Abstract
Radish leaf protein concentrates (RLPC) were prepared by alkaline extraction and characterized for their antioxidant activity, functional properties, mineral content, in-vitro digestibility and microbial stability. Numerical optimization using the 3-factor Box–Behnken Design of response surface methodology suggested that the optimized extraction was obtained at a pH of 9.46, sample/water ratio of 0.075 and time of extraction 46.89 min resulting in 12.12% yield of RLPC with protein content of 87.64%. Glutelins (41.49%), prolamins (24.96%) and albumins (20.43%) were found to be the three major fractions of the protein concentrate, while globulins (13.00%) contributed as a minor component. The apparent molecular weights of these protein fractions ranged between 14 and 60 kDa. Antioxidant activities (FRAP, ABTS and DPPH) were higher in RLPC as compared to the isolated fractions. Functional properties like water holding capacity, oil holding capacity, emulsifying capacity and emulsion stability of the RLPC were 352, 280, 48.1 and 47.8%, respectively. Ca and Fe were the most abundant major and trace minerals, respectively, present in the RLPC. In-vitro protein digestibility was found to be 93.51% and its microbial load remained in acceptable limits during 42 days of storage under both refrigerated and ambient temperature conditions. Our results indicated that the protein concentrates extracted from radish leaves have considerable antioxidant activity, functional properties, mineral content, digestibility and microbial stability. The results highlight the potential of RLPC for use in functional foods as a safe and cost-effective source of protein.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radish (Raphanus sativus L.) belongs to the Cruciferae family, grown as a quick growing, cold season biennial crop having its origin in the Mediterranean and Asia. This crop of tropical and temperate regions is cultivated worldwide for its succulent taproot with a total production of approximately 7 million tons per year [1]. The leaves of radish plant, which constitute 30–50% of its total weight, are usually discarded as kitchen waste. These leaves are a good source of protein and have a biological value of 76.6 with the digestibility coefficient of 73.5% due to the presence of nitrogenous fraction of various amino acids [2]. However, the use of radish leaves as a source of protein has not been well researched and documented so far, since much focus has been placed on the root or the seeds. As the consumers’ anxieties and concerns about the food security, protein deficiency and ever rising cost of animal-based proteins are increasing, the interest of researchers in natural plant-based proteins is also increasing simultaneously.
Leaf Protein Concentrate (LPC) is an unconventional source of plant-derived protein which has been strongly considered as a functional food for children and as a protein supplement for various food formulations [3]. Due to low prices and relative abundance of leaves, the LPCs isolated from their tissues can serve as an alternative protein source to supplement the dietary needs of people suffering from protein malnutrition [4]. The leaf material is subjected to various extraction procedures with an aim to eliminate considerable amount of toxic and anti-nutritional agents along with the fibrous part, making consumption of leaves feasible. LPCs can be prepared by employing various methods viz. chemical extraction [5], enzyme-assisted extraction [6] and heat coagulation [7] among various others. All these extraction methods mainly involve mechanical disruption of tissue, heat and/or pH aided protein precipitation and ultimately protein concentration [8]. The commonly used protein extraction protocol includes subjecting the tissue of interest to the exposure of distilled water or other weak buffers, which then causes rupture of cells with concomitant release of intracellular proteins due to hypotonic effect that emerges gradually [9]. However, due to the presence of hydrophobic groups and disulfide connections between protein molecules, proteins in plant cells are rarely water soluble. For other alternatives, aqueous salt or alkaline extraction is one of the most implemented techniques for the isolation of plant-based proteins because high alkalinity assists well in extracting leaf protein by breaking down the hydrogen bonds, disrupting the leaf tissue and enhancing protein solubility [10]. Alkaline extraction method is a tested approach employed to improve protein extraction in a more economic and environment friendly way in comparison to other organic solvents [11]. Moreover, under alkaline conditions, the cell wall degradation and subsequent solubilization of lignins and carbohydrates (including pectin, cellulose, hemicellulose) lead to a higher protein yield due to the efficient release of well protected proteins within the cell wall [12]. Alkali (NaOH) can extract proteins by breaking down inter-protein interactions, such as covalent (intermolecular disulphide bonds) or non-covalent (hydrogen and hydrophobic) bonds. The NaOH concentration and parameters like pH and time of extraction affect the content of the extracted protein and optimization of these extraction conditions is a major objective of the present research.
Development of value-added products from protein concentrate and its subsequent use as an alternative protein require information on its physical, structural and biochemical properties along with its microbial stability to understand the nutritive value, health benefits and storage life of the same. Proteins have a substantial role in product development and food processing since they are significantly responsible for many functional properties such as emulsification, fat and water absorption, gelation and whipping properties, which strongly influence consumer acceptance of food products [13]. These functional properties are specific physicochemical properties that affect the organoleptic characteristics and quality of the food they are incorporated in, interfering in its behaviour and appearance, from its preparation to its storage. For the LPCs to be incorporated into food products, their functional properties need to be assessed. At present, to the best of researchers’ knowledge, no information on the optimization of process parameters of alkaline extraction of LPCs from radish leaves and their characterization is available in literature. Therefore, the present investigation was undertaken with an objective to optimize the process parameters for extraction of radish leaf protein concentrate (RLPC) and evaluation of its antioxidant activity, functional properties, mineral content, in-vitro digestibility and microbial stability for its potential use as food supplement.
Materials and methods
Radish leaves (Raphanus sativus var. Punjab Safed) grown in the fields of Department of Vegetable Science, Punjab Agricultural University, Ludhiana were used for the study. The leaves were manually separated from their stalks, washed, dried and ground into a fine powder which was stored in air-tight containers at room temperature (25–42 °C) for further experimentation. Chemical reagents used in this study for various experiments were of analytical grade and were purchased from Molychem, Pvt. Ltd. (Mumbai, India), Sisco Research Laboratories Pvt. Ltd. (Mumbai, India) and MP Biomedicals, Pvt. Ltd. (Mumbai, India). A high range protein marker (14–220 kDa), catalogue number 99625, Sisco Research Laboratories Pvt. Ltd., Mumbai, India) was used as the molecular weight marker for electrophoresis.
Preparation of radish leaf protein concentrate
The RLPCs were prepared by modifying the method of Jiamyangyuen et al. [5]. The radish leaf powder (10 g for each treatment) was added to deionized water (variable sample/water ratio) and stirred to obtain homogenous slurry. The pH of the slurry was adjusted, between 7 and 12, according to the experimental plan in order to maintain alkaline conditions by adding 1 N NaOH and homogenized at room temperature for a set period of time. After the homogenization, the slurries were centrifuged (5000×g) for 30 min at 4 °C. The pH of the supernatant was then adjusted to 4.5 for isoelectric precipitation and centrifuged again at 5000×g for 30 min. During isoelectric precipitation where solubility of protein is minimum, both amine and carboxyl groups along with equivalent charges are equal and hence isolates can be recovered from the solution as precipitate. Precipitates were washed using deionized water and freeze–dried. This final product referred to as RLPC was then stored for further analysis.
