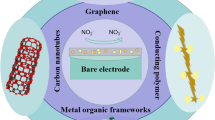
Abstract
Nitrite contamination in food and the environment has attracted incredible public attention owing to its detrimental effects on living systems. It is crucial to develop rapid, easy-to-use, accurate, and cheap analytical techniques to monitor nitrite levels. Metal-organic frameworks are a type of inorganic-organic hybrid crystal material known for their ultrahigh surface area, ordered porous structure, ease of function, as well as their catalytic activity or luminescence in certain situations. This allowed researchers to design a diverse array of sensors for the measurement of nitrite. This article highlights recent progress on the application of metal-organic frameworks towards the sensing of nitrite based on their unique electrical and optical features. According to the design strategies, electrochemical sensors based on metal-organic frameworks can be classified as pristine metal-organic frameworks, metal-organic framework composites, or metal-organic framework derivatives, while optical sensors can be divided into two classes, luminescent metal-organic frameworks, and metal-organic frameworks with luminescent guests. Each category was herein discussed and examples were provided. At the end of the review, current challenges and future trends are also addressed.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrite has been extensively used in food or environmental areas including food preservation, flavour enhancement and fertilizer application. It has also appeared in water distribution systems such as in groundwater, lakes, and seas [1]. Thus, it easily accumulates in vegetables, fruits, poultry, processed meats, and drinking water and is present in our daily diets [2]. Excessive nitrite intake by people can result in the onset of severe diseases. Nitrite induces the irreversible reaction of haemoglobin to form methemoglobincarbon polyhedron, which disrupts the oxygen delivery system. Furthermore, it combines with amines to form N-nitrosamines which are likely to cause gastric cancer [3]. The International Agency for Cancer Research has classified nitrite as a probable carcinogen (Group 2 A) to humans [4]. The maximum contamination level of nitrite in drinking water is 3 mg·L−1, defined by the World Health Organization [5]. Therefore, the sensitive and efficient analysis of nitrite in food and the environment is of particularly interest.
Traditional analytical techniques including spectrophotometry, chromatography and capillary electrophoresis, have been adopted to monitor nitrite in various food commodities and precise detection results have been obtained [6]. However, the limitations of these conventional methods cannot be ignored. For example, the most widely adopted spectrophotometric method for nitrite analysis is the Griess Assay. However, this method is slow and has low sensitivity due to interference by other ions [7]. Chromatography has the benefit of high sensitivity, but the need for sample pre-treatment, complicated operation procedures, well-trained operators and expensive instruments make this approach costly [3]. Regarding capillary electrophoresis, the limited capacity of the capillary columns causes low sensitivity and hinders reproducibility. The method also requires prior sample preparation and a complex apparatus [8]. The disadvantages of conventional methods are a key limitation for the measurement of nitrite. On the contrary, the use of a sensor for the detection of nitrite has drawn increasing attention because of its simplicity, high effectiveness, rapid detection, and low cost. With recent advances in nanotechnology, nanomaterials with outstanding features play an important role in sensor development [9,10,11]. Over the past decade, many nanomaterials like graphene [12], quantum dots [13, 14], noble metal nanoparticles (NPs) [15] and polymers [16] have been successfully used in sensors for nitrite analysis with greatly improved detection performances. Among the various kinds of nanomaterials, metal-organic frameworks (MOFs) have emerged as a crystalline material with significant promise for application in sensor development [17].
MOFs are advanced highly porous materials built from diverse metal ions or clusters and organic ligands. Beyond traditional porous materials like zeolites and mesoporous silicas, MOFs possess organized porosity, superior internal surface area, tailorable structure, and ease of modification [18]. Moreover, they can serve as good supports to be integrated with other functional substances and excellent templates to be pyrolyzed, creating novel MOF composites and MOF derivatives [19]. Thus, increasing efforts have been devoted to the applications of MOF-based materials in various fields including catalysis [20], drug delivery [21], energy storage [22], and gas separation [23]. Besides these applications, research on the design of MOFs-based sensors towards diverse objects has rapidly expanded in the last few years. Although plenty of MOF-based sensors have been summarized in previous reviews [24,25,26], there is a lack of a systematic overview of MOF-based sensors focusing on the analysis of nitrite. To address this, a short article summarizing the emerging applications of MOFs for the control of nitrite is highly required.
Generally, the analytical strategies can be divided into two categories: MOF-based electrochemical sensors and MOF-based optical sensors. In this review, we first summarize the design of MOF-based electrochemical sensors for nitrite analysis. For electrochemical sensing, the selection of suitable electrocatalysts is the key factor to ensuring high sensitivity [27]. MOF-based electrocatalysts are mainly classified into three types: pristine MOFs, MOF composites and MOF derivatives. Several studies have shown that pure MOFs such as MOF-525 [28] and MIL-53 [29] exhibit good catalytic capabilities, originating from the active sites of organic linkers or metal nodes. Besides this, the high porosity of MOFs allows them to be constructed as MOF composites with other active materials [30]. These well-designed MOF composites have improved or even new features when compared to single components because of the cooperative effect. In addition, MOF derivatives can be fabricated through carbonization. They also possess high catalytic activity due to the partially maintained frame structure and novel active sites formed from the precursors [31]. Secondly, the recent applications of MOF-based sensors including luminescent MOFs (LMOFs) and MOF composites in the optical detection of nitrite are summarized and discussed. For optical sensing, it is important to prepare efficient nitrite recognition materials. As a branch of MOFs, LMOFs have attracted much attention since their adjustable structures and high porosity can provide high sensitivity and good selectivity in the field of optical sensing [32]. Apart from the fluorescence produced by LMOFs, other optical components can be incorporated into MOFs to trigger fluorescence and provide a response to nitrite [33]. Finally, personal viewpoints on the challenges and prospects for future studies are given. We expect that this review can provide guidance for scientists to design novel MOF-based sensors for the determination of nitrite and to foster the cooperation between food analysts and material researchers.
