Abstract
This work was carried out to evaluate the microbial and chemical attributes as well as sensory characteristics of turkey breast meat coated with chitosan incorporated with 1% of Origanum vulgare essential oils (oregano EOs) and 1 or 2% of grape seed extract (GSE) stored at refrigerator for 20 days. Gas chromatography–mass spectrometry (GC–MS) assay represented that oregano EO is rich in phenolic compounds mainly carvacrol and thymol. Lipid oxidation, as showed by thiobarbituric acid reactive substances (TBARS) and total volatile basic nitrogen (TVBN) values were significantly (P < 0.05) lower in combined treatments containing both oregano EO and GSE which were 0.71 MDA/kg and 10.04 mg N/100 g, respectively in the chitosan containing 2% GSE and 1% oregano EO treatments. The minimum count of total viable count (TVC), Enterobacteriaceae, Pseudomonas spp., lactic acid bacteria (LAB) and yeast-mold were also determined in these treatments which determined 3.54–4.51 Log CFU/mL on day 20 of cold storage. These combined treatments also obtained the highest sensory scores (the overall acceptability was about 7) due to effective delaying microbial and oxidation activities. Therefore, chitosan-based coating containing GSE and oregano EOs can enhance microbial, chemical and organoleptic properties of fish turkey meat under refrigerated storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poultry meat (chicken and turkey) are considered as a good food commodity in the human diet, because they possess appropriate nutritional values (proper sources of macronutrients and micronutrients), low-fat and -cholesterol quantity and a rather high amount of polyunsaturated fatty acids (PUFA) [1, 2]. Therefore, this type of meat is preferred by consumers and its utilization has raised during the last decades all over the world compared to red meat [3].
However, poultry meat is known to be a perishable food item which is vulnerable to unfavorable microbial activities and oxidative reactions [4, 5]. For instance, it is verified that oxidation of PUFA in poultry products causes rancidity which results in off-flavor, off-odor as well as reduction in nutritional value and production of toxic compounds [6,7,8]. Moreover, microbial activities can cause food spoilage as well as food-borne diseases, which affects safety, sensory and nutritional characteristics of turkey meat [4, 9, 10].
Several methods has been introduced to inhibit the propagation of oxidative and microbial reactions of turkey meat products, including dietary supplementation of poultry with antioxidants before slaughter, application of appropriate packaging to restrict the access of oxygen to meat during storage and using synthetic antioxidants [6, 11].
In spite of extended application of synthetic antioxidants, it is reported that they may cause health risk like cancer for consumers [12, 13]. In consequent, a dramatically increased attention has been dragged towards using the natural plant-based compounds having both antioxidant and antimicrobial effects, instead of synthetic preservatives, in meat industry to extend the shelf life and quality in addition to enhance health benefits of such products [14, 15].
Essential oils (EOs) are secondary metabolites of plants, herbs and spices [16]. EOs and plant extracts are commonly used as natural antioxidant, antimicrobial and flavoring agents to enhance the product quality in addition to extend shelf life by delaying microbial and oxidative reactions [17]. However, they may have undesirable influences on sensorial properties of meat products in relatively high concentrations [18]. A promising approach to overcome this restriction is application of packaging materials as carrier of these agents [7]. Recently, incorporation of EOs and plants extracts into the edible coating and films, named as active packaging, has been investigated in several studies for preservation of food products [1, 4, 5, 19,20,21].
Edible films and coating manufactured from natural components like proteins, polysaccharides and lipids, or their combination, are an excellent alternatives of non-biodegradable plastics used commonly in food packaging because they are biodegradable, edible, environmental friendly and have low prices [22, 23]. Chitosan is a linear, non-toxic, biocompatible and biodegradable polysaccharide produced through deacetylation of the chitin existed in the crustacean shells [7, 24]. It is well known as an antimicrobial and antioxidant compound that is identified as generally recognized as safe (GRAS) [25, 26]. This biopolymer can successfully be used for production of edible films and coatings for food packaging [23].
Grape seed extract (GSE), a by-product of wine and grape juice industries, is known as a potent and natural antimicrobial and antioxidant agent, due to the presence of high amounts of proanthocyanidins, polyphenolic and flavonoids compounds [13, 17, 27, 28]. These components are able to scavenge free and active radical species, which result in antioxidant potentials in GSE, 20 and 50 times greater than vitamin E and vitamin C, respectively [6]. It is a promising alternative to conventional synthetic antioxidants in food sector [29]. Furthermore, it is reported that phenolic compounds present in GSE can prevent the growth of pathogenic and spoilage bacteria [30].
Oregano is a typical spice which has recognized for its antioxidative and antimicrobial activities [31]. It is produced through drying of leaves and flowers of Origanum vulgare species [3, 32]. Oregano EOs are characteristic phenols mainly carvacrol and thymol which show potent antimicrobial properties against both gram negative and gram positive bacteria [33]. Furthermore, it has been used as a natural antioxidant in different meat products [34]. It is worthy to note that Oregano EOs are recognized as GRAS [35, 36].
Recently, the application of different EOs like oregano or GSE directly or their incorporation into the chitosan films and coatings has been studied in different meat and their products including chicken, turkey, fish, lamb, pork and beef to enhance shelf life during cold storage [7, 17, 19, 37,38,39]. However, to best of our knowledge the combined incorporation of GSE and Oregano EOs into chitosan coating in meat and meat products have not been assessed until now. Therefore, the purpose of the present study is to investigate the effects of chitosan coating incorporated with oregano EOs and GSE extract on the microbial, chemical and sensory properties of turkey breast meat during refrigerated storage.