Experimental design and optimization
For the evaluation of maximum protein extraction, Design Expert software -File version 11 (Statease Inc., Minneapolis, USA) was used to draw an experimental design based on three process parameters viz. time of extraction (30–60 min), sample/water ratio (1:10–1:20) and pH of extraction medium (7–12) where two responses i.e., yield% and protein content% were recorded. In alkaline extraction method, the pH value, extraction time, sample/solvent ratio, and the interaction of these factors affect the protein extraction efficiency [14]. Therefore, optimization of the extraction parameters is quite significant for gaining high extraction yields. The experimental plan was designed using response surface methodology (RSM) by adopting a three variable Box and Behnken Design. For the whole experiment, there were a total of 17 treatment combinations of the three independent variables including some repeating combinations at centre points in order to allow curvature of the graph. The three-dimensional response surface plots generated by this software for different interactions between any two independent variables, while holding the value of other variable as constant could give accurate geometrical representation and provide useful information about the behavior of the system within the experimental design. The optimum process parameters were obtained by computer generated response surfaces, according to which the RLPCs were prepared. The model was employed for correlating the response variables to the independent variables by fitting them to a polynomial second-order model as follow:
where, Y = response variable, β0 = offset term. βi = linear coefficient, βii = quadratic coefficient, βij = interaction coefficient and xi, xii = independent variables.
Determination of yield and protein content of RLPCs
Yield of RLPCs was calculated as the percentage of RLPC obtained (g) from the total amount of radish leaf powder used (g). Crude protein content of RLPCs was determined by the standard Kjeldahl method [15]. For the determination of nitrogen, Kelplus Nitrogen Estimation System (Block digestion system with distillation system, Pelican Equipments, Chennai, India) was used. The nitrogen content was multiplied by conversion factor 6.25 to obtain protein content.
Isolation of protein fractions from RLPC
The protein fractions viz. albumins, globulins, prolamins and glutelins were isolated from RLPC by modifying the sequential extraction method of Adebiyi and Aluko [16]. RLPC (5 g) was taken for sequential extraction of protein fractions. First extraction was carried out using 5 ml of distilled water followed by centrifugation at 14000×g for 20 min. The extraction was performed two times. The supernatant so obtained constituted albumins (water soluble). The pellet settled at the bottom of the centrifuge tubes was subjected to extraction using 5 ml of 1.75 M NaCl. The centrifugation was carried out at 14000×g for 20 min and the extraction was repeated twice. The supernatants were pooled and were labeled as globulins (salt soluble). The pellet obtained was subjected to extraction using 5 ml 75% ethanol and centrifugation was done for 30 min at 14000×g. Again, the extraction was repeated twice and the supernatants were pooled and labeled as prolamins (ethanol soluble). The pellet obtained in the previous extraction was subjected to alkali extraction using 5 ml of 0.1 N NaOH followed by centrifugation at 14000×g for 20 min. The extraction was repeated twice and the collected supernatant was labeled as glutelins (alkali soluble). The pellets (non-protein residues) were discarded. The isolated protein fractions were freeze dried and stored. A part of the isolated protein fractions was taken to estimate the proportion of protein (%) in different fractions of RLPC using the method of Lowry et al. [17].
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
The electrophoretic profiles of the polypeptides from RLPCs and their protein fractions were determined according to the method of Laemmli [18]. The protein sample (5 mg) was dissolved in the sample buffer (1 mL) which was composed of distilled water (3.8 mL), 0.5 M Tris HCl buffer (1 mL): pH 6.8, glycerol (0.8 mL), 10% (w/v) SDS (1.6 mL), β-mercaptoethanol (0.8 mL), 0.05% (w/v) bromophenol blue (0.4 mL). The dissolved sample was then heated at 95 °C for 3 min. Once the samples were cooled, a 10 μL aliquot was loaded into the wells of the acrylamide gels (5% stacking, 12% resolving). Coomassie Brilliant Blue dye was used to stain the gel, which was later de-stained using a solution of methanol, acetic acid and distilled water. The electrodes were connected to DC power pack and the current was adjusted to 1.5 mA per cm. For stacking gel, 70 V and for resolving gel, 110 V voltages were employed. A high range protein marker (14–220 kDa) was used as the molecular weight marker for electrophoresis.
Determination of antioxidant properties, free phenols and flavonoids of RLPC
Sample (0.5 g) was mixed with 80% (8 ml) methanol and refluxed at 80 °C for about 10 min. The refluxed sample so obtained was filtered with Whatmann no. 1 filter paper. The supernatant was separated and its final volume was made to 10 ml with 80 % methanol. The methanolic extract thus prepared was then used to estimate free phenolic content, flavonoid content and antioxidant activity. DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity was measured according to the method of Lin et al. [19] by recording a decrease in the absorbance with respect to control. DPPH activity was calculated by preparing the standard curve using trolox and the results were expressed as mg TE/g (TE-Trolox Equivalents). ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) radical scavenging activity was estimated by following the method of Re et al. [20]. A decrease in the absorbance of solution was recorded at 734 nm after 10 min. ABTS scavenging activity of the sample was calculated by preparing the standard curve using trolox and the results were expressed as mg TE/g. FRAP (Ferric reducing/antioxidant power) activity was determined based on the method of Benzie and Strain [21]. The absorbance was measured at 593 nm. The standard curve was prepared simultaneously using FeSO4.7H2O (5–30 µg) and the results were expressed in mg/g dry weight. Free phenols were estimated by the method of Hillis and Swain (1959) [22] by using gallic acid (10–50 µg) as the standard, whereas flavonoids were estimated using the method of Balbaa et. al. [23] by using rutin (40–200 µg) as the standard.
Functional properties of RLPC
Protein solubility (PS) was estimated by method of Yu et al. [24]. Methods of Lin et al. [25] were used for estimation of the water holding capacity (WHC) and oil holding capacity (OHC). Foaming capacity (FC) and foaming stability (FS) were measured as per the methods of Yasumatsu et al. [26]. The method of Coffman and Garcia [27] was used for determining the emulsifying capacity (EC) and emulsion stability (ES). Least gelation concentration was estimated by method of Huda et al. [28].
