Abstract
Kernel of sour cherry (Prunus cerasus L.) is a valuable source of protein generated as byproduct during processing of sour cherries. In this study, optimisation of protein extraction yield from sour cherry kernel was investigated using response surface methodology. Optimum conditions for alkaline solubilisation/isoelectric precipitation extraction were identified as pH 8.5, 1:10 solid-to-solvent ratio, and 1 h extraction time to obtain a protein yield of 63.8% for a protein content of 88.3%. The isoelectric precipitation point of sour cherry kernel protein isolate (SCKPI) was shown to be 4.2 and a high solubility of 85.3% was found at neutral pH. Results showed that SCKPI had a substantially high in vitro protein digestibility (95.7%). Other physicochemical properties such as water- and oil-holding capacities, gelling capacity, emulsion stability, foaming capacity as well as thermal properties were also reported and ensured comparable functionalities indicating a great potential as a valuable plant-based protein source for the food industry.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of Novelty
The utilization of sour cherry kernel (food industry waste) was done by extraction of valuable proteins with promising functional properties as an alternative to commercial plant-based protein sources.
Introduction
Turkey is among the top global sour cherry (Prunus cerasus L.) producers with an annual production of 184,167 tonnes after Russia (232,200 tonnes) and Poland (200,632 tonnes) in 2018 [1]. Sour cherries are exclusively processed into various forms including juice, concentrate, jam, jelly, marmalade, canned or frozen products [2, 3]. Almost forty percent of the total sour cherry crop in Turkey is processed into juice due to consumer preferences [4]. During juice processing, significant amounts of fruit kernels are generated without further use or with very limited utility [5]. According to Bak et al. (2010), sour cherry kernel can be considered as an emerging source of functional food due to its bioactive constituents. No evidence of toxicity has been reported by consuming sour cherry seed in animal subjects [6] and human subjects [7]. Furthermore, cardioprotective, anti-inflammatory, anti-diabetic, antioxidant, dermal photoprotection and therapeutic effects of sour cherry seed kernel have been studied [8, 9]. Sour cherry kernels are rich in carbohydrates, proteins and lipids with their corresponding concentrations of 46.6%, 29.3%, and 17.0%, respectively. Regarding its amino acid composition, sour cherry kernel contains glutamic acid at most, and lysine, a limiting essential amino acid in most cereals, is present in relatively high quantity in sour cherry kernel [10]. Various research groups have investigated utilization of sour cherry kernel as a source of oil [5, 10,11,12,13].
As a result of the diminishing food protein supplies worldwide, there has been an increasing interest for utilization plant proteins as new and low-cost protein sources in food applications [14]. Current trends is pushing industry to find plant-based alternatives to animal-derived ingredients based on consumer perceived fears (e.g., prion disease), religious inhibitions, and dietary and moral preferences associated with consuming animal byproducts [15]. Several protein rich products such as flour, concentrates, or isolates, can be incorporated into food products to improve their functional and/ or nutritional properties [16]. Tuna (2015) indicated that 5% addition of sour cherry kernel flour (SCKF) did not diminish sensory quality of cake formulations [17]. However, protein isolates is of interest rather than the flour of seeds, since they have superior functional properties and are free of indigestible carbohydrates [18]. To the best our knowledge, there is solely one paper reported the impact of pH on protein extraction from defatted sour cherry kernel flour to obtain protein concentrate [19]. Thus, the potential of sour cherry kernel should be investigated in detail in terms of the effects of different extraction parameters for production of protein isolate. In recent studies, soy protein isolate (SPI) has been used as a reference for validation of experimental protein extraction from plant sources due to food industry preferences [20]. The aim of this work is the optimization of conventional alkaline protein extraction from sour cherry kernel in terms of yield and protein functionality and to determine in vitro protein digestibility for the first time. Response surface methodology (RSM) was applied to determine the optimum preparation conditions of extraction pH, liquid-to-solid ratio, and time to obtain sour cherry kernel protein isolates (SCKPI) and physicochemical properties of the SCKPI against the commercially available soybean protein isolate were assessed.
Materials and Methods
SCKF Preparation
Pitted sour cherry kernels (seed part) were supplied from a local fruit byproducts processing company, Hedef Un A.S.(Izmir, Turkey). A commercial SPI (87.8% protein, db, Pingdingshan Tianjing Plant Albumen Co. Ltd., Henan, China) was used as a reference for comparison of functional properties. Double distilled water was used as solvent. All chemicals were reagent-grade and purchased from Sigma–Aldrich Co. LLC (Missouri, United States) unless otherwise mentioned. Pitted sour cherry kernels were ground into flour with liquid nitrogen using IKA T10 basic disperser (IKA Werke, Staufen, Germany) and stored in freezer at − 80 °C until use to avoid lipid oxidation.
Proximate Analysis
Moisture content (AOAC Method 925.09, 1990), oil content using Soxhlet method (AOAC Method 960.39, 1990), total protein content using Kjeldahl method (AOAC Method 950.48, 1990), and ash content (AOAC Method 923.03, 1990) of SCKF were determined. Dietary fiber analysis (Method 32–07.01) was performed according to AACC method [21]. Carbohydrate content was calculated by subtracting total percentage of other components.
Defatting
SCKF samples were defatted prior to extraction according to previously published method with minor modifications [22]. Samples were mixed with hexane (1:3, w/v) for 40 min using magnetic stir plate at 500 rpm followed by hexane decanting. After repeating 3 times, the mixture was filtered through Whatman #1 filter paper (Whatman International Ltd., Maidstone, United Kingdom), and dried under fume hood at room temperature for 16 h. The defatted flour (d-SCKF) was stored at 4 °C until further analysis.