Material and methods
Preparation and analysis of oregano EOs
Origanum vulgare was obtained from Fasa, Fars province, Iran. Then, the dried parts of this herb was hydro-distillated using a Clevenger apparatus for about 3 h. The obtained EOs was dehydrated using anhydrous sodium sulfate and after filtration by nylon filter (0.22 µm) was kept at a refrigerator (4 °C) until analysis. The analysis of oregano EOs was carried out by gas chromatography-mass spectrometry (GC/MS) based on the method of Marriott, et al. (2001) [40].
Preparation of GSE
After purchasing grapes from a local market, the grape seeds were separated, washed and dried in laboratory atmosphere. Then, the seeds were milled using a grinder and passed through a sieve with mesh number of 60. After that, 250 g of homogenized grape seed powder was dissolved in 1 L of ethanol (99.6%, Merck, Germany) with stirring. The solution was placed in a shaker rotary at 150 rpm/min for 6 h and then was filtered through a Whatman paper (number 41). The transparent solution was placed in an evaporator at 40 °C and finally in a desiccator for removing all solvent. The resulting GSE was kept in a dark and sealed vial at 4 °C until application [10].
Turkey breast sample preparation
Skinned turkey breast fillet was purchased from a hyper market (Fasa, Iran). Samples (150 g, 3.5 cm of thickness) were separately prepared by a sterile knife.
Preparation of chitosan coating solution
Chitosan crab shell powder (molecular weight: 340, deacetylation degree: 75–85%) was obtained from Sigma-Aldrich Company, Germany. Chitosan coating solution (2% w/v) was prepared by dissolving powder in acetic acid (1% (w/w)). Glycerol (0.75% v/v), was added into chitosan coating solution, as a plasticizer. Tween 80 was used for coating solution containing oregano EOs, as a surfactant agent [41]. Oregano EOs (1% v/v) and GSE (1% and 2% v/v) were incorporated into chitosan coating solutions. Then, breast turkey fillets were immersed in the obtained chitosan coating solution for 2 min and the coated samples were dried in ambient temperature before packaging. In this study, seven samples were prepared: the blank sample (with sterile distilled water), sample with chitosan coating, and those coated with chitosan containing different amounts of oregano EOs (1%), GSE (1 and 2%) and their combination. The packaged samples were stored at a refrigerator (4 °C) for 20 days and examinations were performed during 4 day intervals.
Chemical analysis
pH measurement
According to Goulas and Kontominas [42], 10 g of minced turkey breast meat was mixed with 100 mL of distilled water thoroughly and filtered using Whatman paper. Then the pH of filtrate was measured using a pH-meter.
Measurement of total volatile basic nitrogen (TVBN)
TVBN was determined according to modified Jeon and Kamilf [43] method. For TVBN determination a micro-Kjeldahl distillation method was applied using digestion of minced samples with sulfuric acid. The TVBN was reported as mg of N per 100 g of turkey sample, using Eq. (1).
where, V1 = volume of sulfuric acid used for sample (mL), V2 = volume of sulfuric acid used for blank (mL), N = normality of sulfuric acid, and W = weight of sample (g).
Measurement of thiobarbituric acid reactive substances (TBARS)
The TMARS assay was conducted according to the method of Pikul et al. [44] with slight modification. This assay carry out normally for determination of secondary oxidation products (Mallon di aldehyde). Minced turkey breast sample (200 mg) was mixed with in a small volume of 1-butanol and then increased to volume 25 ml by the same solvent. After that, 5 mL of this solution was mixed with 10 mL of trichloroacetic acid (0.2%) reagent and kept in a hot water bath (95 °C) for 2 h. After cooling the solution (to room temperature), the absorbance was determined at 532 nm. TBARS value was calculated using Eq. (2).
where, A = Absorbance of sample solution, B = absorbance of blank solution and m = weight of minced sample (mg).
Microbiological analysis
Microbiological determination were conducted using Sallam [45] method with slight modifications. To this end, 10 g of turkey breast sample was aseptically cut and homogenized in a stomacher (for 2–3 min) containing 90 ml sterile peptone water solution (0.1%). Further serial dilutions were made from the prepared solution in the physiological saline solution (0.85% NaCl). The right dilutions were then applied for counting and separation of different particular microorganisms in the turkey samples, on days 0, 4, 8, 12 and 16 and 20 of refrigerated storage. All culture used for microbial determination were purchased from Sigma-Aldrich and Merck companies, Germany.
Total viable count (TVC)
TVC was performed by inoculating 0.1 ml of the solution homogenate, at certain dilutions, into duplicate sterile Plate Count Agar (PCA) using the surface spread plate technique, then the plates were incubated for 24 h at 32 °C [8].
Lactic acid bacteria (LAB)
For enumeration of LAB, diluted samples were plated on de Man, Rogosa, and Sharpe (MRS) agar and kept at 37 °C for 24–72 h under anaerobic conditions [8].
Pseudomonas
Pseudomonas spp. were determined on cephaloridine fucidin cetrimide (CFC) agar and incubated at 20 °C for 48 h [8].
Enterobacteriaceae
Enterobacteriaceae were counted by the pour plating method on Violet Red Bile Agar (VRBA). The plates then incubated at 37 °C for 24 h [8].
Yeast-mold
Yeast and mold counts were enumerated by surface spread plate technique on Rose-Bengal Chloramphenicol Agar. Then the plates were incubated at 25 °C for 72–120 h in dark condition [8].
Sensory analysis
The sensorial properties of roasted (10 min at 100 °C) turkey samples were investigated by ten laboratory trained panelist. The panels were not informed of the experimental evaluation and the samples were unknown. The evaluation of taste, color, odor, texture, and overall acceptability were conducted based on a hedonic scale ranking (1: dislike extremely to 9: like extremely) [46]. The panelist willingness to be participated has been considered.