Mineral analysis, color measurement and in-vitro digestion of RLPC
The RLPC (0.5 g) was digested using 10 ml of conc. HNO3 and HClO3 (v/v 2:1) in Kjeldahl Infra Digestion System using temperature profile: 150 °C for 1 h, 250 °C for 2 h (until clear solution was obtained) followed by addition of 10 ml of double distilled water and filtration [29]. Presence of different minerals was quantitatively determined using inductively coupled plasma optical emission spectroscopy (ICP-OES). The mineral composition of the RLPC was recorded as mg of mineral/100 g of RLPC.
The RLPC was subjected to color measurement using a colorimeter (Miniscan XE plus Hunter lab Colorimeter, U.S.A.) as explained by Znidarcic and Pozrl [30]. Color was measured as L*, a* and b* values which were recorded at D 65/10 °C. Calibration of the colorimeter was done using standard white and black plates.
The in-vitro digestion of RLPC was performed according to the standardized method described by Minekus et al. [31]. Briefly, 1 g of RLPC was mixed with 4 ml of simulated salivary fluid (SSF), 0.5 ml of α-amylase solution at 1500 U/ml in SSF, 25 µl of 0.3 M CaCl2 and 475 µl of ultrapure water. After 2 min of incubation, the mixture was mixed with 8 ml of simulated gastric fluid (SGF), 5 µl 0.3 M CaCl2, at pH 3 adjusted using 1 M HCl, before adding 0.5 ml of pepsin solution (25,000 U/ml in SGF). The gastric mixture was then incubated for 2 h in a water bath at 37 °C. After incubation, 20 ml of gastric mixture was mixed with 85 ml of simulated intestinal fluid (SIF) stock solution, 40 µl 0.3 M CaCl2, with an adjusted pH to 7 using 1 M NaOH, before adding 5 ml of pancreatin solution (800 U/ml in SIF), 2.5 ml of bile solution (160 Mm). The intestinal mixture was also incubated at 37 °C. After the digestion, the samples were cooled and centrifuged at 5000×g for 10 min at 4 °C to separate the soluble bio-accessible fraction from the residual fraction. In vitro protein digestibility was calculated as follows:
Qualitative identification of amino acids and other compounds in RLPC
The investigation of the presence of amino acids and various organic compounds, like phenols and flavonoids was done qualitatively using Liquid Chromatography Mass Spectrometry using Waters Micromass Q-Tof Micro. The instrument used for analysis is hybrid quadrupole time of flight mass spectrometer equipped with electrospray ionization and atmospheric pressure chemical ionization sources having mass range of 4000 amu in quadruple and 20,000 amu in ToF. The Mass Spectrometer is coupled with Waters 2795 HPLC having quaternary pumping configured for flow rates from 0.05 to 5.0 ml/Min. The mobile phase for LC separation was 0.1% formic acid acetonitrile aqueous solution (80% for acetonitrile); 1 μg of sample was dissolved and filtered and was then injected into a C18 column for testing [32]. The results are represented as m/z ratio and relative abundance of the compounds detected which were identified using the standard library database available at SAIF/CIL, PU Chandigarh.
Microbiological analysis of RLPC during storage
To determine the storage stability of the RLPC stored in LPDE (Low Density Polyethylene) bags (100 gauge) under the ambient (Temperature: 30.1–40.6 °C, Relative Humidity: 32–82%) and refrigerated (Temperature: 5 ± 1 °C, relative Humidity: 90%) conditions, its microbial analysis was performed as per Aslam et al. [33] at weekly intervals for 42 days. Potato Dextrose Agar Media (PDA) was used for the estimation of the total yeast and mould count of the stored RLPC. The PDA media was prepared and sterilized at pressure 15 psi for 15 min. Serial dilutions (10−2) were made and microbial count of this dilution was analyzed where 1ml of the dilution was taken in a petri plate on to which 15–20 mL media was poured. Plates were then left for incubation at 27 °C for 48–72 h. The colonies were counted and the results were expressed in log CFU/g. The total plate count of the RLPC was estimated using Nutrient Agar (NA). The NA media was prepared and sterilized at pressure 15 psi for 15 min. Serial dilutions (10−4) were prepared and microbial count of the dilutions was analyzed by taking 1 ml of the dilution pouring on to the petri plate. Plates were then incubated at 37 °C for 24 h, the colonies were counted and the results were expressed in log CFU/g.
Statistical analysis
The experiments were performed in triplicates and the data was expressed as mean ± standard error. Data from Box–Behnken design was processed and analysed using RSM from Design Expert version 11.0. One way analysis of variance (ANOVA) was used to evaluate statistical differences in treatment responses and compositional/microbial analysis. ANOVA was carried out using RSM and SPSS software (IBM SPSS Statistics 20.0 version).
Results and discussion
Effect of treatment combination on extraction of protein concentrates
A wide variation in responses was observed under various experimental conditions i.e., 5.87 to 12.66% for yield and 31.44 to 90.23% in protein content of RLPC. Maximum yield (12.66%) and maximum protein content (90.23%) in RLPC was obtained with sample/water ratio: 0.075, pH of extraction medium 9.5 and extraction time of 45 min, in different replications. Minimum yield of RLPC (5.87%) was observed when extraction was carried out with sample/water ratio 0.1 for extraction time of 45 min and pH of extraction medium 7.0, whereas minimum protein content (31.44%) in RLPC was observed at pH 12, sample water ratio: 0.05 and extraction time: 45 min (Table 1). The yield and protein content in RLPCs increased with increase in pH from 7 to 9.5 and then decreased as the pH went up to 12.0. At first, the increase in alkalinity leads to an increase in protein extractability due to the fact that leaf protein showed higher solubility at higher pH [34]. The decrease in yield and protein content of RLPCs at higher alkaline conditions might be due to the denaturation of proteins under these conditions [5]. Decrease in protein content at higher pH was also found during extraction of protein hydrolysate from de-oiled rice bran [35].
Fitting the models
A prediction model for optimizing the alkaline extraction of RLPC was developed using RSM. The independent and dependent variables, i.e., the process parameters and the responses, respectively, were analysed in order to obtain regression equations that could fit the mathematical models to the experimental data. The quadratic model obtained from the regression analysis for yield% and protein% was developed as follows:
Here A represents pH, B represents sample/water ratio and C represents time of extraction.
The analysis of variance (ANOVA) results for Box–Behnken Design including the sum of squares and P values for both the responses viz. yield of protein and protein content in RLPCs are indicated in Table 2 (a). Values of P (probability value of the regression model) less than 0.05 indicate model terms are significant at 5% level of significance. For the models fitted for yield and protein content of the extracted RLPC, the coefficient of determination i.e., R2, which is a measure of degree of fit, is also presented in Table 2 (a) The value of R2 for yield of RLPC was 0.948 which implied that 94.8% variations could be explained by the fitted model. The R2 value for the protein content of RLPC was 0.9961 indicating that fitted model could determine 99.61% of the total variations. R2 value for a good fit model should adequately be at least 0.80, therefore the developed models fairly represent the relationship between process parameters and responses quite adequately [36].