MOF-based electrochemical sensors
The electrochemical method has gained immense popularity for sensor fabrication owing to its quick response time, high sensitivity, good selectivity, and cost-effectiveness [34,35,36,37]. This method always involves the redox reactions of the target at the electrode surface [38,39,40,41]. Nitrite is electroactive with platinum, gold, copper, and glassy carbon electrodes. However, the utilization of these electrodes is unsatisfactory owing to the high oxidation potential of nitrite on the bare electrodes. The excellent properties of MOF-based materials as electrode modifiers have boosted the development of many electrochemical nitrite sensors, (Fig. 1) and a summary of these sensors is listed in Table 1.
Pristine MOFs
In general, the intrinsic electrochemical activity and the number of active sites of a pristine MOF are of vital importance in its detection performance. The electrocatalysis behaviour of pristine MOFs can be modulated by the careful selection of the metal node and organic linker. Porphyrins are a class of naturally occurring macrocycles in the form of enzyme active sites that are used as building ligands in MOF synthesis [42]. Porphyrin-related MOFs with highly porous structures retain the activity of porphyrin and grant analytes and electrolyte ions access to the activity centres [43]. In 2015, Kung et al. first prepared uniform porphyrin MOF-525 films on fluorine-doped tin oxide substrates [44]. The fabricated MOF-525 films showed high electrocatalytic activity for the oxidation of nitrite, which could be ascribed to the generation of the cation radical state of the porphyrin. In addition, MOF-525 was a Zr-based MOF that exhibited high water stability, indicating potential applicability for nitrite analysis in aqueous systems. Benefiting from their numerous accessible active sites, large surface areas, and short diffusion distances, two-dimensional (2D) MOFs that exhibit excellent electrocatalytic activity have attracted extensive interest in the construction of sensors [45]. Zhao et al. investigated the application of a porphyrin-based 2D Zn-TCPP (TCPP= 4,4,4,4-(Porphine-5,10,15,20-tetrayl)tetrakis(benzoic acid)) nano-disk toward the sensing of nitrite [46]. The size of the synthesized material could be effectively regulated with the help of 4,4’-biphenyldicarboxylic acid (Fig. 2a). The good distribution of the porphyrin ligand and the unique structure of the developed nano-disk exposed more active sites, leading to higher activity. The prepared electrochemical sensor resisted interference from K+, Na+, SO42−, SO32−, and NO3− and showed high selectivity, good reproducibility, and fast response toward nitrite.
a Illustration of the synthesis of Zn-TCPP nano-disk. Reprinted with permission [46]. Copyright 2018, Royal Society of Chemistry. b Schematic illustration of the development of analytical platform depending on Au/Cu-MOF composites and the quantification of nitrite. Reprinted with permission [51]. Copyright 2019, Elsevier. c Illustration of the construction of MOFs-derived α-Fe2O3/carbon nanotubes. Reprinted with permission [63]. Copyright 2018, Elsevier
Aside from the use of the organic linkers of MOFs as catalytic active sites, active centres can also be imported by metal nodes. Because of their outstanding electrochemical properties, Cu-based MOFs have been extensively studied by scientists. An electrochemical sensing platform comprised of a carbon paste electrode decorated with a redox-active Cu-MOF was first constructed for nitrite determination through an amperometry technique [47]. The high surface area and abundant micropores of the Cu-MOF provided good accumulation efficiency and abundant active sites (copper active centres) for nitrite oxidation. Meanwhile, Fe(CN)63+ absorbed on the Cu-MOF could promote the catalytic behaviour of nitrite to magnify the measurement signal. Therefore, the prepared Cu-MOF decorated electrode could significantly improve the detection signal and showed high electrocatalytic ability and good selectivity.
Although the aforementioned instances illustrate that several pristine MOFs can be directly utilized in electrode decoration for the measurement of nitrite, it is worth noting that most MOFs demonstrate unsatisfactory detection performance owing to their low conductivity, relatively poor chemical stability and limited electro-catalytic activity, which would hinder their practical application.
MOF composites
To overcome the inherent defects of pristine MOFs and achieve improved detection performance, incorporating MOFs with guests to form MOF composites is a common strategy. These composites not only compensate for the drawbacks of pure MOFs but also inherit the nature of the guest material, thereby endowing the sensors with higher sensitivity, specificity, and stability [48].
Noble metal NPs are excellent materials for electrochemical analysis due to their high catalytic ability towards nitrite oxidation, high active area, and good electrical conductivity. However, they usually suffer from aggregation issues, causing the degradation of catalytic properties [49]. Incorporating noble metal NPs with MOFs is regarded as a useful way to solve this problem [50]. This maximizes the interfacial area of noble metal NPs and MOFs, enhancing the interaction and electronic transfer between them, and improving their detection properties. For example, Chen et al. coated Au NPs via electrodeposition on the surface of a Cu-MOF to prepare Au/Cu-MOF composites, which integrated both the electrocatalytic activity of Au NPs and the huge surface area of Cu-MOFs (Fig. 2b) [51]. The porosity of Cu-MOF favoured the adsorption of nitrite and made the Au NPs monodisperse distribution. Also, it exhibited a long-term stability, good reproducibility, and anti-interference. The proposed method was successfully applied for the measurement of nitrite and provided satisfactory results.