Surface Charge and Isoelectric Point Determination
The overall surface charge of d-SCKF was determined by measuring the electrophoretic mobility (UE) of d-SCKF dispersions (0.01%, w/w) at pH 2–11 (titrations performed with 0.1 mol/L HCl or NaOH at 22 oC) using Zetasizer NanoS (Stabino™ Zeta Potential Nano-Flex™ Particle Size Distribution Instrument, Meerbusch /Germany). UE was used to calculate the zeta potential (ζ) by applying Henry's Eq. (1):
where ε is the permittivity, f (κα) is a function related to the ratio of particle radius (α) and the Debye length (κ), and η is the dispersion viscosity. For this study, the Smoluchowski approximation f (κα) equalled 1.5.
Protein Extraction
SCKPI was prepared via alkaline solubilisation/isoelectric precipitation extraction [23]. Briefly, d-SCKF was suspended in distilled water which were adjusted to desired pH (8.0–11.0) with 0.1 mol/L NaOH and incubated at designated time and solid/solvent ratio (1/30 to 1/10, w/v) with constant agitation at 250 rpm at room temperature. pH of extracts were adjusted to 4.2 (pI of SCKPI by "Surface Charge and Isoelectric Point Determination" section) with 0.1 mol/L HCl to precipitate proteins. The precipitate was recovered by centrifugation at 3000 × g at 5 °C for 20 min followed by removal of the supernatant via decantation. Protein curd was washed twice with distilled water and centrifuged at 3000 × g for 15 min. Then washed precipitate was freeze-dried as SCKPI, and stored in plastic bags at 4 °C until further analysis.
Experimental Design and Statistical Analysis
The experimental design was constructed by a Design-Expert® 8.0.5 software package (Stat Ease, Inc., Minneapolis, MN, USA, 2010), by Box-Behnken design to determine a set of experimental conditions for the highest protein yield. Seventeen experimental runs were performed consisting of twelve factorial points and 5 replications at the center point. Three factors were selected for optimisation, extraction time (X1, 1-3 h), pH (X2, 8 to 11), and solid/solvent ratio (X3, 30 to 10 g/100 mL), as these parameters affect protein extraction significantly [20, 23, 24]. Although extraction temperature had significant impact on the protein yield in cumin seed, temperatures the changes of protein yield were not significant after 30 °C and were reduced remarkably at 60 °C probably due to protein denaturation. Hence, protein extraction from kernels was performed at room temperature. These independent variables were coded and are represented in Table 1. Mean of triplicate measurements of each run was reported as experimental responses. Experimental runs were randomized to minimize unexpected variability effects in responses. The variables were coded according to the Eq. 2.
where x is the coded, Xi was the corresponding actual, Xo was the actual value in the center of the domain, and ΔX is the increment of Xi corresponding to variation of 1 unit of x. Runs at the central point of design were applied to estimate possible pure error. Protein yield was used as the response variable corresponding to combination of independent variables.
Protein content of isolates was determined using Kjeldahl method. Protein yield was calculated as follows (3):
where WPI and WF are defined as weight of extracted SCKPI, and d-SCKF, respectively.
The responses in terms of protein content yield (Y) was analyzed by RSM to fit the following statistical model (4):
where b0 is the value of the fixed response at the central point of the experiment, which is the point (0,0,0); bn; bnn and bnm, bnnm, bnmm are the linear, quadratic, cross product coefficients, and Xn, Xm are the input variables, respectively.
Verification was achieved by conducting experiments under the selected optimum condition. Experimental and predicted responses were compared. The fitted polynomial equation was expressed as surface and contour plots. SCKPI extracted according to the optimized condition was used to evaluate its physicochemical and functional properties.
Determination of Thermal Properties
Thermal properties of SCKPI and d-SCKF dispersions were evaluated by a differential scanning calorimeter (DSC Q10, New Castle, USA) according to Tan et al. (2014) with minor modifications [24]. Briefly, 0.5 g of SCKPI (extracted under the optimum conditions) was dissolved in 0.5 mL of distilled water and stirred for 20 min to obtain 1:1 slurry (w/w) and equilibrated in refrigerator at 5 ± 1 °C for 16 h. Fifteen mg of the slurry was placed in aluminium pan using microliter pipette. After the pan was hermetically sealed, samples were submitted to heating over a range of temperatures from 27 °C to 157 °C at 5 °C/min with flow rate of 50 mL/min nitrogen. Measurements were performed in triplicate. An empty sealed hermetic pan was used as reference.
Determination of Physicochemical and Functional Properties
Determination of Bulk Density and Color Characteristics
Bulk density and color measurements were performed, as described [25]. Protein samples were placed into a 10 ml graduated cylinder by constant tapping until there was no further change in volume. Contents were weighed and bulk density of samples was calculated.
Color parameters (Hunter L, a, and b values) were measured for d-SCKF, SCKPI, and SPI using a colorimeter Minolta Chroma Meter-400 (Ramsey, New Jersey, USA). A white tile was used L = 96.82, a = + 0.02 and b = + 2.08 as a reference.
Determination of Solubility
The pH-dependent solubility profile was determined according to Chee, Ling, and Ayob (2012) with some modifications [26]. Briefly, 100 mg of sample was dispersed in 10 mL of distilled water. pH of the dispersion was adjusted to pH 2–10 using either 1 M HCl or 1 M NaOH. Protein contents in solution before centrifugation, and in supernatants after centrifugation at 10.000 × g for 30 min, were determined by Bradford method with respect to Eq. (5):
Determination of Water and Oil Absorption Capacity
1 g of either SCKPI or SPI was vortex mixed with 10 mL distilled water for water absorption capacity (WAC) or 10 mL of refined vegetable oil (olive oil, density of 0.89 g/mL) for the determination of oil absorption capacity (OAC) during 30 s. Sample was incubated at room temperaturefor 30 min. The mixture was then centrifuged at 4000 × g and 25 °C for 20 min, and the weight of the supernatant was determined [27]. The weight (g) of water or oil absorbed per gram of isolate was reported.