Effect of process variables on yield and protein content of RLPC
The effect of independent process variables on yield and protein content of RLPC is presented in Table 2 (a). The overall model for yield of RLPCs is significant (P value 0.0010). The results evidently indicate the significant effect of pH on yield of RLPC since its P value is less than 0.05 whereas sample/water ratio and time of extraction showed no significant effect as their probability values accounted to be 0.6495 and 0.6288, respectively (P > 0.05). The pH–pH, ratio–ratio and time–time interactions during extraction of RLPC also showed a significant effect on its yield with P values of 0.0001, 0.0004 and 0.0175, respectively. Surface plots (Fig. 1a–c) illustrate the interactive effects of various process parameters viz. pH, sample/water ratio and time of extraction on the yield of extracted RLPC. The surface plot for the effect of sample/water ratio and pH of extraction is shown in Fig. 1a at constant time of extraction. The results indicated that both sample/water ratio and pH of extraction showed a quadratic effect on the yield of RLPC, which was at maximum at the mid-range of both the factors (optimum conditions). It was followed by a decline in the yield with further increase in sample/water ratio and pH of extraction which might be due to denaturation and hydrolysis of protein at extreme alkaline conditions [37]. The effects of time and sample/water ratio at constant pH of extraction are illustrated in Fig. 1b where both these parameters showed quadratic effect on the response. Similarly, in Fig. 1c, time and pH of extraction at constant sample/water showed a quadratic effect on the response.
The quadratic model for protein content of RLPCs was significant (P < 0.0001). It was significantly affected by all the process variables. The pH of extraction exhibited the most significant effect on protein content of RLPC (P < 0.0001) followed by sample/water ratio (P = 0.0098) and time of extraction (P = 0.0343). The pH–pH and ratio–ratio interaction also showed statistically significant effect on protein content of RLPCs (P < 0.0001) (Table 2 a). An increase in protein content was observed with an increase in pH of extraction till a certain optimum level as shown in Fig. 1d after which it declined with further increase in the pH, thus suggesting its quadratic effect on the response. On the contrary, a linear effect of time of extraction was observed in this interaction when the sample/water ratio was kept constant. However, the interaction between sample/water ratio and pH of extraction at constant time of extraction presented in Fig. 1e showed a quadratic effect for both parameters on the protein content of extracted RLPC. The interaction between time of extraction and sample/water ratio at constant pH of extraction is presented in Fig. 1f. Here the time showed a linear effect while sample/water ratio exhibited a quadratic effect shown by a curve in the surface plot.
Optimization and verification of results
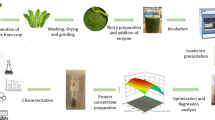
In order to determine the optimal conditions for RLPC extraction considering the responses viz. yield of RLPC and protein content simultaneously, the analysis of results using RSM technique was performed. From the model, the optimum conditions for alkaline extraction of RLPC obtained by computer-generated response surfaces are presented in Table 2(b). Optimum conditions of process parameters predicted were: 0.075 sample/water ratio, 9.46 pH of extraction solvent solution and 46.89 min of extraction time which gave protein concentrate yield of 11.84% and protein content 88.84% RLPC. The experiment was conducted under the predicted conditions to validate the results; the experimental results indicated a yield of RLPC: 12.12% with a protein content 87.66%. The results were found to be close to optimized predicted values with a variation 0.26% in yield and 1.17% in protein content of RLPC. The entire process of preparation of RLPC under these optimized conditions has been summarized in Fig. 2. Optimization of extraction of peanut proteins was performed using RSM with maximum yield of 2.7% and protein concentration of 85.28% under optimum conditions of sample water ratio: 1.8, pH: 8.0 and time: 30 min [38]. Similarly, alkaline extraction of protein from de-oiled rice bran was optimized using RSM which gave 8.63% yield of protein concentrate having protein content of 83.75% where optimum process variables for extraction were 0.175 bran/water ratio, 9.5 pH of solution and 45 min of extraction time [35].
Fractionation of RLPC and SDS-PAGE analysis
The RLPC prepared under the optimized conditions of extraction, as shown in Fig. 2, was subjected to fractionation. Four different types of protein fractions were isolated from the protein concentrates depending upon variable solubility. The alkali soluble fractions (glutelins) constituted the highest proportion (41.49%) followed by ethanol soluble prolamins (24.96%), water soluble albumins (20.43%) and salt soluble glutelins (13.00%) as shown in Fig. 3. This RLPC protein fraction composition was different from other protein isolates; for instance, amaranth, quinoa and chia protein isolates, where the highest proportion of albumins (51%) was observed in amaranth, while globulins (60.2%) and prolamins (53.8%) were reported to be maximum in quinoa and chia protein isolates, respectively [39]. SDS-PAGE analysis was performed to determine apparent molecular weight of polypeptide composition profile of proteins recovered in RLPC and protein fractions isolated from the RLPC. The electrophoretogram generated is presented in Fig. 4 where the five bands in the RLPC were observed with approximate molecular weights of 14, 35, 47, 52 and 60 kDa. These results were comparable to the apparent molecular weights of protein concentrates of sour cherry kernels which varied from 14 to 66 kDa under the same reducing and denaturing conditions [40]. Similar results were reported for alfalfa soluble leaf proteins [41] and protein isolates of the leaves of amaranth, eggplant and fluted pumpkin [42]. Electrophoretogram representing the polypeptide composition profile of different protein fractions isolated from the RLPC showed that albumins and prolamins had a similar banding pattern with their bands ranging between 14 and 55 kDa, whereas the glutelins and globulins appeared as smears of bands between 20 and 30 kDa. The reason behind improper separation of bands might be the limited solubility of these protein fractions in electrophoresis buffer or the heterogeneous nature of polypeptides in the RLPC and protein fractions [43].