Statistical analysis
In this study, all obtained data were analyzed by one way ANOVA and comparison of means was determined by Duncan tests using SPSS software (IBM SPSS statistics 20). P < 0.05 was considered significant. All experiments were done in triplicate and all of the data are reported as the mean ± standard deviation.
Results and discussion
Identification of oregano EOs
Nineteen components were determined in Origanum vulgare leaves by GC–MS method. The major agents of oregano EOs are presented in Table 1. The results showed that carvacrol (52.3%) and thymol (24.2%) were the most active components in oregano EOs. Furthermore, camphene (0.06%) and 1,8-cineole (0.05%) were composed the lowest percent of active compounds.
Van Haute et al. [47] and Karabagias et al. [31] reported similar results to our study. Although, Govaris et al. [48] determined carvacrol and thymol concentrations as 80.15% and 4.82%, respectively. The concentration and the differences between the EOs compositions identified in similar spice could be related to the differences in season, climate, geology and geography of plant' growth conditions and its maturity as well as differences in drying plant method and extraction method in different surveys [46].
Chemical evaluation
Meat products are known to be vulnerable to unwanted chemical reactions that indicate spoilage [49]. During storage, these products are susceptible to lipid oxidation reactions which could result in rancidity, reducing the quality and nutritional value as well as threating consumers' health by production of toxic compounds [7]. Thus, some chemical changes were measured by evaluation the values of pH, total volatile basic nitrogen (TVBN) and thiobarbituric acid reactive substances (TBARS) in all prepared samples during cold storage in days of 0, 4, 8, 12, 16 and 20.
pH value
The changes in pH values of all investigated treatments during the cold storage are illustrated in Fig. 1. The pH amounts of all treatments increased in the course of storage from 6.27–6.55 to 6.31–7.52, probably due to microbial activities. Furthermore, the presence of intrinsic enzymes such as lipase and protease could result in high production of specific amines like trimethylamine and ammonia in turkey meat [8].
Changes in pH values of turkey meat samples during cold storage. Control (C), chitosan (CH), chitosan with oregano (CH-O), chitosan with grape seed extract 1% (CH-GSE 1%), chitosan with grape seed extract 2% (CH-GSE 2%), chitosan with grape seed extract 1% and oregano (CH-GSE 1%-O), chitosan with grape seed extract 2% and oregano (CH-GSE 2%-O)
The pH amounts of the CH-GSE 2% and CH-GSE 2%-O treatments were significantly (P < 0.05) lower than those of other treatments during all cold storage times which reached to 6.32 and 6.31, respectively at the end of cold storage. It is worthy to note that increasing the pH value negatively affect the food quality principally in sensorial characteristics like taste, odor, color and texture. As shown in Fig. 1, it is obvious that treatments containing 2% of GSE (CH-GSE 2% and CH-GSE 2%-O) indicated greater antimicrobial activity in comparison with treatments treated with chitosan, GSE 1% or oregano alone or their combination which determined 7.52, 6.64, 6.64 and 6.56, respectively on day 20. Additionally, the higher pH value of control (6.55–7.31) and chitosan treatments (6.52–7.52) could be due to higher microbial growth amount of these treatments during storage. These results agree with those reported by Paparella et al. [50] for pork meat (containing oregano EOs) and by Amin and Edris [27] for minced beef (containing GSE). Xiong et al. [28] and Guan et al. [32] also suggested that the incorporation of GSE did not affect the pH amount of the coated pork meat and hairtail fish balls, respectively during cold storage.
TVBN
TVBN, which is mainly composed of products of protein breakdown including different types of amines like ammonia, is one of the most common value for quality evaluation of meat and meat products [33]. The Veterinary Organization of Iran reported the safe limit of TVBN in poultry meat as 28 mg/100 g [8]. Endogenous enzymatic activities and microbial growth in these products may lead to increasing in the TVBN value during storage. These activities cause protein decomposition and generation of volatile nitrogen compounds. Subsequently, using antioxidants and antimicrobial agents could prevent lipid oxidation and microbial growth, considerably in such products and then inhibit TVBN increasing during storage.
TVBN changes’ trend are presented in Fig. 2 as a function of treatments during 20 days of storage. The initial amounts of TVBN in the samples were in the range of 8.93–8.96 mg N/100 g. TVBN amounts depicted an raising progressively trend during 20 days of cold storage for all treatments and reached 32.58 mg N/100 g (higher than safe limit) in the control sample (C), the highest amount observed in the present compared to treated samples. The higher microbial counts in the control treatment could probably increase the amount of nitrogen bases in this sample [22]. The amount of TVBN in samples coated with chitosan increased from 8.95 to 19.26 mg N/100 g at the end of storage which was much higher than that in turkey meat samples containing oregano EO and GSE. As depicted in Fig. 2 the TVBN content of samples containing 1% oregano (CH-O) and samples containing 1% GSE (CH-GSE 1%) was 13.58 and 12.69 mg N/100 g on day 20 which means that the effect of GSE is more than oregano EO in inhibition of microbial growth and TVBN increasing. Furthermore, in the 20th days of storage, the lowest TVBN amount was determined in combined treatments i.e. chitosan containing 2% GSE and 1% oregano EO treatment (CH-2% GSE-O) followed by chitosan containing 1% GSE and 1% oregano EO (CH-1% GSE-O) which measured as 10.04 and 11.47 mg N/100 g, respectively. This may have occurred due to prevention of microbial growth and lipid oxidation by oregano EO and GSE to generate TVBN from amine groups. There was a positive relationship between the TVBN and pH values, as increasing in volatile amines results in increasing of pH, which is in accordance with Mehdizadeh and Langroodi [8] study.
