiration. Significant differences in respiration rates and in ethylene production rates among control and treated groups were observed throughout the entire storage time (P < 0.05; Fig. 1g, h). The final respiration and ethylene production rates of treated samples were 99.7–119.7 mg/kg h and 15.2–20.97 µl/kg h lower than the controls, respectively. Among all designated treatments, KG + PFE 0.5% and FG + PFE 0.5% exhibited the best results in terms of respiration and ethylene production rates by 58.9–61.5 mg/kg h and 11.33–12.45 µl/kg h, respectively, after storage and simulated market conditions (P < 0.05). Reduction of respiration rate as a result of the coating containing plant EO or extract compared to uncoated samples has also been found for strawberries (Shahbazi 2018), green-unripe mangoes (Naeem et al. 2018), and banana (Kittur et al. 2001).
Microbial quality changes of bananas
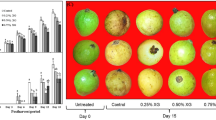
The effects of FG and KG based coatings containing PFE (0, 0.25 and 0.5%) on microbial spoilage (TVC, total coliform, and total yeasts/molds counts) population of bananas exhibited in Fig. 2a–c, respectively. The initial TVC and total coliform count of < 1 log CFU/g in the control samples indicated that the fruits had good initial microbiological quality (Bico et al. 2009). The initial yeasts/molds count of fresh bananas was found to be 1.11 log CFU/g, which is inconsistent with those reported by Soradech et al. (2017). These authors reported that the initial total yeasts/molds count of bananas were in the ranges of 1.07–1.60 log CFU/g. A recent study reported that microbiological quality of bananas during processing, handling and storage in the market could be influenced by contaminated handling tools, polluted water and air, temperature abuse, and other conditions during bananas storage (Issouffou et al. 2018). An increase in the microbial population of control group during storage time was observed in the present study (P < 0.05; Fig. 2a–c). The microbial population of samples coated with KG alone was always less than untreated groups. The TVC, total coliform, and total yeasts/molds counts of banana coated with FG and KG solutions containing PFE (0.25 and 0.5%) were significantly lower than uncoated controls (P < 0.05).
Changes in total viable count (TVC) (a), total coliform count (b) and total yeasts/molds count (c) of bananas coated with Farsi gum (FG) and Kurdi gum (KG) based-coatings containing Prosopis farcta extract (PFE; 0.25 and 0.5%) during storage. Each bar represents the mean of three samples taken from different experiments. Each sample was analyzed in triplicate. For each sampling day, different lowercase letters indicate significant differences among treatments (P < 0.05)
Sensory analysis of bananas
Sensory evaluation results stored bananas treated with KG or FG with or without PFE after 21 days of storage plus 7 days at simulated marketing conditions are given in Fig. 3. The poorest sensory attributes were found in the untreated control bananas (P < 0.05). The sensory attributes of the samples treated with KG or FG, with or without PFE were all significantly higher than the controls (P < 0.05). Odor, color, and overall acceptability attributes showed positive correlations with the microbiological properties, evidenced by R2 = 0.8352–0.8849, R2 = 0.8014–0.8538, and R2 = 0.7932–0.8542, respectively. The corresponding sensory scores were correlated to the chemical findings in the ranges of R2 = 0.8248–0.8599, R2 = 0.9235–0.9753, and R2 = 0.8692–0.8835, respectively. It was found that incorporating essential oils/extracts such as lemongrass (Azarakhsh et al. 2014), cinnamon (Maqbool et al. 2011), clove (Ranasinghe et al. 2005), and mint (Shahbazi 2018) to fresh fruits gave more acceptable sensory scores compared to the untreated samples.

taken from different experiments. Each sample was analyzed in triplicate. For each measured characteristic, different lowercase letters indicate significant differences among treatments (P < 0.05)
Sensory properties (appearance, texture and overall acceptability) of banana samples coated with Farsi gum (FG) and Kurdi gum (KG) based-coatings with or without Prosopis farcta extract (PFE; 0.25 or 0.5%) after 21 days of storage plus 7 days of simulated market conditions. Each bar represents the mean of three samples
Conclusion
The application of KG or FG solutions enriched with PFE retarded microbial growth and physicochemical changes in bananas stored at 13 °C, 80% RH for up to 21 days plus 7 days at simulated market conditions (25 °C, 60% RH). Application of KG and FG solutions greatly improved the sensory characteristics of the bananas as compared with untreated controls, and the addition of PFE (0.25 or 0.5%) further improved the sensory characteristics of the bananas. Therefore, application of KG and FG coatings enriched with PFE may be useful to increase the commercialization of bananas during prolonged storage.