Characterization of RLPC and protein fractions
Antioxidative properties, free phenols and flavonoids of RLPC
Ferric reducing antioxidant activity [FRAP] assay measures the capacity of an antioxidant in reduction of an antioxidant probe (ferric to ferrous reduction) in acidic pH, where the degree of change of color is proportional to the concentration of antioxidant [44]. The reducing power was observed to be 38.24 mg/100 g in the RLPC which means the presence of antioxidants in the samples causes the reduction of the Fe2+/ferricyanide complex to the ferrous form. Prolamins, among all the protein fractions, showed the highest FRAP activity (36.66 mg/100 g) which was at par with the RLPC while minimum activity (16.84 mg/100 g) was exhibited by albumins. The difference in their reducing power can be attributed to the difference in their polyphenolic content [45]. The RLPC exhibited a DPPH activity (11.87 mg TE/g) higher than the activity exhibited by all the isolated fractions. However, the DPPH activity exhibited by the globulins was at par with the RLPC which could possibly contain some substrates which were electron donors and could react with free radicals to convert them into more stable products and terminate the radical chain reaction [46]. Among protein fractions, the least DPPH scavenging potential (2.75 mg TE/g) was exhibited by glutelins. The reports of Delfanian et al. [47] indicate that high free radical scavenging activity was a result of high phenolic compounds in the extract; with increasing concentrations of phenolic compounds, the number of hydroxyl groups available in the reaction medium also increases, thus the possibility of hydrogen donation to free radicals increases. The differences in the radical scavenging ability found might be attributed to the difference in composition and/or nature, type and pattern of distribution of amino acids within protein molecules [45]. Glutelins showed the highest ABTS radical scavenging activity among all the protein fractions (Table 3). Table 4 shows the free phenolic and flavonoid content (mg/g) in RLPC which are in comparable range with leaf protein concentrates of Daucus carota [48].
Functional properties of RLPC
Water holding capacity
The value of WHC of the RLPC was 352% (Fig. 5a) which exceeded the values reported for protein concentrates and isolates of other plant products, for instance soy protein isolate (130%) [49], leaf protein concentrates of different varieties of cassava (118–200%) [7], apricot kernel protein concentrate (140%) [50] and sour cherry kernel protein isolate (242%) [40]. High WHC of RLPC of radish leaves might be due to the presence of a large number of polar groups in the proteins which are responsible for enhancing their hydration by interacting with water molecules [51]. WHC is an important property of proteins for their incorporation in viscous foods (e.g., soups, gravies, sauces, etc.), baked products, confectionery, etc. High value of WHC indicated that the extracted RLPC can be used as a functional ingredient in aqueous food formulations of these products.
Functional properties of extracted radish leaf protein concentrate (RLPC): a water holding capacity (WHC) and oil holding capacity (OHC), b foaming capacity (FC), foaming stability (FS), emulsifying capacity (EC), emulsion stability (ES) and least gelation concentration (LGC) and, c Solubility profile of RLPC at varying levels of pH (Error bars indicate standard error from the mean of values)
Oil holding capacity
OHC has a significant role in flavour retention of food products and it also affects the food formation processes, especially in the meat industry [52]. The OHC value of alkali extracted RLPC was 280% (Fig. 5a) which is higher than 110% in soy protein isolate [49], 254% in hyacinth bean protein isolate [53], 207% in sunflower flour [24] and 191–227% in chickpea protein concentrate [54]. Therefore, the extracted RLPC showed a high potential to be used as flavour retainers, stabilization agents and meat extenders in food products such as sausages, salad dressings, soups, etc [55].
Foaming capacity and stability
Foaming properties are important for the incorporation of gases in fortified food products like bread, cookies etc. which are related to the extent of absorption of molecules to the air-liquid interfaces [56]. The FC of the RLPC was 20.4% evaluated at pH 7.4 (Fig. 5b) which is comparable to the FC of apricot kernel protein concentrate (21%) [50] and mung bean protein isolates (26%) [57]. However, this value was much lower than the FC of cowpea protein isolates (82–93%) [58]. Similarly, the FS of the extracted RLPC (34%) as shown in Fig. 5b was lower as compared to 55% in cashew protein isolate [12], 56 % in bayberry kernel protein isolates [59] and 76.9% in mung bean protein isolate [57]. FS affects the strength of the protein film as well as its permeability for the gases [60]. The lower values of FC and FS indicated that the RLPC prepared from radish leaves are not highly suitable to be used as foaming ingredients and whipping agents in bakery products, drinks and ice-creams.
Emulsion capacity and stability
Another functional property, namely EC is the measure of the capacity of a protein to form an emulsion, whereas ES is a measure of the ability of a protein to form a stable emulsion for a critical period of time. Both of these properties are critical determinants for fat-emulsion production and stabilization. The EC (48.1%) and ES (47.8%) of the extracted RLPC (Fig. 5b) are evidently higher than 7 and 11% reported for wheat flour [24]. The emulsifying properties of cassava leaf meals (27.4% EC, 41.2% ES) and cassava leaf protein concentrates (32.5% EC, 42.9% EC) suggested the use of cassava leaf to enhance the protein quality of various flours from cereals and stabilize them [7]. Therefore, higher EC and ES of the protein concentrates extracted from radish leaves can also be used as additives for the stabilization of emulsions in food products.
Least gelation concentration
Gel formation in necessary for the matrix production that holds water, sugars, flavours and other ingredients of food products such as emulsion meat items (salami, sausage, etc). For a given protein, a critical concentration is required for the formation of the gel which is referred to as LGC. The LGC of RLPC was 9% (w/v) as shown in Fig. 5b, which is lower as compared to 12% in pigeon pea protein concentrate [51] and 14–16% in chickpea protein concentrate [54]. The lower the LGC, the better the gelation characteristics of protein isolate [40]. Hence, the RLPC demonstrated superior gelation characteristics and it might be useful as an additive in food products for gel formation.
Protein solubility
Protein solubility is an important functional property since it owes its effect on other properties like gelling, foaming and emulsification. The RLPC showed maximum solubility at pH 12 (74%) and minimum solubility at pH 2 (61%). In the solubility profile of RLPC (Fig. 5c), a linear pattern was observed where the solubility increased from pH 2 to pH 12. The protein solubility results of mung bean protein isolates [57], defatted peanut flour and peanut protein isolates [61] and black bean protein isolates [62] showed variable solubility with varying pH presenting a zig–zag profile unlike the RLPC prepared from radish leaves. The values of protein solubility of RLPC were found to be higher than the corresponding values of other protein concentrates; the maximum solubility at pH 12 suggesting their use in alkaline foods [55].