This study exhibited lower TVBN amounts in treatments containing natural antimicrobial and antioxidative agents including oregano EO or GSE and specially both of them, in accordance with the studies done by Raeisi et al. (2020) [49] and Mehdizadeh and Langroodi [8].
The results observed in the present study revealed that treatments containing chitosan incorporated with oregano EO and GSE significantly decreased the generation of TVBN compounds due to the increased antibacterial activities. It is worthy to mention that, all treatments kept at 4 °C up to the end of the storage time had acceptable amount of TVBN, except control treatment. Vatavali et al. [33] studied the combined effects of chitosan and oregano EO on the quality of red porgy stored in ice. In treatments containing both chitosan and oregano EO, TVBN amounts were found lower significantly compared to other samples. Raeisi et al. (2015) [46] studied the effect of carboxymethyl cellulose edible coating containing Zataria multiflora EO (ZEO) and GSE on chemical properties of rainbow trout meat and reported that coating incorporated with ZEO and GSE had positive effect on decline in the production of TVBN.
TBARS
Malondialdehyde, the secondary products of lipid oxidation, considers as an indicator of the oxidation status and degree of rancidity [14]. TBARS value, is one of the most common index for evaluation of the lipid oxidation and the amount of malondialdehyde in meat [32]. The lipid oxidation has negative impacts on the organoleptic, functional, and nutritional characteristics of meats which can affect the shelf life of such products [4]. TBARS changes for all turkey breast treatments during 20 days of cold storage are shown in Fig. 3. The TBARS amounts for all treatments were in the range of 0.57–0.62 mg MDA/kg turkey meat at the initial time of storage. It should bear in mind that chitosan coating could efficiently prevent lipid oxidation as showed in the results of the present study while combination of chitosan with natural extract and EOs had stronger antioxidative effects on meat products [41].
As observed in Fig. 3, TBARS amounts were increased for all treatments throughout the storage period. This incremental trend for treatments CHO-2% GSE-O followed by CHO-1% GSE-O and CHO-2% GSE was lower than other groups which reached 0.71, 0.79 and 0.94 mg MDA/kg, respectively representing a very low level of lipid oxidation. This value exceeded to 5.89 mg MDA/kg at the 20th days of storage for control sample which is the highest amount compared to other treatments. The TVBN level of samples coated with chitosan (CH) also reached to 1.56 mg MDA/kg at the end of storage. After day eight, the TBARS value in the treatments contained both oregano and GSE (CH-GSE 1%-O and CH-GSE 2%-O), were significantly lower than other treatments (P < 0.05) which were 0.62 mg MDA/kg for both treatments. These results could be because of presence of high amounts of antioxidant agents like thymol and carvacrolere in oregano and proanthocyanidins, gallic acid, catechin and epicatechins in GSE, which had high antioxidant activity for inhibition of the lipid oxidation [32]. They probably show their antioxidant effects by scavenging of reactive oxygen species and chelating metals such as iron with their active groups. Reddy et al. [29] and guan et al. [32] study also revealed that the TBARS amount in hairtail fish balls sample treated with grape seed, sage and oregano extracts were significantly lower than that in control during 15 days of storage. Sogut and Syedim [51] also reported that chitosan film containing 15% GSE was effective in limiting lipid oxidation in chicken breast fillets which is in line with Reddy et al. [29] study. Xiong et al. [28] results also complied with those of the present study. They used chitosan–gelatin coating incorporated with nisin and GSE for preservation of fresh pork. They reported that coated samples containing GSE had the lowest TBARS values at day 20 due to high levels of phenolic compounds in the GSE acting as a strong antioxidant.
It is worthy to note that lipid oxidation is associated with off-flavor in meat products during storage time [31]. It is recommended that, TBARS value of 2–5 mg MDA/kg is considered the threshold for perceiving off-flavor for humans [2, 31, 52]. Such high TBARS amounts were not observed in the current study except for control treatment at the end of storage (5.89 mg MDA/kg). It is worthwhile to mention that, our results showed that TBARS amounts are correlated positively with TVBN values similar to Vatavali et al. [33] study. We also observed that GSE had more antioxidant effect than oregano (Fig. 2 and Fig. 3). As presented in Fig. 3 the TVBN content of CH-O and CH-GSE 1% was 1.29 and 1.16 mg MDA/kg, respectively.
Microbial evaluation
Alterations in TVC, LAB, Enterobacteriaceae, Pseudomonas spp., and yeast-mold counts of turkey meat samples are presented in Figs. 4, 5, 6, 7 and 8, respectively during 20 days of refrigerated storage. Microbial data are in good agreement with TVBN amounts as reported in Vatavali et al. [33] study. Spoilage of fresh meat is resulted from the specific spoilage organisms’ activity which generate metabolites causing off-flavors and off-odors and finally lead to food rejection by consumers. The shelf life of different types of meat including fresh poultry meat are usually restricted by microbial spoilage which are normally prevalent in spoiled meat flora [53]. The counts of these microorganisms have found better association with the shelf life of fresh meat than the TVC [45].