References
Ahmed ZF, Palta JP (2016) Postharvest dip treatment with a natural lysophospholipid plus soy lecithin extended the shelf life of banana fruit. Postharvest Biol Technol 113:58–65
Al-Qurashi AD, Awad MA, Mohamed SA, Elsayed MI (2017) Postharvest chitosan, trans-resveratrol and glycine betaine dipping affect quality, antioxidant compounds, free radical scavenging capacity and enzymes activities of ‘Sukkari’ bananas during shelf life. Sci Horticult 219:173–181
Andrade R, Skurtys O, Osorio F (2015) Drop impact of gelatin coating formulated with cellulose nanofibers on banana and eggplant epicarps. LWT-Food Sci Technol 61:422–429
AOAC (1995) Official methods of analysis, 16th edn. Association of Official Analytical Chemists, Arlington
Asadollahi A, Sarir H, Omidi A, Torbati MBM (2014) Hepatoprotective potential of Prosopis farcta beans extracts against acetaminophen-induced hepatotoxicity in wister rats. Int J Prev Med 5:1281
Azarakhsh N, Osman A, Ghazali HM, Tan CP, Adzahan NM (2014) Lemongrass essential oil incorporated into alginate-based edible coating for shelf-life extension and quality retention of fresh-cut pineapple. Postharvest Biol Technol 88:1–7
Bhushette PR, Annapure US (2018) Physicochemical, functional and rheological investigation of Soymida febrifuga exudate gum. Int J Biol Macromol 111:1116–1123
Bico S, Raposo M, Morais R, Morais A (2009) Combined effects of chemical dip and/or carrageenan coating and/or controlled atmosphere on quality of fresh-cut banana. Food Control 20:508–514
Bozorgi M, Memariani Z, Mobli M, Salehi Surmaghi MH, Shams-Ardekani MR, Rahimi R (2013) Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): a review of their traditional uses, phytochemistry, and pharmacology The Scientific World Journal 2013
Chen C, Ramaswamy H (2002) Color and texture change kinetics in ripening bananas. LWT-Food Sci Technol 35:415–419
Ghalem BR, Mohamed B (2009) Bactericidal activity of Pistacia atlantica. Desf mastic gum against certain pathogens. African Journal of Plant Science 3:013–015
Hadian M, Hosseini SMH, Farahnaky A, Mesbahi GR (2017) Optimization of functional nanoparticles formation in associative mixture of water-soluble portion of Farsi gum and beta-lactoglobulin. Int J Biol Macromol 102:1297–1303
Hajinezhad M, Esmaeel Zadeh Bahabadi S, Miri H, Davari I, Darvish Sargazi M (2015) Effect of hydroalcoholic extract of Prosopis farcta pod on liver histopathology and malondialdehyde level in streptozotocin diabetic rats. Horizon Med Sci 21:31–36
Huang H, Jing G, Wang H, Duan X, Qu H, Jiang Y (2014) The combined effects of phenylurea and gibberellins on quality maintenance and shelf life extension of banana fruit during storage. Sci Hortic 167:36–42
Issouffou C, Suwansri S, Salaipeth L, Domig KJ, Hwanhlem N (2018) Synergistic effect of essential oils and enterocin KT2W2G on the growth of spoilage microorganisms isolated from spoiled banana peel. Food Control 89:260–269
Joukar F, Hosseini SMH, Moosavi-Nasab M, Mesbahi GR, Behzadnia A (2017) Effect of Farsi gum-based antimicrobial adhesive coatings on the refrigeration shelf life of rainbow trout fillets. LWT-Food Sci Technol 80:1–9
Khorram F, Ramezanian A, Hosseini SMH (2017) Shellac, gelatin and Persian gum as alternative coating for orange fruit. Sci Hortic 225:22–28
Kittur F, Saroja N, Tharanathan R (2001) Polysaccharide-based composite coating formulations for shelf-life extension of fresh banana and mango. Eur Food Res Technol 213:306–311
Lo’ay A, Dawood H (2017) Minimize browning incidence of banana by postharvest active chitosan/PVA Combines with oxalic acid treatment to during shelf-life. Sci Hortic 226:208–215