Mineral analysis of RLPC
The contents of the minerals Zn, Cu, Mn, Cr, Fe, Ca, K, Mg and P were estimated (mg/100 g) in RLPC where calcium was found to be the most abundant mineral (623.22 mg/100 g), while chromium was the least abundant with a value of 2.56 mg/100 g. The calcium levels in RLPC can easily meet the value of Ca required as per the recommended daily allowance for both children and adults [63]. The calcium content in RLPC was much higher than LPCs of common leafy vegetables like Solanum sp., amaranth, fluted pumpkins, bitter gourd leaf (68.8, 65.4, 52.1 and 111.3 mg/100 g, respectively) as reported by [55]. Therefore, RLPC can be used as effective source of calcium. Likewise, the potassium levels are also considerably high in RLPC (336.82 mg/100 g), which is at par with 333.63 mg/100 g potassium reported in moringa leaves [64]. Magnesium and phosphorus contents were also in appreciable amounts in the RLPC. The values of Mg content in RLPC were found to be higher than 20.8 mg/100 g in LPCs of Centrosema pubescens (butterfly pea) [65]. The P levels of RLPC were comparable to that observed in the protein concentrates of moringa leaves [64]. The RLPC is a very rich source of Fe (175.21 mg/100 g) which is a very important element for hemoglobin formation in the blood. These values far exceed the Fe content in leaf protein concentrates of Glyricidia sepium (92.22 mg/100 g) and Mucuna puriens (71.3 mg/100 g) [66]. Mn content in RLPC was 19.76 mg/100 g which is lower than 52.1 mg/100 g present in moringa leaves [64]. Copper, on the other hand is present in appreciable amounts in the RLPC (25.74 g/100 g). Cu present in Albizia lebbek leaves was in comparable amounts as that present in the RLPC [67].
Color measurement of RLPC
In Hunter scale, L* measures lightness and varies from 100 for perfect white to zero for black, whereas a* measures redness when positive, gray when zero and greenness when negative. On the other hand, b* measures yellowness when positive, gray when zero and blueness when negative [30]. From Table 4, it can be observed that the value of L* for RLPC is 49.70, a* is − 5.8 indicating its greenness and b*value is positive (11.70) showing its slight yellowness. The color values of the RLPC were compared to a commercial soy protein concentrate. Toews et al. [68] indicated that the L*, a* and b* values of the soy protein concentrate were 88.40, 1.10, and 14.56. The L* value of the RLPC was lower than the soy protein concentrate. In other words, the RLPC was relatively darker than the commercial soy protein concentrate. The reason for this is thought to be caused by the chlorophyll degradation during protein extraction from the radish leaves [69]. Furthermore, the RLPC showed low redness and yellowness compared to the soy protein concentrates, which are encouraging in the food industry [36]. The color values (L*, a* and b*) may vary according to the color pigments of the raw material [68], the particle size of the powder product [70], the pH value of the extraction medium [71], and the protein isolation method [68].
In-vitro disgestibity of RLPC
While all the methods employed till now determine the quality of the RLPC, the protein digestibility shows the utilization potential of the protein concentrate [72]. According to the results (Table 4), it can be extrapolated that the RLPC underwent a virtually high protein digestibility (93.51%) compared to 64.7% reported as the digestibility of Moringa Oleifera leaf isolate [73]. The digestibility of RLPC was also higher than that of commercial protein concentrate supplements investigated by Corgneau et al. [74]. High protein digestibility value has been attributed to the ease of access of the protease to the peptide bonds aided by the lower amount of non-protein materials. The protein extraction and concentration through the optimized chemical process may have affected the native structure of solubilized proteins and made them more accessible to the digestive enzymes, thereby facilitating the overall digestibility of the RLPC [73].
Identification of amino acids and phenolic compounds in RLPC
LC–MS/MS with electrospray ionization and chemical ionization enables the sensitive and simultaneous detection and identification of a large number of (even co-eluting) compounds from a single chromatogram and is therefore the method of choice when libraries are available. MS also enables reductions in the process of sample preparation from extracts [75]. Various amino acids were tentatively identified from the spectrum where the peaks depicting the m/z ratio were processed as raw data to calculate molecular formulae on the basis of the monoisotopic mass of the ions. With high-resolution MS, the relative abundances along with the m/z signals were also measured in isotropic pattern [76]. Among all the amino acids identified as shown in Fig 6a tryptophan, threonine and phenylalanine were found to be the most abundant. Similarly, the presence of phenolic acids (caffeic acid, cinnamic acid, chlorogenic acid, syringic acid, trans-ferullic acid, quinic acid, etc.) and flavonoids (quercetin, kaempferol, luteolin, hesperetin) in RLPC is depicted in Fig 6b. Caffeic acid and quercetin were present more abundantly relative to the other compounds.
Microbiological analysis of RLPC during storage
The RLPC was analyzed for its microbial contamination from day 0 till 6 weeks to determine its storage stability (Table 5). Under ambient conditions the yeast/mould count increased from 2.28 log CFU/g at day 7 to 3.03 log CFU/g at day 42. However, under refrigerated conditions, no growth was detected up to 14 days of storage. The yeast/mould count under refrigerated conditions was 2.11 log CFU/g at day 21 which increased to 2.68 log CFU/g at day 42. The yeast/mould counts of the RLPC were in acceptable limits during 42 days of storage under both refrigerated and ambient conditions. The total plate count for the RLPC was not detected till 21 days and 35 days of storage under ambient and refrigerated conditions, respectively. However, the total plate count was 4.06 log CFU/g at day 28 which increased to 4.39 log CFU/g at day 42 under ambient storage conditions. At day 42, the plate count was 4.13 log CFU/g under refrigerated conditions which was again under acceptable limits in accordance with the FSSAI specifications [77] for dehydrated vegetable products. Kaur et al. [34] reported similar results during the storage of protein concentrates extracted by heat coagulation from radish leaves under both refrigerated and ambient conditions. Sasikumar et al. [78] studied the microbial count for the spray dried samples of blood fruit seed protein isolate extracted using KOH, which was less than 5 log CFU/g under similar storage conditions.
Conclusion
The extraction conditions for the preparation of RLPC were optimized by using the 3-factor, 3-level Box–Behnken Design of response surface methodology, and the optimized conditions were as follow: pH 9.46, sample/water ratio of 0.075 and time of extraction 46.89 min, which gave a protein yield of 12.12% and protein content of 87.64%. The pH of extraction had a significant effect on the yield of RLPC, whereas all the three process parameters (pH, sample/water ratio and time of extraction) had a significant effect on the protein content of the concentrates. Glutelins constituted the major proportion of the extracted RLPC followed by prolamins and albumins. Prolamins, among all the fractions, showed the highest FRAP activity, globulins showed the maximum DPPH activity, whereas glutelins showed the maximum ABTS radical scavenging activity. The microbial contamination of the stored RLPC was in acceptable range for a considerable time period and thus they are deemed safe for consumption. Moreover, the presence of several essential amino acids and phenolic compounds was detected. However, a quantitative analysis of identified phytocompounds needs to confirm the nutritional value of the RLPC. Overall, the present results confirm that the protein concentrate extracted from the underestimated radish plant leaves can be an alternative source of protein because of its high protein content, an appropriate yield, desirable functional properties, considerable mineral content, antioxidant activity, in-vitro digestibility and microbial stability.