Carbon-based nanomaterials are promising candidates for supporting MOFs because of their various structures (e.g., 0D quantum dot, 1D carbon nanotube and 2D graphene structures) and can remove the weakness of MOFs through their high electrical conductivity, superior mechanical strength, and good chemical and thermal robustness [52]. A multi-walled carbon nanotubes (MWCNTs)-Zr-MOF composite was prepared and its performance in sensing nitrite is investigated [53]. The introduction of MWCNTs facilitated electron transfer, resulting in better conductivity, and contributed to the improved stability of the electrode. The developed sensor exhibited suitable stability, repeatability, and selectivity for the recognition of nitrite. The spiked samples demonstrated good recovery results and the relative standard deviation (RSD) was 0.34%. In another case, with the help of ultrasonic treatment, Cu-MOF anchored graphene oxide (GO) hybrids were synthesized for the determination of nitrite [54]. Taking advantage of the sufficient oxygen-containing functional groups of GO, Cu-MOFs were size-controllably synthesized and possessed colloidosome-like morphology. The original properties of each component in this composite were preserved, while the synergistic effects of Cu-MOFs and GO can promote the quantification performance. The modified electrode improved the oxidation current signal by two-fold as compared to the electrode without surface modification and showed a fast response (less than 3 s) to nitrite.
Benefiting from the extremely large surface area and high porosity of MOFs, multiple functional components can be simultaneously combined with them to broaden the scope of MOF composites and achieve enhanced electrochemical sensing performance [55]. An analysis platform was introduced to monitor nitrite levels by encapsulating Au NPs and electrochemically reduced graphene oxide (ERGO) within the Cu-TDPAT (TDPAT=2,4,6-tris(3,5-dicarboxylphenylamino)-1,3,5-triazine) [56]. Electron transport between nitrile and the electrode surface was significantly enhanced with the assistance of Au NPs and ERGO. The oxidation signal of nitrite on the decorated electrode was enhanced by 1.83 times compared to the bare electrode and showed a low oxidation potential of 0.77 V. Due to the cooperative effect of the Au NPs, ERGO, and Cu-TDPAT, the presented sensor exhibited good stability, wide linear range, low detection limit, and could quantify nitrite in tap water, milk, sausage, and pickled vegetable samples with recoveries in the range of 91.4-100.2%.
There are growing interests in the building of biosensors for nitrite detection due to their high selectivity and specificity. Generally, a redox electroactive protein or enzyme like cytochrome c (Cyt c), myoglobin, or haemoglobin should be integrated [57]. Nevertheless, their intrinsically fragile nature limits their practical application. Employing porous MOFs as the matrices for protein immobilization can improve the stability of the proteins and facilitate nitrite transport without negative effects on activity. Tong et al. prepared an electrochemical sensor for the determination of nitrite by a Co-TCPP/ionic liquid/Cyt c bio-composite [58]. In their study, Cyt c was absorbed onto Co-TCPP followed by the deposition of ionic liquid. Co-TCPP not only had a large surface area to immobilize more Cyt c, leading to high sensitivity, but also enhanced the repeatability and reusability of the developed biosensor. After storage for 14 days at 4℃ at pH 7.0, the constructed sensor retained 91% of its initial response, indicating its high stability.
MOF derivatives
An alternative strategy to overcome the poor conductivity and instability of common MOFs is pyrolyzing MOFs to obtain their derivatives. These materials not only preserve the inherited structural properties of the parent MOFs but also enhance several of their features including electric conductivity, stability and catalytic activity, demonstrating their great potential in electrochemical analysis [59].
Generally, when carbonizing MOFs in an inert atmosphere, the organic ligands will transform into carbons that serve as a reductant to reduce the metal nodes in situ. This occurs because of the confinement effect of MOFs, which causes the generation of carbon-supported metal composites [60]. These novel composites possess highly ordered structures and high electrocatalytic activity with the presence of well-dispersed metallic NPs, which contribute to nitrite detection. By directly carbonizing ZIF-67 in argon, a magnetic Co@carbon hybrid was prepared and then deposited on the electrodes for electrochemical detection of nitrite [61]. The developed hybrid retained its original rhombic dodecahedral shape, with uniformly distributed cobalt nanoparticles in the porous carbon. Benefiting from its porous structure and high activity, the Co@carbon hybrid showed an outstanding electrochemical response. The reproducibility of the sensor was investigated by employing five independent electrodes (RSD = 3.4%).
If the thermal decomposition of precursor MOFs is conducted under an air atmosphere, porous metal oxides with intriguing properties will form [62]. Such MOF-derived metal oxides and carbons exhibit large surface areas and interlinked pores, which make them desirable for application in electrochemical sensing. An α-Fe2O3/carbon nanotubes nanocomposite was fabricated using MIL-101(Fe) as both a template and as a precursor assembled onto carbon nanotubes for the quantification of nitrite (Fig. 2c) [63]. Thanks to the high electrocatalytic activity of α-Fe2O3, the good conductivity of the carbon nanotubes, and the synergistic effect between them, the electrochemical performance of the studied α-Fe2O3/carbon nanotubes decorated electrode for the oxidation of nitrite displayed high responsivity, good linearity, outstanding selectivity, and superior stability. In tap water and pond water, the recoveries were 97.4-102.2% and 96.1-103.6% respectively, demonstrating the potential application of this method in real samples.
To further improve the performance of the MOF derivatives-based sensors, post-modification appeared to be an attractive strategy. In a recent study, Zhang et al. reported Co, N co-doped carbon polyhedron (CP) obtained from ZIF-8@ZIF-67 by carbonization under argon, followed by the electrodeposition of Ni NPs onto them to produce a Ni/Co, N-CP composite as an absorbing platform for analysing nitrite [64]. The high porous Co, N-CP nanoparticles could effectively immobilize Ni NPs without aggregation and provide plenty of active sites for electrochemical detection. Meanwhile, the introduction of Ni NPs could facilitate electron transportation and improve electronic conductivity. The proposed strategy in this work revealed that the Ni/Co, N-CP modified electrode showed good performance for monitoring nitrite.