Determination of Emulsifying Properties
Emulsifying activity (EA) and emulsion stability (ES) were determined based on the procedure of literature [24]. Briefly, 10 mL of 1% (w/v) protein isolate suspensions were prepared in distilled water prior to homogenization (Ultra Turrax T25, Staufen, Germany) at speed 4 which corresponds to 12,000 rpm, for 2 min at room temperature. Approximately 10 mL of vegetable oil was added to slurries in falcon tubes of 50 ml, and homogenized for 2 min. Emulsions were centrifuged at 1200 × g for 5 min. The emulsifying activity was determined as in Eq. (6):
For the emulsion stability analysis, the emulsion was re-centrifugated at 1200 × g for 5 min after heating the tubes in water bath at 80 °C for 30 min, and then ES was calculated as in Eq. (7):
Determination of Foaming Properties
A slightly modified method was used to measure foaming capacity (FC) and stability (FS) [23]. Approximately 20 mL of 1% (w/v) protein solution was mixed for 2 min with a homogenizer at 12,000 rpm. Samples were transferred into 250 mL graduated glass cylinders (inner diameter = 37 mm; height distance between adjacent units = 2 mm; as measured by a digital calliper). The foaming capacity was calculated using the Eq. (8).
For the determination of FS, foam volume changes in the graduated cylinder were recorded after standing 10, 30, and 60 min at room temperature, according to Eq. 9:
Determination of Gelation Properties
Gelation properties of samples were determined by employing standard methods [28]. Suspensions with a protein fraction of 2–20% were prepared with an interval of 2 in distilled water. Samples were heated for 1 h in boiling water followed by rapid cooling in an ice bath for 10 min, and then cooled further at 4 °C for 2 h. The least gelation concentration (LGC), which is the concentration above which the sample did not fall down or slip when the test tube was inverted, was determined as g/100 g protein basis.
Determination of in vitro Protein Digestibility
In vitro protein digestibility in d-SCKF and SCKPI were determined based on a published method [29]. Briefly, simulated saliva solution was prepared by dissolving 2.38 g Na2HPO4, 0.19 g KH2PO4, and 8 g NaCl, in 1 L of distilled water. Solution pH was adjusted to 6.75, and α-amylase (EC. 3.2.1.1) was added to obtain 200 U/mL of enzyme activity. For gastric digestion, 300 U/mL of pepsin (from porcine stomach mucosa, pepsin A, EC. 3.4.23.1, Merck KGaA, Darmstadt, Germany) solution was prepared in 0.03 mol/L NaCl at pH 1.2. Further, simulated intestinal juice was prepared by dissolving 0.05 g of pancreatin (from porcine pancreas, activity equivalent 4 USP, EC. 232.468.9) and 0.3 g of bile extract (from porcine, EC. 232.369.0) in 35 mL 0.1 mol/L NaHCO3. d-SCKF and SCKPI were subjected to simulated gastrointestinal digestion as follows: 1 g of each sample was homogenized in a Stomacher laboratory blender for 1 min to simulate mastication in presence of 15 mL of simulated salivary fluid; and subsequently shaken for 10 min at 37 °C. The samples were adjusted to pH 1.2 using 5 mol/L HCl, then 15 mL of simulated gastric fluid was added. The samples were shaken for 60 min at 37 °C. After digestion with the gastric fluid, pH of the samples were adjusted to 6.0 with 0.1 mol/L of NaHCO3 and then 15 mL of a mixture of bile extract and pancreatin was added. pH of the mixture was adjusted to pH 7.0 with 1 mol/L NaOH and finally 5 mL of 0.12 mol/L NaCl and 5 mL of 0.12 mol/L KCl were added to each sample. Once prepared, the samples were submitted to in vitro digestion for 120 min at 37 °C in dark. After centrifugation, at 4000 × g and 5 °C for 20 min, supernatants were collected.
Protein content from supernatants was determined with Bradford method using BSA (bovine serum albumin, EC. 232–936-2) as standard protein. The in vitro protein digestibility was evaluated on the basis of total soluble protein content and the protein content determined after in vitro digestion with respect to Eq. (10):
where PD (%) represents in vitro protein digestibility; Pt, total protein content; Pr, protein content after in vitro simulated gastrointestinal digestion.
Results and Discussion
Proximate Composition
The protein content of ground d-SCKF was determined as 43.58% prior to experiments by Kjeldahl method (Table 2). The kernels were found to be rich in lipids, proteins, and dietary fiber with concentrations of 39.4%, 35.5%, and 20.3%, respectively (Table 2). The lipid composition was nearly consistent previous findings [6]. In the study of Yılmaz and Gökmen (2013) an oil content of 17.0% was described for sour cherry seed kernels whereas significantly different oil contents were reported for SCKF as 27–36% [17] and 34.8% [19]. For the protein content of SCKF, there has been a wide range of findings previously reported in literature (29.3–45.0%) covering the protein composition of this study (35.5%), which may be explained by varietal, seasonal, or geographical differences.