References
A. Rani, Y. Arfat, R.S. Aziz, L. Ali, H. Ahmed, S. Asim, M. Rashid, C.H. Hocart, Environ. Technol. Innov. 23, 1–13 (2021). https://doi.org/10.1016/j.eti.2021.101620
Ankita, K. Prasad, Pharm. Lett. 7, 269–279 (2015)
K.V. Badar, A.U. Kulkarni, Curr. Bot. 2, 5–7 (2011)
A.E. Ghaly, F.N. Alkoaik, Am. J. Appl. Sci. (2010). https://doi.org/10.3844/ajassp.2010.331.342
S. Jiamyangyuen, V. Srijesdaruk, W.J. Harper, Extension 27, 56 (2005)
S. Tang, N.S. Hettiararchy, S. Eswaranandam, P. Crandall, Food Sci. (2003). https://doi.org/10.1111/j.1365-2621.2003.tb05696.x
A.O. Fasuyi, V.A. Aletor, Pak. J. Nutr. (2005). https://doi.org/10.3923/pjn.2005.43.49
P.F. Coldebella, S.D. Gomes, J.A. Evarini, M.P. Cereda, S.R. Coelho, A. Coldebella, Eng. Agric. 33, 1223–1233 (2013)
M.H. Soo, N.A. Samad, D.N.A. Zaidel, Y.M.M. Jusoh, I.I. Muhamad, Z. Hashim, Chem. Eng. Trans. (2021). https://doi.org/10.3303/CET2189043
S. Rawdkuen, Food Appl. Biosci. J. 8, 43–67 (2020)
M. Contreras, A. Lama-Muñoz, J.G.P. Manuel, F. Espínola, M. Moya, E. Castro, Bioresour Technol. (2019). https://doi.org/10.1016/j.biortech.2019.02.040
C. Zhang, J.P.M. Sanders, T.T. Xiao, M.E. Burns, PLoS ONE (2015). https://doi.org/10.1371/journal.pone.0133046
S.O. Ogunwolu, F.O. Henshaw, H.P. Mock, A. Santros, S.O. Awonorin, Food Chem. (2009). https://doi.org/10.1016/j.foodchem.2009.01.011
A.A. Wani, D.S. Sogi, L. Grover, D.C. Saxena, Biosyst. Eng. (2006). https://doi.org/10.1016/j.biosystemseng.2006.02.004
AOAC, Official Methods of Analysis, 17th edn. (Association of Official Analytical Chemists, Washington, DC, 2000)
A.P. Adebiyi, R.E. Aluko, Food Chem. (2011). https://doi.org/10.1016/j.foodchem.2011.03.116
O.H. Lowry, N.J. Rosebrough, A.L. Farr, R.J. Randall, J. Biol. Chem. 193, 265 (1951)
U.K. Laemmli, Nature 227, 680–685 (1970)
C.W. Lin, C.W. Yu, K.H. Yih, J. Food Drug Anal. 5, 386–395 (2009)
R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, C. Rice-Evans, Free Radic. Biol. Med. (1999). https://doi.org/10.1016/s0891-5849(98)00315-3
I.F. Benzie, J.J. Strain, Anal. Biochem. (1996). https://doi.org/10.1006/abio.1996.0292
W.E. Hillis, T. Swain, J. Sci. Food Agric. 10, 135–144 (1969). https://doi.org/10.1002/jsfa.2740100211
I.S. Balbaa, A.Y. Zaki, A.M. El Shamy, J. Assoc. Off. Anal. Chem. 57, 752–755 (1974). https://doi.org/10.1093/jaoac/57.3.752
J. Yu, M. Ahmedna, I. Goktepe, Food Chem. (2007). https://doi.org/10.1016/j.foodchem.2006.08.012
M.J.Y. Lin, E.S. Humbert, F.W. Sosulski, J. Food Sci. (1974). https://doi.org/10.1111/j.1365-2621.1974.tb02896.x
K. Yasumatsu, K. Sawada, S. Moritaka, M. Misaki, J. Toda, T. Wada, K. Ishii, Agric. Biol. Chem. (1972). https://doi.org/10.1080/00021369.1972.108603212
C.W. Coffman, V.V. Garcia, J. Food Tech. 12, 473–484 (1977)
N. Huda, A. Abdullah, A.S. Babji, Int. J. Food Sci. Technol. (2001). https://doi.org/10.1046/j.1365-2621.2001.00473.x
J.F. Pedler, D.R. Parker, D.E. Crowley, Planta (2000). https://doi.org/10.1007/s004250000270
D. Znidarcic, T. Pozrl, Acta. Agric. Slov. 87, 235–243 (2006)
M. Minekus, M. Alminger, P. Alvito, S. Balance, Brodkorb A. Food Funct. (2014). https://doi.org/10.1039/C3FO60702J
V. Nour, I. Trandafir, S. Cosmulescu, J. Chrom. Sci. 51, 883–890 (2013). https://doi.org/10.1093/chromsci/bms180
R. Aslam, M.S. Alam, S. Singh, S. Kumar, LWT 151, 112183 (2021). https://doi.org/10.1016/j.lwt.2021.112183
G. Kaur, S. Bhatia, Int. J. Agric. Sci. (2021). https://doi.org/10.15740/HAS/IJAS/17.2/185-193
G.S. Mann, S. Bhatia, M.S. Alam, Agric. Eng. Int. CIGRJ. 4, 243–251 (2016)
A. Akyuz, S. Ersus, Food Chem. 335, 127673 (2021). https://doi.org/10.1016/j.foodchem.2020.127673
M. Wang, N.S. Hettiarachchy, M. Qi, W. Burks, T. Siebenmorgen, J Agric. Food Chem. (1999). https://doi.org/10.1021/jf9806964
I.S. Rustom, M.H. Lopex-Leiva, B.M. Nair, Food Sci. (1991). https://doi.org/10.1016/0308-8146(91)90031-I
D.N. López, M. Galante, M. Robson, V. Boeris, D. Spelzini, Int. J. Biol. Macromol. (2018). https://doi.org/10.1016/j.ijbiomac.2017.12.080
M. Çelik, M. Güzel, M. Yildirim, J. Food Sci. Technol. (2019). https://doi.org/10.1007/s13197-019-03785-8
B.P. Lamsal, R.G. Koegel, S. Gunasekaran, LWT Food Sci. Technol. (2007). https://doi.org/10.1016/j.lwt.2006.11.010
A.A. Famuwagun, A.M. Alashi, S.O. Gbadamosi, K.A. Taiwo, D.J. Oyedele, O.C. Adebooye, R.E. Aluko, Int. J. Food Prop. (2020). https://doi.org/10.1080/10942912.2020.1772285
A.P. Adebiyi, A.O. Adebiyi, Y. Hasegawa, T. Ogawa, K. Muramoto, Eur. Food Res. Technol. (2009). https://doi.org/10.1007/s00217-008-0945-4