MOF-based optical sensors
Optical sensors have gained huge interest by virtue of their good sensitivity, convenient operation, fast response time, low cost, and easy visualization [65]. In light of their exceptional characteristics, MOFs as fluorescent probes are also taken into account in the construction of luminescent sensors for nitrite measurement (Table 2). Generally, the fluorescence can originate from LMOFs themselves or luminescent guests on MOFs (Fig. 3).
LMOFs
Lanthanide MOFs (Ln-MOFs), a specific subset of LMOFs constructed from lanthanide ions and organic linkers, have been widely employed as promising luminescent probes in analytical chemistry, combining the unique structural properties of MOFs and the intrinsic luminescent properties of lanthanide ions [66]. Recent studies indicated that energy transfer between some lanthanide ions and nitrite causes a quenching effect on the luminescence, making Ln-MOFs ideal candidates for nitrite detection. Among different lanthanide ions, Tb3+ is the most frequently used. In a recent report, a novel Tb-MOF was prepared using terbium(III) nitrate hexahydrate, o-phenanthroline and pyridine-3,5-dicarboxylic acid [67]. This Tb-MOF showed a strong green emission when excited at 242 nm. The emission intensity of Tb-MOF was quenched upon the introduction of nitrite (Fig. 4a). The probe could distinguish nitrite from other anions such as NO3−, CO32− and HCO3−, demonstrating its function as a promising sensor. The Tb-MOF also exhibited tolerance to some organic solvents and acid-base solutions. Interestingly, the sensor showed similar behaviour for ferric ions.
a Schematic illustration of the preparation of Tb-MOF and the quantification of nitrite. Reprinted with permission [67]. Copyright 2021, Elsevier. b Illustration of the quantification of nitrite by Tb3+@In-MOF. Reprinted with permission [68]. Copyright 2018, American Chemical Society. c Illustration of the quantification of nitrite by Rh110@MOF-801. Reprinted with permission [69]. Copyright 2019, Springer
MOFs with luminescent guests
The intriguing porous structure of MOFs allows for the incorporation of emissive guests dispersedly to prevent aggregation-induced quenching and form novel photofunctional probes. As mentioned above, Tb3+ is used to distinguish nitrite in most cases. Apart from studies on LMOFs fabricated with Tb3+ as the metal centre, the development of luminescent sensing can also be achieved by doping Tb3+ into the MOF framework via post-synthetic modification. 2,2’-bipyridine-5,5’-dicarboxylic acid and In(NO3)3·6H2O were reported to synthesize In(OH)bpydc, an In-MOF [68]. After coordinating Tb3+ with the uncoordinated sites of bipyridine in the framework, the developed Tb3+@In-MOF emits green light and could be applied to monitor nitrite through fluorescence quenching (Fig. 4b). Apart from sensing, the prepared material was also examined for nitrite absorption behaviour and showed a good adsorption capacity (3.45 mg·g−1).
Besides lanthanide ions, fluorescent dyes can also be encapsulated in the MOFs to produce luminescent nitrite sensors. Huang et al. reported the immobilization of rhodamine 110 (Rh110) dye in MOF-801 through a one-pot route [69]. The strong interactions between MOF-801 and the dye offered strong support for Rh110 to prevent it from escaping. Because of the porous nature of MOF-801, the aggregation of dyes can be inhibited and allows for contact with nitrite. The emission intensity of Rh110@MOF-801 was gradually quenched upon the incremental addition of nitrite (Fig. 4c). Compared with Rh110 alone, the Rh110@MOF-801 showed a higher quenching constant and a faster response time.
Conclusions and further perspectives
Food safety is closely related to human health. Among numerous food hazards, nitrite is known to be one of the most common because of its toxicity to humans. Therefore, developing highly efficient analytical methods for the detection of nitrite is an important scientific pursuit. Conventional spectroscopic analysis remains the predominant approach in monitoring nitrite since it is cheap and feasible, but it is always hampered by interferences and long operation times. While chromatography and capillary electrophoresis provide high sensitivity, they need high-cost devices. MOF-based sensors address many of the shortcomings of conventional analytical methods and have recently become a competitive alternative for the rapid, sensitive, and cost-effective quantification of nitrite. In this review, state-of-the-arts of MOF-based electrochemical and optical sensors for nitrite were summarized and discussed. Although much progress has been made in the use of MOFs for nitrite pollution control, the following obstacles remained to be taken into account in the near future.
A current literature survey indicated that some pristine MOFs with electrocatalytic activity were employed in the construction of sensors for nitrite analysis. However, their performances are still far from satisfactory, as most detection limits can only reach the micromolar level. To develop electrochemical sensors with the needed sensitivity, regulation of the morphology of MOFs is a promising strategy to improve their surface area. This would expose more catalytic active sites and promote analyte-catalyst interaction, thereby resulting in higher activity. Another potential strategy is to construct bimetallic MOFs to strengthen their catalytic and electronic properties. Such a strategy is also feasible for the design of MOF derivatives. In recent years, numerous attempts have been made to design high-stability and highly conductive MOFs. However, attention was only focused on material synthesis while the applicability of these materials in the field of food safety was ignored. Bridging nanotechnology and food science would create more possibilities and may lead to more directed approaches.