Thermal Properties
DSC profile of dispersions containing SCKPI (extracted under optimum conditions) at 1:1 (w/w) proportion with distilled water is displayed in Fig. 1. Heat flow was demonstrated as function of sample temperature with an endothermic response oriented upwards in the plot. Denaturation temperatures show protein thermo-stability, while ΔH is an indication of hydrophobic/hydrophilic interactions and compactness of proteins [30]. The protein denaturation transition of SCKPI (88.3% protein) was observed as doublet, an endothermic peak at 83.2 °C with an onset temperature of 82.9 °C and a conclusion temperature of 90.7 °C, a second endothermic peak at 109.4 °C with an onset temperature of 99.4 °C and a conclusion temperature of 122.5 °C, as shown in Table 3. A DSC thermogram of d-SCKF (43.6% protein) exhibited a wider peak (data not shown) at the interval of first transition temperature starting at 61.1 °C possibly due to starch gelatinization. Although starch constituent of SCKF has not been reported before; low amounts of starch present in nut and kernel flours can undergo gelatinization upon heating and affect the thermal properties [31]. The high denaturation temperatures of proteins indicate high thermal stability [32]. Some authors indicated single denaturation point for SPI at 92.5 °C under similar experimental conditions [24], which demonstrated higher thermal stability compared to first transition temperature of SCKPI. On the other hand, researchers indicating doublet denaturation temperatures for SPI (75.0 °C and 94.8 °C) have implied that the number of peaks can stem from varietal differences [20].
Isoelectric Point
The isoelectric point (pI) of d-SCKF, the point at which surface net charge is zero, was found to be at around pH 4.2. When pH was deviated from the pI, the net charge on protein chains were increased. Similar net charges observed between different starting pH points, as the pH was gradually increased from pH 2.5 to 10 and vice versa (Fig. 2a). The pI of the sour cherry kernel proteins was assumed to be pH 4.5 due to the formation of a firmer precipitate than pH 4.0 in which yield of protein extraction was the minimum in a previous study [19]. The experimental pI of SCKPI is consistent with the pI of plant based proteins in literature [20].
Extraction Conditions
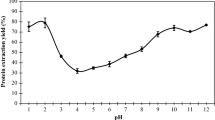
SCKF was extracted for its protein following 17 combinations of three independent variables (pH, extraction time, solid/solvent ratio) (Table 1). Results revealed that the experimental protein yield varied from 25.4% and 79.6%. A similar range of yield (%) has been described for isoelectric precipitated and ultrafiltered proteins derived from pulse flours in the literature [33]. The maximum yield (79.6%) was obtained under experimental conditions of X1 (pH) = 9.5, X2 (solid/solvent ratio) = 1/30 g/mL, and X3 (time) = 3 h (Fig. 3). Similar results (74.1 ± 2.50%) has been reported at pH 10.0 recently [19].
Model Fitting
The application of RSM yields the following regression equation, which is an empirical relationship between protein yield and the test variable in coded units, as given in Eq. (11).
The predicted values (Table 1) of protein yields are calculated using the regression model and compared with experimental values. The predicted values (Table 1) of yield are calculated using the regression models and compared with experimental values. The values of the determination coefficient R2 was 0.9891, and adjusted determination coefficient R2adj was 0.9563 indicating that more than 98% of the variation was explained by the models [23].
Analysis of variance (ANOVA) of independent variables was performed. The statistical analysis data revealed that the contribution of reduced cubic model was significant with respect to ANOVA table (Table 4). The coefficients of variation (CV) for protein yield, which is a standard deviation expressed as a percentage of the mean, a sign of good precision and high reliability and reproducibility when below 10%, was measured as 4.54%. The statistical analysis data revealed that linear, quadratic and interaction terms of solid/solvent ratio and extraction time are significant (p < 0.05) on the extraction yield of protein (Table 4). The pH and two-way interaction of pH and time (X1X3) are insignificant (p > 0.05) under the experimental conditions used in this study, however other quadratic and interactions of pH demonstrated significant effects (p < 0.05) on protein yield. Previous RSM studies for plant proteins indicated that both pH and solid/solvent ratio were important factors affecting protein yield [34,35,36].
The insignificance of the change in pH on protein yield may stem from high solubility of SCKPI at alkaline pH intervals (8–11). Since the solubility of SCKPI was above 85% ("Physicochemical and Functional Properties" section) for each pH values studied, a high extraction yield can be performed without significant differences between conditions related to pH within the ranges in this study.
Interpretation of RSM
The 3D surface plots were drawn to illustrate the main and interactive effects of the independent variables on the dependent one. These graphs were obtained by fixing two variables at coded zero level (Table 1) while varying the remaining two variables and predicting the response variable (protein yield). 3D surface plots corresponding extraction yields and interaction effect of process variables of pH and solid/solvent ratio, extraction time are presented in Fig. 3.
The time of extraction has an effect on protein yield; when it was kept constant at 1 h as an optimum condition, the protein yield changed from 35 to 65% and the rise in solid/solvent ratio positively affected the yield response (Fig. 3) which is consistent with literature indicating that 1 h extraction time would produce higher yield of protein as long as the extraction temperature kept under room temperature to eliminate protein denaturation risk [36].
No significant (p > 0.05) changes observed in protein yield with the change of solution pH for any response surfaces in consistency with ANOVA (Table 4). The averages of protein yield are 49.10, 59.12, and 42.53 mg/g at pH of 8, 9.5 and 11, respectively. Moreover, the averages of protein yield are 59.93, 46.23, and 61.86 mg/g at solid/solvent ratio of 1/30, 1/20, 1/10, respectively. The isoelectric precipitation method of protein extraction relies on high solubility of protein constituents at alkaline pH [15], the solubility of SCKF protein was high above 85% and did not change drastically within the interval of pH 8–11. Although the maximum yield of protein (79.6%) was obtained under the conditions including extraction time of 3 h, the extraction time has been reported in previous studies as responsible from the formation of undesired protein–phytic acid complexes and fall in solubility upon rise in extraction time [37]. Moreover, it is targeted herein to obtain higher extraction yield within a shorter time with the intention of waste utilization and cost reduction.