Generally, incorporation of GSE and EOs into coating materials intensify the antibacterial potent of chitosan against several spoilage microorganisms as well as food-borne pathogens [54]. The combination of both oregano and GSE showed a synergistic effect on the inhibition of the microbial growth in turkey meat (Figs. 4, 5, 6, 7 and 8). The synergistic effects of EOs and different extracts is not completely discovered although it is likely to inhibit bacteria by increasing the number and size of pores in cell membranes [8]. Phenolic agents of GSE and oregano decreased the growth rate of the microorganism [53]. The microbial counts were considerably different between the control and treated samples (Figs. 4, 5, 6, 7 and 8); representing a strong antimicrobial activity for oregano and particularly for GSE. The results of present study showed that the GSE has more intense antibacterial activity than oregano EO.
Previous studies have reported antibacterial effects of oregano EOs on different food pathogens and bacterial spoilage. Its antibacterial activity is demonstrated as an enhancement of permeability of cell membrane and a destruction of the cellular transmission [50]. For instance, the existence of components like thymol and carvacrol in oregano (Table 1) are main contributors to the promotion of antimicrobial activities [55]. The ability of oregano EO incorporated in different films and coating matrices like chitosan, to reduce microbial populations in food, has been investigated in several studies [36]. Antimicrobial mechanism of these compounds is correlated to their capacity to break the outer membrane of gram-negative bacteria, discharging lipopolysaccharides and enhancing the permeability of the cytoplasmic membrane. However, EOs are more affective against Gram-positive bacteria through phosphate ion leakage in their membrane [9, 54].
The antimicrobial characteristics of GSE can be triggered via interactions between its toxic phenolic and sulfhydryl groups of proteins in the bacterial cells. GSE could be mainly effective against different kinds of microbiota, with Gallic acid as the major active agent [53].
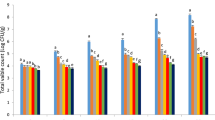
TVC
Figure 4 shows the TVC of turkey breast meat in all treatments during 20 days of cold storage period. The initial TVC varied from 3.68 to 7.91 Log CFU/g and the counts increased in the all samples throughout the cold storage time and reached to 4.32–7.91 Log CFU/g indicating good meat quality. TVC reached the values of 7.91 and 7.04 Log CFU/g on day 20 for the control (C) and chitosan coated (CH) samples which are significantly higher compared to other samples in all storage times. Unacceptable TVC (almost 6–8 CFU/g) were found in control and chitosan samples (from day 12) which determined 6.11 and 4.81 Log CFU/g, respectively. Significant differences (P < 0.05) in TVC between all treatments were observed from day 4 which varied between 3.68 and 5.02 Log CFU/g, respectively. The results of present study showed that GSE shows more antimicrobial activity than oregano. In this sense, the increase rate of TVC in groups containing 1% GSE (CH-GSE 1%) (1.14 Log CFU/g) were considerably lower than samples containing 1% oregano (CH-O) (2.15 Log CFU/g). Therefore, the addition of 1% oregano was not much sufficient to postpone the growth of total aerobic bacteria in raw turkey breast, coated in chitosan and stored up to 20 days cold storage. Obviously, simultaneous presence of GSE and oregano in combined treatments (CH-GSE 1%-O and CH-GSE 2%-O) resulted in the lowest TVC compared to other treatments during cold storage which were 4.43 and 4.32 Log CFU/g, respectively. Similar results have been observed for beef packed in chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus EO which were enhanced by increasing their concentrations [55]. Karimnezhad et al. [38] also reported that the increase rate of TVC in groups of chicken fillet treated with chitosan film containing oregano EO were notably lower compared to the control and chitosan alone groups.
Regarding to the amount of 7 Log CFU/g, which is the acceptable level of TVC amount for fresh poultry meat, the incorporation of both GSE and oregano into the chitosan coating was more efficient in decreasing the microbial growth of turkey breast meat during cold storage. In the study carried out by Paparella et al. [50] also proved that the initial TVC in control sample was 4.8 Log CFU/g and reached around 7 Log CFU/g at day 13 of cold storage. Their results showed that the combination of chitosan and 4% oregano EO restricted the growth of TVC during the storage in the packaged pork [50]. Furthermore, Raeisi et al. (2015) [46] reported that the TVC of control sample was much higher than 7 log CFU/g after 10 days of storage, while TVCs of fish fillet coated with carboxyl methyl cellulose containing different concentration of GSE and Zataria multiflora EO, did not surpass 7 Log CFU/g.
LAB counts
LAB are facultative anaerobic bacteria that regarded as a notable part of the poultry meat microflora. In present study, the initial LAB number of samples ranged from 3.18 Log CFU/g, in CH-GSE 2%-O, to 3.27 Log CFU/g in sample containing only chitosan. The LAB numbers reached to 7.32 and 7.22 Log CFU/g on day 20 of storage for the control and chitosan coated samples, respectively (Fig. 5). By incorporation of oregano and GSE in chitosan coating, this incremental trend reduced in all treatments. On the end of storage, the use of oregano, GSE 1% and GSE 2% resulted in a reduction of LAB counts by 0.44, 2.09 and 2.29 Log CFU/g, respectively, while the combination of oregano-GSE 1% and oregano-GSE 2% resulted in a reduction in LAB counts by 2.71 and 2.98 Log CFU /g, respectively. However, in the Chaleshtori and Chaleshtori [19] study, chitosan stopped growth of LAB, Psychrophilics and Enterobacteriacae in chicken meat, possibly due to the reaction between their amine groups and anionic groups of bacterial cell membrane, which will finally result in the death of bacteria.
CH-GSE 1%-O and CH-GSE 2%-O samples showed significantly lower (P < 0.05) LAB counts (2.81 and 3.08 Log CFU/g, respectively) in comparison to the control sample. Additionally, the growth profile of LAB demonstrated that control was the highest at 20th day (7.32 Log CFU/g), followed by chitosan (CH) sample (7.22 Log CFU/g). Furthermore, the LAB count of samples coated with chitosan containing 1% oregano EO (CH-O) and those incorporated with GSE 1% (CH-GSE 1%) was 6.78 and 5.13 Log CFU/g, respectively at the end of storage which represents higher antimicrobial activity of GSE than that oregano EO.