Emerging studies have employed MOF composites in the construction of sensors owing to their superior properties and have achieved fairly good results. However, the accurate control of composite preparation is difficult. A deeper fundamental understanding of the interaction between MOFs and guest materials is needed in the design of MOF composites to maximize their synergistic effects, leading to better detection performance. In addition, we observed that noble metals are widely used as guests while other metals or metal oxide NPs are rarely reported. Previous reports have illustrated the possibility of the use of different metals and metal oxide NPs such as Cu, NiO and CeO2 for the electroanalysis of nitrite. The fabrication of nanohybrids between these and MOFs and them is an attractive alternative as it can reduce costs.
Compared with pure MOFs, MOF derivatives not only offer some of the same merits as MOFs, such as tuneable catalytic active sites and high porosity but also possess a higher conductivity and better stability. However, studies on MOF derivatives-based electrochemical nitrite sensors are few and recent. How their composition, structure and morphology influence the detection performance remain unexplored. For example, by carefully designed regulation means, MOF derivatives in different structures such as hollow, core-shell, and yolk-shell structures can be obtained. It is difficult to predict which is most suitable for monitoring nitrite. Moreover, single-site atom catalysis, which can also be prepared by MOF derivation, may improve detection performance, and should receive attention in this regard.
Compared with electrochemical sensing, there are relatively few studies on optical sensors. Most optical sensors are depending on LMOFs rather than MOFs with luminescent guests. However, pure LMOFs are sometimes unstable in water or harsh conditions. The strategy of combining luminescent guests with carefully selected water-stable MOFs may solve this problem and needs further exploration. Until now, almost all the reported sensors function through a “turn-off” mechanism. Luminescence enhancement (signal-on) is preferable over luminescence quenching (signal-off) when the detection process is carried out in a dark background. Considering the advantages of higher sensitivity and practicability, developing sensors based on the “turn-on” mechanism deserves more attention. Other analytical modes such as colourimetric assay and ratiometric assay should also be investigated. For instance, the ratiometric method can eliminate environmental interference and enhance the accuracy of the determination method. Furthermore, a survey of the literature revealed that some studies did not carry out simulated real sample analysis. Thus, it is difficult to evaluate the feasibility of these sensors in practical applications. Future research should include the application of fabricated sensors in real samples to evaluate their practicality.
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- NPs:
-
Nanoparticles
- MOFs:
-
Metal-organic frameworks
- LMOFs:
-
Luminescent MOFs
- 2D:
-
Two-dimensional
- TCPP:
-
4,4,4,4-(Porphine-5,10,15,20-tetrayl)tetrakis(benzoic acid)
- MWCNTs:
-
Multi-walled carbon nanotubes
- RSD:
-
Relative standard deviation
- GO:
-
Graphene oxide
- ERGO:
-
Electrochemically reduced graphene oxide
- TDPAT:
-
2,4,6-tris(3,5-dicarboxylphenylamino)-1,3,5-triazine
- Cyt c:
-
Cytochrome c
- CP:
-
Carbon polyhedron
- Ln-MOFs:
-
Lanthanide MOFs
- Rh110:
-
Rhodamine 110
- LR:
-
Linear range
- LOD:
-
Limit of detection
References
Z. Liu, V.S. Manikandan, A. Chen, Curr Opin Electroche (2019). https://doi.org/10.1016/j.coelec.2019.05.013
W. Bedale, J.J. Sindelar, A.L. Milkowski, Meat Sci (2016). https://doi.org/10.1016/j.meatsci.2016.03.009
Q.H. Wang, L.J. Yu, Y. Liu, L. Lin, R.G. Lu, J.P. Zhu et al., Talanta (2017). https://doi.org/10.1016/j.talanta.2016.12.044
M. Flores, F. Toldra, Meat Sci (2021). https://doi.org/10.1016/j.meatsci.2020.108272
T. Ramakrishnappa, K. Sureshkumar, M. Pandurangappa, Mater Chem Phys. (2020) https://doi.org/10.1016/j.matchemphys.2020.122744