Verification of Predictive Model
The optimum condition for SCKF protein extraction determined by numerical optimization feature of Design Expert® 8.0.5 software (Stat Ease, Inc., Minneapolis, MN, USA, 2010) targeting the highest protein yield in a short time was mild alkaline pH (8.5), solid/solvent ratio (1/10), and time (1 h) for economic reasons. The verification experiment was carried out with protein extracts at optimum conditions. The predicted (62.3%) and experimental yield (63.8%) were consistent with each other, the experimental results were within the range of 95% confidence level. The protein content of optimally extracted SCKPI was 88.28% (w/w), which indicated its high potential to be utilized in foods meeting high protein content at low cost.
Physicochemical and Functional Properties
The results of the physicochemical and functional property experiments of SCKPI (extracted at optimum conditions described in "Extraction Conditions" section) are summarized in Table 5.
Solubility
The pH dependent (2.0–10.0) protein solubility profile of SCKPI and SPI was shown at Fig. 2b. The resultant U-shaped curve fits in the extant paper on sour cherry protein [19]. SCKPI had the lowest solubility at range of pI (4.0–5.0) similar to many plant based proteins such as SPI [20, 38]. A rise in solubility was observed at pH above 5.0 and below 4.0 exhibiting a V-shaped curve as in SPI (Fig. 2b). At pH 3.0, SCKPI demonstrated moderate solubility (55.1%), which increased through more acidic pH’s, reached up to 71.8% at pH 2.0 in accordance with the previous study [19]. At neutral pH, SCKPI showed solubility of 85.3%, higher than commercial SPI (64.0%). At alkaline pH, the highest solubility values, more than 95%, were observed for SCKPI. Although the solubility at pH 10 was 0.8% lower than commercial SPI, with the maximum value of 99.2% at pH 9, SCKPI exhibited superior solubility profile at alkaline pH compared to SPI which was used as a reference in this study (86.7–90.1%) [20]. The protein solubility is phenomenon related to interactions of proteins with solvents and themselves, and the surface hydrophobic–hydrophilic balance of them [39]. Hydrogen bonds and electrostatic interactions stem from intra and inter-molecular bonds in the presence of charged, polar, and nonpolar groups of protein amino acid residues on the protein surface; whereas, nonpolar amino acid residues stays in the protein interior [40]. Thus, the comparable solubility profile of SCKPI to SPI at alkaline pH can be associated with its content of amino acid residues like Asp (7.6%), Gly (6.5%), and Ser (4.5%) [10], and favorable for its potential applications.
Bulk Density
Bulk densities of SCKPI and SPI were 0.32 g/ml and 0.34 g/ml, respectively (Table 5). Many parameters can affect bulk density of a sample like particle size obtained by different drying and grinding procedures. Grinding with liquid nitrogen and freeze-drying methods used in this study are noted for generating very finite particles [41], they promote unexceptionally lower bulk densities as in the case of SCKPI. Hunter color parameters indicated darkening and lowered yellowness for SCKPI upon protein extraction.
Water and Oil Absorption Capacity
The water absorption capacity (WAC) of SCKPI (1.7 g/g proteins) was significantly lower than SPI (3.1 g/g protein) samples used in this study (Table 5). Different water holding capacity values were reported previously for proteins derived from sour cherry kernel (2.42 g/g) [19] and soybean (1.3 g/g) [42]. The position of polar amino acid groups can be responsible for protein–water interaction [40] in such a way that the positioning of polar groups such as Gly and Ser [10] on SCKPI surface lead to high WAC. The oil absorption capacity (OAC) of SCKPI was found as 3.6 g/g protein, significantly lower than commercial SPI (7.5 g/g protein). The hydrophobic groups, mainly non-polar amino acids on protein surface have interaction with oil, determine the OAC which is a required property in food applications such as bakery products enhancing flavor retention and improved palatability [39].
Emulsifying Properties
Emulsifying activity (EA) and emulsion stability (ES) values of SCKPI was 44.9% and 96.6%, respectively, which were comparable to previously reported SPI (47.4% and 100%) under similar experimental conditions [24], but significantly different from commercial SPI tested in this study (64.5% and 61.6%). EA values may be affected by surface hydrophilicity-hydrophobicity, and solubility of SCKPI. Highly soluble protein would have high EA as argued in previous works [20]. A higher solubility of SCKPI at alkaline pHs (above 85%) may help the proteins to hydrate sufficiently, in turn, show higher EA. Moreover, the surface hydrophobic–hydrophilic balance of SCKPI may favor the interactions of oil and aqueous medium so that the stable emulsions were formed.
Foaming Properties
The foaming capacity (FC) and stability (FS) values of SCKPI extracted at optimum conditions were 77.6% and 77.6% (at 60 min), respectively. The FS measurements were performed at 10, 30, and 60 min. At 10 min, the FS value was 88.8% and it decreased up to 77.6% at 30 min and stayed same at 60 min. The FC of SCKPI (77.6%) was significantly higher compared to commercial SPI (39.4%), significantly lower than lentil (LPI) (60.0%) under similar experimental conditions [23]. All samples of SCKPI demonstrated high FS regardless of the protein fraction, more than 78% of the formed foams remained stable after standing for 30 min at room temperature. FS value in this study is absolutely higher compared to other studies in literature such as lentil (80%) and commercial SPI (92%) under similar experimental conditions. The foaming capacity of a protein can be related to its high solubility and unique amino acid composition with balanced hydrophilic and hydrophobic segments so that it can be adsorbed at the air–water interface with hydrophobic segments oriented towards the air and vice versa [40].