The results of Langroodi et al. [4], Chaleshtori and Chaleshtori [19] and Raeisi et al. (2015) [46] studies, regarding to LAB, are similar to our study. They reported that the incremental trend in LAB value of control samples were significantly higher than coated samples containing Zataria multiflora, lemon and oregano EOs, sumac extract or GSE. Completely uniform with our results, Mehdizadeh and Langroodi [8] informed approximately 1.5 Log cycle decline in LAB number after coating with chitosan containing Zataria multiflora EO and propolis extract.
Enterobacteriaceae count
The variations of Enterobacteriaceae count in all samples was similar to LAB count and TVC (Fig. 6). Enterobacteriaceae family is considered as a hygiene value in the meat products and form one of the main microflora of such products [56]. The initial number of Enterobacteriaceae were 3.11–3.31 and reached to 3.52–7.38 Log CFU/g at the end of storage. Moreover, significantly lower Enterobacteriaceae counts (P < 0.05) were observed for CH-GSE 1%-O and CH-GSE 2%-O treatments stored during 20 days of storage at 4 °C which were 3.71 and 3.52 Log CFU/g, respectively. Among all the treatments in the present study, CH-GSE 2%-O followed by CH-GSE 1%-O treatments were determined to be the most efficient in prohibition division of Enterobacteriaceae in turkey meat, resulting in approximately around 4 Log cycle reduction compared to chitosan coated and control samples, probably due to the synergistic antimicrobial influence of chitosan, GSE and oregano EO. On day of 20, Enterobacteriaceae counts reached the amount of 7.38, 7.14 and 5.16 Log CFU/g for control (C), chitosan (CH) and chitosan containing oregano samples (CH-O), respectively. Furthermore, Enterobacteriaceae counts were reached to 5.16 Log CFU/g in the presence of GSE 1%, to 4.39 Log CFU/g in the presence of GSE 2%. However, these counts in the combined samples containing both GSE and oregano i.e. CH-GSE 1%-O and CH-GSE 2%-O were reduced by ca. 3.43 and 3.62 Log CFU/g, respectively on the same day which showed higher antimicrobial activity compared to other treatments. Furthermore, the number of Enterobacteriaceae in the oregano containing samples (CH-O) was higher than those containing 1% GSE at the end of storage which were 6.23 and 5.16 Log CFU/g, respectively. Shekarforoush et al. [57] survey revealed that the chitosan coating containing oregano EO decline the growth of LAB, Psychrophilics, Enterobacteriacae and E. coli in chicken products. They also reported that chitosan alone did not demonstrate any inhibitory impact on the spoilage bacteria and Escherichia coli, although it was efficient against Listeria monocytogenes. In agreement with Mayeli et al. [56], the counts of Enterobacteriaceae bacteria during the time of storage in the control sample was significantly higher than other treated samples. Vatavali et al. [33] also reported that, the combination of chitosan and oregano EO resulted in a reduction of 1.8 Log CFU/g (P < 0.05) of Enterobacteriacae population in red porgy stored in ice.
Pseudomonads counts
Pseudomonas species, as one of the main microflora of poultry meat in the preservation storage which have proteolysis properties, are responsible for spoilage of raw meat when their numbers are between 7 and 8 Log CFU/g [8]. The Pseudomonads counts were presented in Fig. 7 over the 20 days of cold storage. In the present survey, the initial pseudomonas counts were ranged from 3.94 Log CFU/g, in CH-GSE 2% treatment, to 4.11 Log CFU/g in the control group. Both oregano EO and GSE showed high antimicrobial activity against the Pseudomonads. As shown in Fig. 7, the Pseudomonads counts of CH-O and CH-GSE 1% was similar indicating same effect of them in inhibition growth of Pseudomonas which was determined 4.35 and 4.25 Log CFU/g, respectively at the end of cold storage. Furthermore, using chitosan coating led to reduction of 1.87 Log CFU/g in the Pseudomonads counts compared to control sample. The pseudomonas count of all the samples increased until the 20th day in exception of combined treatments i.e. CH-GSE 1%-O and CH-GSE 2%-O. Until day 20 of storage the Pseudomonads counts in combined treatments i.e. CH-GSE 1%-O and CH-GSE 2%-O were lower significantly (P < 0.05) than those in other treatments which declined from 3.95 and 3.94 to 3.89 and 3.81 Log CFU/g, respectively. These outputs are in accordance with previous investigations that stated the diminution of Pseudomonas spp. in fresh meat coated with chitosan and EOs [38, 58]. These results are also in agreement with Mehdizadeh and Langroodi [8] study which showed strong antibacterial effect of chitosan containing both propolis extract and Zataria multiflora EO. In the Sogut and Syedim [51] and Alves et al. [37] studies, the Pseudomonas number in the control fish sample also reached the highest count at the end of storage, and the treated samples showed significantly lower counts, undoubtedly due to the combined antimicrobial effect of GSE and chitosan throughout the period of storage.