X. Li, J. Ping, Y. Ying, TrAC Trends Anal. Chem. (2019) https://doi.org/10.1016/j.trac.2019.01.008
J. Sun, X. Zhang, M. Broderick, H. Fein, Sensors (2003). https://doi.org/10.3390/s30800276
T. Baciu, I. Botello, F. Borrull, M. Calull, C. Aguilar, TrAC Trends Anal. Chem. (2015) https://doi.org/10.1016/j.trac.2015.05.011
J.-M. Liu, Y. Hu, Y.-K. Yang, H. Liu, G.-Z. Fang, X. Lu et al., Trends Food Sci Tech. (2018) https://doi.org/10.1016/j.tifs.2017.11.005
Y. Orooji, M. Haddad Irani-Nezhad, R. Hassandoost, A. Khataee, S. Rahim Pouran, S.W. Joo, Spectrochim Acta A. (2020) https://doi.org/10.1016/j.saa.2020.118272
A. Khataee, H. Sohrabi, O. Arbabzadeh, P. Khaaki, M.R. Majidi, Food Chem. Toxicol. (2021) https://doi.org/10.1016/j.fct.2021.112030
G. Li, Y. Xia, Y. Tian, Y. Wu, J. Liu, Q. He et al., J Electrochem Soc. (2019) https://doi.org/10.1149/2.0171912jes
W. Li, S. Huang, H. Wen, Y. Luo, J. Cheng, Z. Jia et al., Anal Bioanal Chem. (2020) https://doi.org/10.1007/s00216-019-02325-9
R. Jalili, A. Khataee, M.R. Rashidi, A. Razmjou, Food Chem (2020). https://doi.org/10.1016/j.foodchem.2020.126172
Z. Yang, X. Zhou, Y. Yin, W. Fang, Anal Lett (2021). https://doi.org/10.1080/00032719.2021.1897134
O. Salhi, T. Ez-zine, M. El Rhazi, Electroanal (2021). https://doi.org/10.1002/elan.202100033
R. Darabi, M. Shabani-Nooshabadi, H. Karimi-Maleh, A. Gholami, Food Chem (2022). https://doi.org/10.1016/j.foodchem.2021.130811
A. Magri, M. Petriccione, T.J. Gutiérrez, Food Chem (2021). https://doi.org/10.1016/j.foodchem.2021.129533
M.-S. Yao, W.-H. Li, G. Xu, Coordin Chem Rev. (2021) https://doi.org/10.1016/j.ccr.2020.213479
S. Dou, X. Li, X. Wang, ACS Mater Lett. (2020). https://doi.org/10.1021/acsmaterialslett.0c00229
H.D. Lawson, S.P. Walton, C. Chan, ACS Appl Mater Interfaces (2021). https://doi.org/10.1021/acsami.1c01089
Y. Li, M. Cui, Z. Yin, S. Chen, T. Ma, Chem Sci. (2020) https://doi.org/10.1039/d0sc04684a
X. Zhao, X. Yu, X. Wang, S. Lai, Y. Sun, D. Yang, Chem. Eng. J. (2021) https://doi.org/10.1016/j.cej.2020.127221
Y. Xu, H. Wang, X. Li, X. Zeng, Z. Du, J. Cao et al., Compr Rev Food Sci Food Saf. (2021) https://doi.org/10.1111/1541-4337.12675
W. Cheng, X. Tang, Y. Zhang, D. Wu, W. Yang, Trends Food Sci Tech. (2021) https://doi.org/10.1016/j.tifs.2021.04.004
T. Du, L. Huang, J. Wang, J. Sun, W. Zhang, J. Wang, Trends Food Sci Tech. (2021) https://doi.org/10.1016/j.tifs.2021.03.013
Z. Khorablou, F. Shahdost-Fard, H. Razmi, M.L. Yola, H. Karimi-Maleh, Chemosphere (2021). https://doi.org/10.1016/j.chemosphere.2021.130393
J.W. Brown, Q.T. Nguyen, T. Otto, N.N. Jarenwattananon, S. Glöggler, L.-S. Bouchard, Catal. Commun. (2015) https://doi.org/10.1016/j.catcom.2014.09.040
L. Zhang, X. Ma, H. Liang, H. Lin, G. Zhao, J Mater Chem B. (2019) https://doi.org/10.1039/c9tb01832h
H. Karimi-Maleh, M.L. Yola, N. Atar, Y. Orooji, F. Karimi, P. Senthil Kumar et al., J. Colloid Interface Sci. (2021) https://doi.org/10.1016/j.jcis.2021.02.066
X. Yan, L. Chen, H. Song, Z. Gao, H. Wei, W. Ren et al., New J Chem. (2021) https://doi.org/10.1039/d1nj03227e
Y. Feng, Y. Wang, Y. Ying, Coordin Chem Rev. (2021) https://doi.org/10.1016/j.ccr.2021.214102
B.B. Guo, J.C. Yin, N. Li, Z.X. Fu, X. Han, J. Xu et al., Adv Opt Mater. (2021) https://doi.org/10.1002/adom.202100283
H. Karimi-Maleh, F. Karimi, L. Fu, A.L. Sanati, M. Alizadeh, C. Karaman et al., J. Hazard. Mater. (2021) https://doi.org/10.1016/j.jhazmat.2021.127058
C. Karaman, O. Karaman, B.B. Yola, İ Ülker, N. Atar, M.L. Yola, New J Chem. (2021) https://doi.org/10.1039/d1nj02293h
H. Medetalibeyoğlu, M. Beytur, S. Manap, C. Karaman, F. Kardaş, O. Akyıldırım et al., ECS J Solid State Sc. (2020) https://doi.org/10.1149/2162-8777/abbe6a
H. Sohrabi, O. Arbabzadeh, P. Khaaki, A. Khataee, M.R. Majidi, Y. Orooji, Crit Rev Food Sci. (2021) https://doi.org/10.1080/10408398.2021.1887077
S. Tajik, Y. Orooji, F. Karimi, Z. Ghazanfari, H. Beitollahi, M. Shokouhimehr et al., J Food Meas Charact. (2021) https://doi.org/10.1007/s11694-021-01027-0
A.A. Ensafi, H. Karimi-Maleh, S. Mallakpour, Electroanal (2011). https://doi.org/10.1002/elan.201000741