Emulsion and foaming properties of SCKPI can head light on its potential as emulsifier or foaming agent, while determining its amount, sensory properties should also be considered. Tuna et al. added SCKF into cake formulations, and reported similar sensory analysis scores with control cake up to 5% addition [17]. Having light yellowish color and somewhat acrid taste, SCKPI may be added to food products in lower amounts compared to SPI.
Gelation Properties
The gelling capacity of food hydrocolloids is a crucial functional attribute for manufacturing gel-like or emulsion-type foods. Heating a protein solution results in molecular unfolding, which leads first to aggregation and then to gelation when the amount of aggregated protein exceeds a critical concentration [43]. Least gelation concentration (LGC) is the usual index of heat-induced gelation capacity [33]. In this study, LGC of 6.0 g/100 g for SCKPI was observed which was much lower than the value observed for the commercial SPI (12 g/100 g). Similarly, 8% LGC has been shown for sour cherry proteins by different authors at pH 7.0 [19]. A range of LGC values that is 4 and 28% has been described for different oilseeds and kernels in the literature [44]. For example, wheat protein has been reported to be gelatinized at 8% (w/v), which was lower than soybean protein (12%) and pea protein (14%) [45]. Lower LGC indicates a better gelation ability, means that SCKPI possesses a great potential as a gelling agent than SPI or other plant based sources in emulsion-type food products. The gelation properties depend upon the nature of the protein and other non‐protein components of the sample [46]. For proteins, gelation properties are based on exposition of the functional groups such as peptide bonds, side chain amide groups or hydrophobic groups. They interact with each other under heat induced gelation and form three dimensional gel network. A low LGC is advantageous when targeting certain textural attributes at a lower concentration [40].
In Vitro Protein Digestibility
After being digested by alpha-amylase, pepsin, and pancreatin; protein content of SCKF almost vanished. The PD% values for SCKF and SCKPI was found to be 93.9% and 95.7%, respectively; which is higher than the value previously reported for SPI (85.2%) [24], yet upon digestion by chymotrypsin and pancreatin enzymes. Plant proteins are known to be less digestible in general that lowers the amino acid availability for the satiety signal in the body [47]. A higher PD% of protein stems from its composition like storage proteins that are easily digested and utilized throughout the bodily functions [48]. PD% of SCKPI was very close to apricot kernel proteins, another stone fruit from Prunus genus, which depended barely on the enzyme system involved [49]. Performing in vitro digestion with a multi-enzyme system for the estimation of protein digestibility mimicking the metabolic activities throughout the human body provide an insight on bioactivity of the concerned protein [50]. Proteins derived from nuts, cereals, seeds and legumes consist of the source of plant proteins. Lower in vitro protein digestibility values were reported in beans such as 70.8% and 68.1% for faba and and kidney beans, respectively [51]. Furthermore, Li et al. (2018) indicated that coconut cake protein isolate has 86.97% in vitro digestibility that is the main byproduct of the coconut milk and oil industry [52]. Hence, with high PD%, SCKPI can be added to food products without any further need for digestion related modifications such as deamidation, hydrolysis, or high pressure applications.
Conclusion
In this study, the great potential of sour cherry kernel –a food industry waste that is intensively discharged–to recover plant-based proteins at low cost. The extraction yield of SCKPI was optimized with a high protein content of 88.3% and extraction yield of 63.8% under following conditions: pH 8.5, solid/solvent ratio 1/10 g/mL, and a short duration of 1 h when conventional alkaline solubilisation/isoelectric precipitation extraction performed. Solubility and gelation properties of SCKPI were found to be superior to the values measured for commercial SPI, whereas other properties like solubility, water absorption, foaming were similar except for oil absorption. In addition, allergenicity and GMO issues generate bad reputation for the utilization of soy based products worldwide. Functional food products without a dark color is also desirable in food industry and can easily be achieved using SCKPI. Having promising functional properties and good digestibility, SCKPI can be added to food formulations as an alternative to any other commercial proteinaceous products. The results of this study can be utilized for further isolation and purification attempts from sour cherry kernel.