Yeast-mold counts
The alterations in yeast-mold count of turkey meat as a function of treatment and storage period is presented in Fig. 8. The primary number of yeast-mold in turkey meat samples were 3.15–3.24 Log CFU/g. The yeast-mold counts of all turkey samples increased significantly throughout the 20 days of cold storage. Not surprisingly, the yeast-mold counts of control sample (C) exhibited the fastest growth during the cold storage and was significantly greater (P < 0.05) than treated samples since day 8 and reached to 7.21 Log CFU/g on day 20. Using chitosan for coating samples resulted in 1.64 decline cycle in the number of yeast-mold during 20 days of cold storage. Furthermore, our results showed that 1% GSE is a little effective in inhibition of yeast-mold growth than 1% oregano EO which reached to 4.83 and 4.62 Log CFU/g, respectively. In this study, strong antibacterial effects of chitosan coating containing both GSE and oregano EOs were shown due to their synergistic effects as well as their controlled release during storage. In this regard, the CH-GSE 1%-O and CH-GSE 2%-O samples showed the lowest yeast-mold counts compared to other samples and reached to 4.39 and 4.27 Log CFU/g, respectively at the end of storage time. Yan et al. [54] also reported that application of EOs also meaningfully enhanced the antifungal activity of chitosan coating. Similar to our results, Langroodi et al. [4] also reported that, the highest counts of yeasts-molds were found in control, followed by chitosan treatment and the lowest numbers were observed in combined samples containing both sumac extract and Zataria multiflora EO which were in good agreement with Cleshtori and Chaleshtori [19], Chouliara et al. and Chouliara et al. [3] studies.
Sensory evaluation
Table 2 is shown the changes in sensory properties of different treatments of turkey meat all over the storage time at refrigerated temperature. All samples were investigated based on nine points hedonic scale. The scores lower than 7 are considered as unacceptable for consumers. As presented in this table, the sensory scores indicated a significant reduction (P < 0.05) in all the samples during 20 days of the cold storage due to the microbial activities and chemical alterations in the treatments.
The initial scores for sensory evaluation (taste, color, odor, texture, and overall acceptance) of the turkey meat treated were slightly altered by the oregano and GSE addition except for color which determined 6.4–9. Regarding to initial scores of turkey meat, addition of oregano and GSE, especially in combined treatments, significantly affects the color and decreases scores and reached to 0–7. The color and overall characteristics of control sample were obtained unacceptable scores after 4 days (6.4 and 5.3, respectively), while the texture and odor properties were unacceptable after 8 days of cold storage (6.5 and 6.2, respectively). Regarding to all sensory characteristics, the results showed that the coated samples containing both oregano and GSE i.e. CH-GSE 1%-O and CH-GSE 2%-O depicted higher consumer scores compared with other samples which were 4.7–7.4. In the present study, the control sample followed by chitosan samples obtained lower scores in comparison to the other treatments during the storage time which were 0–1.9.
In the case of overall acceptability, it is concluded that the shelf life of turkey meat can be increased using chitosan enriched with GSE and oregano, by approximately 16 (CH-GSE2 2% and CH-GSE 1%-O) and 20 (CH-GSE 2%-O) days. The scores calculated for overall acceptability of CH-GSE 2%, CH-GSE 1%-O and CH-GSE 2%-O were 5.8, 6.6 and 7, respectively. The sensory evaluation results presented to be associated with chemical and microbial investigations. Given the high microbial counts along with lipid oxidation, the control samples of turkey meat showed lower scores regarding to sensory characteristics. Our results in good agreement with Mayeli et al. [56], Rezaeifar et al. [18] and Mehdizadeh and Langroodi [8] studies.
Conclusion
The present study concluded that coating of turkey breast meat with chitosan incorporated with GSE and oregano EOs was effective against the propagation of different kinds of spoilage microorganisms including TVC, Enterobacteriaceae, Pseudomonas spp., lactic acid bacteria and yeast-mold counts. It also postponed lipid oxidation and maintained sensory properties and subsequently prolonged the shelf life of the fresh turkey meat during cold storage. Therefore, chitosan incorporated with oregano EOs and GSE could be utilized as efficient coating organic for preservation of turkey meat under refrigerated storage.
References
G. Vasilatos, I. Savvaidis, Chitosan or rosemary oil treatments, singly or combined to increase turkey meat shelf-life. Int. J. Food Microbiol. 166(1), 54–58 (2013)
R. Bagheri, P. Ariaii, A. Motamedzadegan, Effects of chitosan incorporated with basil seed gum and nettle (Urtica dioica L.) essential oil on the quality of beef burger during refrigerated storage. J. Food Meas. Charact. 15(1), 256–264 (2020)
E. Chouliara, A. Karatapanis, I.N. Savvaidis, M.G. Kontominas, Combined effect of oregano essential oil and modified atmosphere packaging on shelf-life extension of fresh chicken breast meat, stored at 4 C. Food Microbiol. 24(6), 607–617 (2007)