Maqbool M, Ali A, Ramachandran S, Smith DR, Alderson PG (2010) Control of postharvest anthracnose of banana using a new edible composite coating. Crop Prot 29:1136–1141
Maqbool M, Ali A, Alderson PG, Zahid N, Siddiqui Y (2011) Effect of a novel edible composite coating based on gum arabic and chitosan on biochemical and physiological responses of banana fruits during cold storage. J Agric Food Chem 59:5474–5482
Minaiyan M, Karimi F, Ghannadi A (2015) Anti-inflammatory effect of Pistacia atlantica subsp. kurdica volatile oil and gum on acetic acid-induced acute colitis in rat. Res J Pharmacogn 2:1–12
Morkhade DM (2017) Evaluation of gum mastic (Pistacia lentiscus) as a microencapsulating and matrix forming material for sustained drug release. Asian J Pharm Sci 12:424–432
Naeem A, Abbas T, Ali TM, Hasnain A (2018) Effect of guar gum coatings containing essential oils on shelf life and nutritional quality of green-unripe mangoes during low temperature storage. Int J Biol Macromol 113:403–410
Nunes CN, Yagiz Y, Emond J-P (2013) Influence of environmental conditions on the quality attributes and shelf life of ‘Goldfinger’bananas. Postharvest Biol Technol 86:309–320
Pratiwi AS, Dwivany FM, Larasati D, Islamia HC, Martien R (2015) Effect of chitosan coating and bamboo FSC (fruit storage chamber) to expand banana shelf life. In: AIP conference proceedings. AIP Publishing, vol 1, p 100005
Ranasinghe L, Jayawardena B, Abeywickrama K (2005) An integrated strategy to control post-harvest decay of Embul banana by combining essential oils with modified atmosphere packaging. Int J Food Sci Technol 40:97–103
Rezaei F, Shahbazi Y (2018) Shelf-life extension and quality attributes of sauced silver carp fillet: a comparison among direct addition, edible coating and biodegradable film. LWT-Food Sci Technol 87:122–133
Shahbazi Y (2017) The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int J Biol Macromol 99:746–753. https://doi.org/10.1016/j.ijbiomac.2017.03.065
Shahbazi Y (2018) Application of carboxymethyl cellulose and chitosan coatings containing Mentha spicata essential oil in fresh strawberries. Int J Biol Macromol 112:264–272. https://doi.org/10.1016/j.ijbiomac.2018.01.186
Sharifi MS, Hazell SL (2011) GC-MS Analysis and Antimicrobial activity of the essential oil of the trunk exudates from Pistacia atlantica kurdica. J Pharm Sci Res 3:1364
Siriwardana H, Abeywickrama K, Kannangara S, Jayawardena B, Attanayake S (2017) Basil oil plus aluminium sulfate and modified atmosphere packaging controls Crown rot disease in Embul banana (Musa acuminata, AAB) during cold storage. Sci Hortic 217:84–91
Soradech S, Nunthanid J, Limmatvapirat S, Luangtana-anan M (2017) Utilization of shellac and gelatin composite film for coating to extend the shelf life of banana. Food Control 73:1310–1317
Taran M, Mohebali M, Esmaeli J (2010) In vivo efficacy of gum obtained Pistacia atlantica in experimental treatment of cutaneous leishmaniasis. Iranian J Public Health 39:36
Vilaplana R, Pazmiño L, Valencia-Chamorro S (2018) Control of anthracnose, caused by Colletotrichum musae, on postharvest organic banana by thyme oil. Postharvest Biol Technol 138:56–63
Acknowledgements
We acknowledge Razi University for the use of their facilities and instrumentations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Human and animal rights
The article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahbazi, Y., Shavisi, N. Application of active Kurdi gum and Farsi gum-based coatings in banana fruits. J Food Sci Technol 57, 4236–4246 (2020). https://doi.org/10.1007/s13197-020-04462-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04462-x