J. Dai, R.J. Mumper, Molecules (2010). https://doi.org/10.3390/molecules15107313
O. Kadiri, C.T. Akanbi, B.T. Olawoye, S.O. Gbadamosi, Int. J. Food Prop. (2017). https://doi.org/10.1080/10942912.2016.1230874
Z. Xie, J. Huang, X. Xu, Z. Jin, Food Chem. (2008). https://doi.org/10.1016/j.foodchem.2008.03.078
M. Delfanian, S.M. Razavi, M.H.H. Khodaparast, R.E. Kenari, S. Golmohammadzadeh, Food Res. Int. (2018). https://doi.org/10.1016/j.foodres.2018.03.043
A. Sodamade, S.M. Raimi, A.D. Owonikoko, A.T. Adebimpe, IJTSRD. 36, 57–68 (2019)
A. Fernández-Quintela, M.T. Macarulla, A.S. Del Barrio, J.A. Martínez, Plant Food Hum. Nutr. 51, 331–341 (1997)
P.C. Sharma, B.M.K.S. Tilakratne, A. Gupta, J. Food Sci. Technol. (2010). https://doi.org/10.1007/s13197-010-0096-z
S.K. Sathe, S.S. Deshpande, D.K. Salunkhe, J. Food Sci. (1982). https://doi.org/10.1111/j.1365-2621.1982.tb10110.x
S. Damodaran, Food Sci. Technol. 1, 1–24 (1997)
A. Subagio, Food Chem. (2006). https://doi.org/10.1016/j.foodchem.2004.12.042
A.M. Ghribi, I.M. Gafsi, C. Blecker, S. Danthine, H. Attia, S. Besbes, J. Food Eng. (2015). https://doi.org/10.1016/j.jfoodeng.2015.06.021
O. Aletor, A. Oshodi, K. Ipinmoroti, Food Chem. (2002). https://doi.org/10.1016/S0308-8146(01)00376-4
R. Chatterjee, T.K. Dey, M. Ghosh, P. Dhar, Food Bioprod. Process 94, 70–81 (2015)
M. Du, J. Xie, B. Gong, X. Xu, W. Tang, X. Li, C. Li, M. Xie, Food Hydrocoll. (2018). https://doi.org/10.1016/j.foodhyd.2017.01.003
K. Shevkani, N. Singh, A. Kaur, J.C. Rana, Food Hydrocoll. 43, 679–689 (2015)
J. Cheng, S. Zhou, D. Wu, J. Chen, D. Liu, X. Ye, Food Chem. (2009). https://doi.org/10.1016/j.foodchem.2008.05.106
F. Garcia-Moreno, E. Solórzano, J. Banhart, Soft Matter 7, 9216–9223 (2011)
H. Wu, Q. Wang, T. Ma, J. Ren, Food Res. Int. (2009). https://doi.org/10.1016/j.foodres.2008.12.006
T.G. Kudre, S. Benjakul, H. Kishimura, J. Sci. Food Agric. (2013). https://doi.org/10.1002/jsfa.6052
NRC, National Research Council (1989) https://doi.org/10.17226/1349
S.A. El Sohaimy, G.M. Hamad, S.E. Mohamed, M.H. Amar, R.R. Al-Hindi, Global Adv. Res. J. Agric. Sci. 4, 188–199 (2015)
J.O. Agbede, J. Sci. Food Agr. (2006). https://doi.org/10.1002/jsfa.2491
J.K. Mensah, R.I. Okoli, J.O. Ohaju-Obodo, K. Eifediyi, Afr. J. Biotechnol. 7, 14 (2008)
L.H. Khan, V.K. Varshney, J. Diet Suppl. (2018). https://doi.org/10.1080/19390211.2017.1349232
R. Toews, N. Wang, Food Res. Int. (2013). https://doi.org/10.1016/j.foodres.2012.12.009
G. Pumilia, M.J. Cichon, J.L. Cooperstone, G. Dugo, S.J. Schwartz, Food Res. Int. (2014). https://doi.org/10.1016/j.foodres.2014.05.047
J.Y. Han, K. Khan, Cereal Chem. 67, 384–390 (1990)
Y.A. Adebowale, I.A. Adeyemi, A.A. Oshodi, K. Niranjan, Food Chem. (2007). https://doi.org/10.1016/j.foodchem.2006.11.05
T.A. Aderinola, A.M. Alashi, I.D. Nwachukwu, T.N. Fagbemi, T.N. Enujiugha, R.E. Aluko, Food Hydrocoll. (2020). https://doi.org/10.1016/j.foodhyd.2019.105574
T. Benhammouche, A. Melo, Z. Martins, M.A. Faria, S.C. Pinho, I.M. Ferreira, F. Zaidi, Food Chem. (2021). https://doi.org/10.1016/j.foodchem.2020.128858
M. Corgneau, C. Gaiani, J. Petit, Y. Nikolova, S. Banon, L. Riti’e-Pertusa, D.T.L. Le, J. Scher, Int. J. Dood Sci. Technol. (2019). https://doi.org/10.1111/ijfs.14170
P. Goufo, I. Cortez, Biology (2020). https://doi.org/10.3390/biology9090268
R. Flamini, Mass Spec. Rev. 22, 218–250 (2008). https://doi.org/10.1002/mas.10052
FSSAI Specification (2018) for dehydrated vegetable products. https://archive.fssai.gov.in
R. Sasikumar, K. Vivek, A.K. Jaiswal, J. Food Process. Preserv. (2021). https://doi.org/10.1111/jfpp.15568
Acknowledgements
Authors are grateful to Dr Tarsem Singh Dhillon, Associate Director (Seeds), Punjab Agricultural University, Ludhiana for providing radish leaves and Department of Processing and Food Engineering, Punjab Agricultural University, Ludhiana for providing facilities for carrying out the research experiments. The authors are also thankful to Dr Amrit Kaur Mahal (Professor of Statistics) for her guidance in statistical analysis during research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, G., Bhatia, S. Radish leaf protein concentrates: optimization of alkaline extraction for production and characterization of an alternative plant protein concentrate. Food Measure 16, 3166–3181 (2022). https://doi.org/10.1007/s11694-022-01411-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01411-4