References
FAOSTAT [Internet]. Annual Production of Crops. 2018 [cited 2020 Jun 21]. Available from: http://www.fao.org/faostat/en/#data/QC
McLellan, M.R., Padilla-Zakour, O.I.: Sweet Cherry and Sour Cherry Processing. In: Barrett DM, Somogyi L, Ramaswamy HS, editors. Processing Fruits: Science and Technology [Internet]. 2nd ed. CRC Press; [cited 2020 Jun 23]. p. 497 (2004)
Yılmaz, F.M., Görgüç, A., Karaaslan, M., Vardin, H., ErsusBilek, S., Uygun, Ö., et al.: Sour cherry by-products: compositions, functional properties and recovery potentials—a review. Crit. Rev. Food Sci. Nutri. 59(22), 3549–3563 (2019)
Damar, I., Ekşi, A.: Antioxidant capacity and anthocyanin profile of sour cherry (Prunus cerasus L.) juice. Food Chem. 135(4), 2910–2914 (2012)
Górnaś, P., Rudzińska, M., Raczyk, M., Mišina, I., Soliven, A., Segliņa, D.: Composition of bioactive compounds in kernel oils recovered from sour cherry (Prunus cerasus L.) by-products: Impact of the cultivar on potential applications. Ind. Crops Prod. 82, 44–50 (2016)
Bak, I., Lekli, I., Juhasz, B., Varga, E., Varga, B., Gesztelyi, R., et al.: Isolation and analysis of bioactive constituents of Sour Cherry (Prunus cerasus ) seed Kernel: an emerging functional food. J Med Food 13(4), 905–910 (2010)
Csiki, Z., Papp-Bata, A., Czompa, A., Nagy, A., Bak, I., Lekli, I., et al.: Orally delivered sour cherry seed extract (SCSE) affects cardiovascular and hematological parameters in humans. Phyther. Res. 29(3), 444–449 (2015)
Bak, I., Czompa, A., Csepanyi, E., Juhasz, B., Kalantari, H., Najm, K., et al.: Evaluation of systemic and dermal toxicity and dermal photoprotection by sour Cherry Kernels. Phyther. Res. 25(11), 1714–1720 (2011)
Mahmoud, F., Haines, D., Al-Awadhi, R., Dashti, A.A., Al-Awadhi, A., Ibrahim, B., et al.: Sour cherry (Prunus cerasus) seed extract increases heme oxygenase-1 expression and decreases proinflammatory signaling in peripheral blood human leukocytes from rheumatoid arthritis patients. Int. Immunopharmacol. 20(1), 188–196 (2010)
Yilmaz, C., Gökmen, V.: Compositional characteristics of sour cherry kernel and its oil as influenced by different extraction and roasting conditions. Ind. Crops Prod. 1(49), 130–135 (2013)
Popa, V.-M., Misca, C., Bordean, D., Raba, D.-N., Stef, D., Dumbrava, D.: Characterization of sour cherries (Prunus cerasus) kernel oil cultivars from Banat. J. Agroaliment Process. Technol. 17(4), 398–401 (2011)
Özcan, M.M., Ünver, A., Arslan, D.: A research on evaluation of some fruit kernels and/or seeds as a raw material of vegetable oil industry. Qual. Assur. Saf. Crop Foods 7(2), 187–191 (2015)
Chandra, A., Nair, M.G.: Characterization of pit oil from montmorency cherry (Prunus cerasus L.). J. Agric. Food Chem. 41, 879–881 (1993)
Khalid, E.K., Babiker, E.E., EL Tiny, A.H.: Solubility and functional properties of sesame seed proteins as influenced by pH and/or salt concentration. Food Chem. 82(3), 361–366 (2003)
Karaca, A.C., Low, N., Nickerson, M.: Emulsifying properties of canola and flaxseed protein isolates produced by isoelectric precipitation and salt extraction. Food Res. Int. 44(9), 2991–2998 (2011)
Oreopoulou, V., Tzia, C.: Utilization of plant by-products for the recovery of proteins, dietary fibers, antioxidants, and colorants. In: Oreopoulou, V., Russ, W. (eds.) Utilization of By-Products and Treatment of Waste in the Food Industry, pp. 209–232. Springer, Boston (2007)
Tuna HE. :Utilization Of Cherry Kernel, Pomegrante Seed, Pumpkin Seed And Apricot Kernel Flours For Cake Production [Internet]. Fen Bilimleri Enstitüsü; [cited 2020 Jun 23]. (2015)
Mune Mune, M.A., Minka, S.R., Mbome, I.L.: Response surface methodology for optimisation of protein concentratepreparation from cowpea [Vigna unguiculata (L.) Walp]. Food Chem. 110(3), 735–41 (2018)
Çelik, M., Güzel, M., Yildirim, M.: Effect of pH on protein extraction from sour cherry kernels and functional properties of resulting protein concentrate. J. Food Sci. Technol. 56(6), 3023–3032 (2019)
Horax, R., Hettiarachchy, N., Kannan, A., Chen, P.: Protein extraction optimisation, characterisation, and functionalities of protein isolate from bitter melon (Momordica charantia) seed. Food Chem. 124(2), 545–550 (2011)
AACC: Approved Methods of the American Association of Cereal Chemists, 18th edn. AACC, St. Paul (1986)
Stone, A.K., Karalash, A., Tyler, R.T., Warkentin, T.D., Nickerson, M.T.: Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res Int. 76(P1), 31–38 (2015)
Jarpa-Parra, M., Bamdad, F., Wang, Y., Tian, Z., Temelli, F., Han, J., et al.: Optimization of lentil protein extraction and the influence of process pH on protein structure and functionality. LWT Food Sci Technol. 57(2), 461–469 (2014)
Tan, E.S., Ying-Yuan, N., Gan, C.Y.: A comparative study of physicochemical characteristics and functionalities of pinto bean protein isolate (PBPI) against the soybean protein isolate (SPI) after the extraction optimisation. Food Chem. 152, 447–455 (2014)
Turan, D., Altay, F., Çapanoǧlu, G.E.: The influence of thermal processing on emulsion properties of defatted hazelnut flour. Food Chem. 167(15), 100–106 (2015)
Chee, K.L., Ling, H.K., Ayob, M.K.: Optimization of trypsin-assisted extraction, physico-chemical characterization, nutritional qualities and functionalities of palm kernel cake protein. LWT Food Sci. Technol. 46(2), 419–427 (2012)