H. Karimi-Maleh, M. Keyvanfard, K. Alizad, M. Fouladgar, F. Gholami-Orimi, Int J Electrochem Sc 6, 6141 (2011)
T. Zabihpour, S.-A. Shahidi, H. Karimi-Maleh, A. Ghorbani-HasanSaraei, J Food Meas Charact. (2020) https://doi.org/10.1007/s11694-019-00353-8
S.S. Rajasree, X. Li, P. Deria, Commun Chem (2021). https://doi.org/10.1038/s42004-021-00484-4
X. Zhang, M.C. Wasson, M. Shayan, E.K. Berdichevsky, J. Ricardo-Noordberg, Z. Singh et al., Coordin Chem Rev. (2021) https://doi.org/10.1016/j.ccr.2020.213615
C.-W. Kung, T.-H. Chang, L.-Y. Chou, J.T. Hupp, O.K. Farha, K.-C. Ho, Electrochem. Commun. (2015) https://doi.org/10.1016/j.elecom.2015.06.003
Y. Xue, G. Zhao, R. Yang, F. Chu, J. Chen, L. Wang et al., Nanoscale (2021). https://doi.org/10.1039/d0nr09064f
Y. Zhao, L. Jiang, L. Shangguan, L. Mi, A. Liu, S. Liu, J Mater Chem A. (2018) https://doi.org/10.1039/c7ta07911g
B. Yuan, J. Zhang, R. Zhang, H. Shi, X. Guo, Y. Guo et al., Int. J. Electrochem. Sci. 10, 4899 (2015)
J.W. Yoon, J.H. Kim, C. Kim, H.W. Jang, J.H. Lee, Adv Energy Mater. (2021) https://doi.org/10.1002/aenm.202003052
L. Wang, X. Li, R. Yang, J.-J. Li, L.-B. Qu, Food Anal Method. (2020) https://doi.org/10.1007/s12161-020-01823-2
J. Guo, Y. Wan, Y. Zhu, M. Zhao, Z. Tang, Nano Res. (2020) https://doi.org/10.1007/s12274-020-3182-1
H.Y. Chen, T. Yang, F.Q. Liu, W.H. Li, Chem Sensor Actuat (2019). https://doi.org/10.1016/j.snb.2018.10.036
A. Cernat, M. Tertis, R. Sandulescu, F. Bedioui, A. Cristea, C. Cristea, Anal. Chim. Acta. (2015) https://doi.org/10.1016/j.aca.2015.05.044
B.P. Suma, M. Pandurangappa, Electroanal (2020). https://doi.org/10.1002/elan.202060091
P. Arul, N.S.K. Gowthaman, S.A. John, H.N. Lim, ACS Omega (2020). https://doi.org/10.1021/acsomega.9b03829
F.-Y. Yi, R. Zhang, H. Wang, L.-F. Chen, L. Han, H.-L. Jiang et al., Small Methods. (2017) https://doi.org/10.1002/smtd.201700187
B.S. He, D.D. Yan, Food Control (2019). https://doi.org/10.1016/j.foodcont.2019.04.001
A. Gahlaut, V. Hooda, A. Gothwal, V. Hooda, Crit Rev Anal Chem. (2019) https://doi.org/10.1080/10408347.2018.1461551
M. Tong, S. Dong, K. Wang, G. Suo, J Electrochem Soc. (2017) https://doi.org/10.1149/2.0911706jes
J.M. Gonçalves, P.R. Martins, D.P. Rocha, T.A. Matias, M.S.S. Julião, R.A.A. Munoz et al., J Mater Chem C. (2021) https://doi.org/10.1039/d1tc02025k
S. Fu, C. Zhu, J. Song, D. Du, Y. Lin, Adv Energy Mater. (2017) https://doi.org/10.1002/aenm.201700363
X. Zhou, Y. Zhou, Z. Hong, X. Zheng, R. Lv, Electroanal (2018). https://doi.org/10.1016/j.jelechem.2018.07.038
R.R. Salunkhe, Y.V. Kaneti, Y. Yamauchi, ACS Nano. (2017). https://doi.org/10.1021/acsnano.7b02796
K. Wang, C. Wu, F. Wang, C. Liu, C. Yu, G. Jiang, Electrochim Acta (2018). https://doi.org/10.1016/j.electacta.2018.07.228
W. Zhang, C. Ge, L. Jin, S. Yoon, W. Kim, G. Xu et al., Electroanal Chem. (2021) https://doi.org/10.1016/j.jelechem.2021.115163
H. Du, Z. Li, Y. Wang, Q. Yang, W. Wu, Food Rev Int. (2020) https://doi.org/10.1080/87559129.2020.1740733
L. Shi, N. Li, D. Wang, M. Fan, S. Zhang, Z. Gong, TrAC Trend Anal Chem. (2021) https://doi.org/10.1016/j.trac.2020.116131
X. Yang, M. Zhang, J. Xu, S. Wen, Y. Zhang, J. Zhang, A. Spectrochim Acta (2021). https://doi.org/10.1016/j.saa.2021.119553
J.-X. Wu, B. Yan, Ind. Eng. Chem. Res. (2018) https://doi.org/10.1021/acs.iecr.8b00762
C.J. Huang, Y.X. Ye, L.W. Zhao, Y.S. Li, J.L. Gu, J Inorg Organomet Polym (2019). https://doi.org/10.1007/s10904-019-01111-5
C.-H. Su, C.-W. Kung, T.-H. Chang, H.-C. Lu, K.-C. Ho, Y.-C. Liao, J Mater Chem A. (2016) https://doi.org/10.1039/c6ta03547g
D. Cheng, X. Li, Y. Qiu, Q. Chen, J. Zhou, Y. Yang et al., Anal Methods. (2017) https://doi.org/10.1039/c6ay03164a
S. Lu, H. Jia, M. Hummel, Y. Wu, K. Wang, X. Qi et al., RSC Adv. (2021) https://doi.org/10.1039/d0ra10522h
L.-M. Shi, J.-X. Pan, B. Zhou, X. Jiang, J Mater Chem B. (2015) https://doi.org/10.1039/c5tb01361e
S. Dong, D. Zhang, G. Suo, W. Wei, T. Huang, Anal. Chim. Acta. (2016) https://doi.org/10.1016/j.aca.2016.05.040
C.-W. Kung, Y.-S. Li, M.-H. Lee, S.-Y. Wang, W.-H. Chiang, K.-C. Ho, J Mater Chem A. (2016) https://doi.org/10.1039/c6ta02563c
M. Saraf, R. Rajak, S.M. Mobin, J Mater Chem A (2016). https://doi.org/10.1039/c6ta06470a
D.K. Yadav, V. Ganesan, P.K. Sonkar, R. Gupta, P.K. Rastogi, Electrochim Acta (2016). https://doi.org/10.1016/j.electacta.2016.03.092
J. Yang, L. Yang, H. Ye, F. Zhao, B. Zeng, Electrochim Acta. (2016) https://doi.org/10.1016/j.electacta.2016.10.071
B. Yuan, J. Zhang, R. Zhang, H. Shi, N. Wang, J. Li et al., Sensors Actuat B-Chem. (2016) https://doi.org/10.1016/j.snb.2015.08.100
S. Dong, M. Tong, D. Zhang, T. Huang, B.-Chem Sensors Actuat. (2017) https://doi.org/10.1016/j.snb.2017.05.047
A.T. Ezhil Vilian, B. Dinesh, R. Muruganantham, S.R. Choe, S.-M. Kang, Y.S. Huh et al., Microchim. Acta. (2017) https://doi.org/10.1007/s00604-017-2513-8
Y.C. Chen, W.H. Chiang, D. Kurniawan, P.C. Yeh, K.I. Otake, C.W. Kung, ACS Appl Mater Interfaces. (2019) https://doi.org/10.1021/acsami.9b11447
J. Liu, J. Int, Electrochem Sci. (2019) https://doi.org/10.20964/2019.02.16
B.P. Suma, M. Pandurangappa, J. Solid State Electr (2019). https://doi.org/10.1007/s10008-019-04454-8
S. Salagare, P. Shivappa Adarakatti, Y. Venkataramanappa, J. Int Environ An Ch. (2020) https://doi.org/10.1080/03067319.2020.1796989
Y.-C. Wang, Y.-C. Chen, W.-S. Chuang, J.-H. Li, Y.-S. Wang, C.-H. Chuang et al., ACS Appl Nano Mater. (2020) https://doi.org/10.1021/acsanm.0c02052
P. Arul, S.-T. Huang, V. Mani, Y.-C. Hu, Electrochim. Acta. (2021) https://doi.org/10.1016/j.electacta.2021.138280
T.-E. Chang, C.-H. Chuang, C.-W. Kung, Electrochem. Commun. (2021) https://doi.org/10.1016/j.elecom.2020.106899
J. Chen, S. Li, F. Xu, Q. Zhang, Electroanal (2021). https://doi.org/10.1002/elan.202100209
S. Huang, M. Lu, L. Wang, RSC Adv. (2021). https://doi.org/10.1039/d0ra09551f
H. Lei, H. Zhu, S. Sun, Z. Zhu, J. Hao, S. Lu et al., Electrochim. Acta. (2021) https://doi.org/10.1016/j.electacta.2020.137375
H.Y. Liu, J.J. Wen, H.X. Xu, Y.B. Qiu, Z.Z. Yin, L.H. Li et al., J AOAC Int. (2021) https://doi.org/10.1093/jaoacint/qsaa089
W. Qiu, H. Tanaka, F. Gao, Q. Wang, M. Huang, Adv Powder Technol. (2019) https://doi.org/10.1016/j.apt.2019.06.022
Z. Zhao, J. Zhang, W. Wang, Y. Sun, P. Li, J. Hu et al., Appl Surf Sci. (2019) https://doi.org/10.1016/j.apsusc.2019.04.202
S. Dong, Z. Li, Y. Fu, G. Zhang, D. Zhang, M. Tong et al., Electroanal Chem. (2020) https://doi.org/10.1016/j.jelechem.2019.113785
P. Lei, Y. Zhou, R. Zhu, S. Wu, C. Jiang, C. Dong et al., Microchim. Acta. (2020) https://doi.org/10.1007/s00604-020-04545-8
X. Meng, X. Xiao, H. Pang, Front Chem (2020). https://doi.org/10.3389/fchem.2020.00330
P.Y. Du, W. Gu, X. Liu, Dalton Trans (2016). https://doi.org/10.1039/c6dt01360k
Z. Qi, Q. You, Y. Chen, Anal. Chim. Acta. (2016) https://doi.org/10.1016/j.aca.2015.10.031
J. Sahoo, S.B. Waghmode, P.S. Subramanian, M. Albrecht, Chemistry Select (2016). https://doi.org/10.1002/slct.201600533
B. Ding, Y. Cheng, J. Wu, X.M. Wu, H.M. Zhang, Y. Luo et al., Dyes Pigments. (2017) https://doi.org/10.1016/j.dyepig.2017.07.044
X. Hao, Y. Liang, H. Zhen, X. Sun, X. Liu, M. Li et al., J Solid State Chem. (2020) https://doi.org/10.1016/j.jssc.2020.121323
H. Min, Z. Han, M. Wang, Y. Li, T. Zhou, W. Shi et al., Inorg Chem Front. (2020) https://doi.org/10.1039/d0qi00780c
P. Tapangpan, K. Panyarat, C. Chankaew, K. Grudpan, A. Rujiwatra, Inorg. Chem. Commun. (2020) https://doi.org/10.1016/j.inoche.2019.107627
S. Zhu, L. Zhao, B. Yan, J. Microchem (2020). https://doi.org/10.1016/j.microc.2020.104768
Y. Li, Y. Zhao, W. Zhang, K. Shao, H. Zhou, Z. Anorg. Allg. Chem. (2021) https://doi.org/10.1002/zaac.202100040
Funding
The authors acknowledge financial support from the Innovative and Entrepreneurial Talent of Jiangsu Province; the Science and Technology Innovation Cultivation Fund of Yangzhou University under Grant 2019CXJ189; and the “Lvyang Jinfeng” talents attracting plan.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Z., Zhong, Y., Zhou, X. et al. Metal-organic framework-based sensors for nitrite detection: a short review. Food Measure 16, 1572–1582 (2022). https://doi.org/10.1007/s11694-021-01270-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-01270-5