Sridaran, A., Karim, A.A., Bhat, R.: Pithecellobium jiringa legume flour for potential food applications: studies on their physico-chemical and functional properties. Food Chem. 130(3), 528–535 (2012)
Yuliana, M., Truong, C.T., Huynh, L.H., Ho, Q.P., Ju, Y.H.: Isolation and characterization of protein isolated from defatted cashew nut shell: Influence of pH and NaCl on solubility and functional properties. LWT Food Sci. Technol. 55(2), 621–626 (2014)
Świeca, M., Baraniak, B., Gawlik-Dziki, U.: In vitro digestibility and starch content, predicted glycemic index and potential in vitro antidiabetic effect of lentil sprouts obtained by different germination techniques. Food Chem. 138(2–3), 1414–1420 (2013)
Ma, C.Y., Harwalkar, V.R.: Thermal analysis of food proteins. Adv. Food Nutr. Res. 35(C), 317–66 (1991)
Correia, P., Beirão-Da-Costa, M.L.: Effect of drying temperatures on starch-related functional and thermal properties of chestnut flours. Food Bioprod Process. 90(2), 284–294 (2012)
Acevedo, B.A., Avanza, M.V., Cháves, M.G., Ronda, F.: Gelation, thermal and pasting properties of pigeon pea (Cajanus cajan L.), dolichos bean (Dolichos lablab L.) and jack bean (Canavalia ensiformis) flours. J Food Eng. 119(1), 65–71 (2013)
Boye, J.I., Aksay, S., Roufik, S., Ribéreau, S., Mondor, M., Farnworth, E., et al.: Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res Int. 43(2), 537–546 (2010)
Du, M., Xie, J., Gong, B., Xu, X., Tang, W., Li, X., et al.: Extraction, physicochemical characteristics and functional properties of Mung bean protein. Food Hydrocoll. 76, 131–140 (2018)
Yücetepe, A., Saroğlu, Ö., Özçelik, B.: Response surface optimization of ultrasound-assisted protein extraction from Spirulina platensis: investigation of the effect of extraction conditions on techno-functional properties of protein concentrates. J Food Sci Technol. 56(7), 3282–3292 (2019)
Siow, H.L., Gan, C.Y.: Functional protein from cumin seed (Cuminum cyminum): optimization and characterization studies. Food Hydrocoll. 41, 178–187 (2014)
Eromosele, C.O., Arogundade, L.A., Eromosele, I.C., Ademuyiwa, O.: Extractability of African yam bean (Sphenostylis stenocarpa) protein in acid, salt and alkaline aqueous media. Food Hydrocoll. 22(8), 1622–1628 (2018)
Sharma, P.C., Tilakratne, B.M.K.S., Gupta, A.: Utilization of wild apricot kernel press cake for extraction of protein isolate. J. Food Sci. Technol. 47(6), 682–685 (2019)
Ozdal, T., Capanoglu, E., Altay, F.: A review on protein-phenolic interactions and associated changes. Vol. 15, Food International, vol. 51, pp. 954–70. Elsevier, Amsterdam (2019)
Damodaran, S.: Food proteins: an overview. In: Damodaran, S., Paraf, A. (eds.) Food Proteins and Their Applications, pp. 1–24. CRC Press, Boca Raton (1997)
George, R.M.: Freezing proceseses used in the food industry. Trends Food Sci. Technol. 4(5), 134–138 (1997)
Fernández-Quintela, A., Macarulla, M.T., Del Barrio, A.S., Martínez, J.A.: Composition and functional properties of protein isolates obtained from commercial legumes grown in northern Spain. Plant Foods Hum. Nutr. 51(4), 331–341 (1997)
Maltais, A., Remondetto, G.E., Subirade, M.: Mechanisms involved in the formation and structure of soya protein cold-set gels: a molecular and supramolecular investigation. Food Hydrocoll. 22(4), 550–559 (2018)
Moure, A., Sineiro, J., Domínguez, H., Parajó, J.C.: Functionality of oilseed protein products: a review. Food Res Int. 39(9), 945–963 (2006)
Zhao, H., Shen, C., Wu, Z., Zhang, Z., Xu, C.: Comparison of wheat, soybean, rice, and pea protein properties for effective applications in food products. J Food Biochem. 44(4), e13157 (2020)
Omowaye-Taiwo, O.A., Fagbemi, T.N., Ogunbusola, E.M., Badejo, A.A.: Effect of germination and fermentation on the proximate composition and functional properties of full-fat and defatted cucumeropsis mannii seed flours. J Food Sci Technol. 52(8), 5257–5263 (2020)
Gilbert, J.A., Bendsen, N.T., Tremblay, A., Astrup, A.: Effect of proteins from different sources on body composition. Nutr Metab Cardiovasc Dis. 21(Suppl 2), B16-31 (2011)
Damodaran S. :Structure-function relationship of food proteins. In: Hettiarachchy NS, Ziegler GR, editors. Protein Functionality in Food Systems. CRC Press. p. 1–37 (1994)
El-Aal, M.H.A., Hamza, M.A., Rahma, E.H.: In vitro digestibility, physico-chemical and functional properties of apricot kernel proteins. Food Chem. 19(3), 197–211 (1986)
Chen, N., Zhao, M., Sun, W.: Effect of protein oxidation on the in vitro digestibility of soy protein isolate. Food Chem. 141(3), 3224–3229 (2013)
Alonso, R., Aguirre, A., Marzo, F.: Effects of extrusion and traditional processing methods on antinutrients and in vitro digestibility of protein and starch in faba and kidney beans. Food Chem. 68(2), 159–165 (2000)
Li, Y., Zheng, Y., Zhang, Y., Xu, J., Gao, G.: Antioxidant activity of coconut (Cocos nucifera L.) protein fractions. Molecules. 23(3), 707 (2018)
Funding
This research was financially supported by the grant from İTÜ BAP Project No.39325 (Istanbul Technical University Scientific Research Projects Department).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kasapoğlu, K.N., Demircan, E., Eryılmaz, H.S. et al. Sour Cherry Kernel as an Unexploited Processing Waste: Optimisation of Extraction Conditions for Protein Recovery, Functional Properties and In Vitro Digestibility. Waste Biomass Valor 12, 6685–6698 (2021). https://doi.org/10.1007/s12649-021-01417-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01417-x