A.M. Langroodi, H. Tajik, T. Mehdizadeh, M. Moradi, E. Moghaddas Kia, A. Mahmoudian, Effects of sumac extract dipping and chitosan coating enriched with Zataria multiflora Boiss. oil on the shelf-life of meat in modified atmosphere packaging. LWT-Food Sci. Technol. 98, 372–380 (2018)
M. Mielnik, E. Olsen, G. Vogt, D. Adeline, G. Skrede, Grape seed extract as antioxidant in cooked, cold stored turkey meat. LWT-Food Sci. Technol. 39(3), 191–198 (2006)
X. Ao, I. Kim, Effects of grape seed extract on performance, immunity, antioxidant capacity, and meat quality in Pekin ducks. Poultry Sci. 99(4), 2078–2086 (2020)
M. Hashemi, S. Daneshamooz, M. Raeisi, B. Jannat, S. Taheri, S.M.A. Noori, An overview on antioxidants activity of polysaccharide edible films and coatings contains essential oils and herb extracts in meat and meat products. Adv. Anim. Vet. Sci. 8(2), 198–207 (2020)
T. Mehdizadeh, A.M. Langroodi, Chitosan coatings incorporated with propolis extract and Zataria multiflora Boiss. oil for active packaging of chicken breast meat. Int. J. Biol. Macromol. 141, 401–409 (2019)
M. Boskovic, N. Zdravkovic, J. Ivanovic, J. Janjic, J. Djordjevic, M. Starcevic, M.Z. Baltic, Antimicrobial activity of thyme (Tymus vulgaris) and oregano (Origanum vulgare) essential oils against some food-borne microorganisms. Procedia Food Sci. 5, 18–21 (2015)
M. Moradi, H. Tajik, S.M. Razavi Rohani, A.R. Oromiehie, Effectiveness of Zataria multiflora Boiss. essential oil and grape seed extract impregnated chitosan film on ready-to-eat mortadella-type sausages during refrigerated storage. J. Sci. Food Agric. 91(15), 2850–2857 (2011)
X. Tang, D.A. Cronin, The effects of brined onion extracts on lipid oxidation and sensory quality in refrigerated cooked turkey breast rolls during storage. Food Chem. 100(2), 712–718 (2007)
M.R. Loizzo, R. Tundis, F. Menichini, G. Duthie, Anti-rancidity effect of essential oils, application in the lipid stability of cooked turkey meat patties and potential implications for health. Int. J. Food Sci. Nutr. 66(1), 50–57 (2015)
J.M. Lorenzo, R.M. González-Rodríguez, M. Sánchez, I.R. Amado, D. Franco, Effects of natural (grape seed and chestnut extract) and synthetic antioxidants (buthylatedhydroxytoluene, BHT) on the physical, chemical, microbiological and sensory characteristics of dry cured sausage “chorizo.” Food Res. Int. 54(1), 611–620 (2013)
S. Khorshidi, T. Mehdizadeh, M. Ghorbani, The effect of chitosan coatings enriched with the extracts and essential oils of Elettaria cardamomum on the shelf-life of chicken drumsticks vacuum-packaged at 4 °C. J. Food Sci. Technol. (2020). https://doi.org/10.1007/s13197-020-04794-8
S. Kaur, S. Kumar, Z.F. Bhat, A. Kumar, Effect of pomegranate seed powder, grape seed extract and tomato powder on the quality characteristics of chicken nuggets. Nutr. Food Sci. (2015). https://doi.org/10.1108/NFS-01-2015-0008
M. Oussalah, S. Caillet, L. Saucier, M. Lacroix, Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci. 73(2), 236–244 (2006)
M. Ghanbari, A. Motallebi, N. Rokni, A. Anvar, Evaluation of red grape seed essential oil nanoemulsion (Vitis vinefera) on the shelf life of fresh packaged chicken fillets during refrigerated storage at 4 °C. Arch. Pharm. Pract. 1, 120 (2020)
M. Rezaeifar, T. Mehdizadeh, A. Mojaddar Langroodi, F. Rezaei, Effect of chitosan edible coating enriched with lemon verbena extract and essential oil on the shelf life of vacuum rainbow trout (Oncorhynchus mykiss). J. Food Saf. 40, e12781 (2020)
F.S. Chaleshtori, R.S. Chaleshtori, Antimicrobial activity of chitosan incorporated with lemon and oregano essential oils on broiler breast meat during refrigerated storage. Nutr. Food Sci. (2017). https://doi.org/10.1108/NFS-08-2016-0123
S. Jafarzadeh, S.M. Jafari, A. Salehabadi, A. Nafchi Mohammadi, U.S. Uthaya, A. Khalil, Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. (2020). https://doi.org/10.1016/j.tifs.2020.04.017
L.X. Mei, A. Nafchi Mohammadi, F. Ghasemipour, E. Azhar Mat, S. Jafarzadeh, A.A. Al-Hassan, Characterization of pH sensitive sago starch films enriched with anthocyanin-rich torch ginger extract. Int. J Biol. Macromol. 164, 4603–4612 (2020)
M. Esmaeili et al., Comparison of coating and nano-coating of chitosan-Lepidium sativum seed gum composites on quality and shelf life of beef. J. Food Meas. Charact. 15(1), 341–352 (2020)
E. Montaño-Sánchez, B.D.M. Torres-Martínez, R.D. Vargas-Sánchez, N. Huerta-Leidenz, A. Sánchez-Escalante, M.J. Beriain, G.R. Torrescano-Urrutia, Effects of chitosan coating with green tea aqueous extract on lipid oxidation and microbial growth in pork chops during chilled storage. Foods 9(6), 766 (2020)
S.R. Kanatt, M.S. Rao, S.P. Chawla, A. Sharma, Effects of chitosan coating on shelf-life of ready-to-cook meat products during chilled storage. LWT-Food Sci. Technol. 53(1), 321–326 (2013)
M. Gökmen, Ü. Gürbüz, Use of chitosan in Turkish sausage (sucuk) production and effects on quality. Kafkas Univ. Vet. Fak. Derg. 17(Suppl A), 67–71 (2011)
V.K. Juneja, H. Thippareddi, L. Bari, Y. Inatsu, S. Kawamoto, M. Friedman, Chitosan protects cooked ground beef and turkey against Clostridium perfringens spores during chilling. J. Food Sci. 71(6), 236–240 (2006)
R.A. Amin, S.N. Edris, Grape seed extract as natural antioxidant and antibacterial in minced beef. PSM Biol. Res. 2(2), 89–96 (2017)
Y. Xiong, M. Chen, R.D. Warner, Z. Fang, Incorporating nisin and grape seed extract in chitosan-gelatine edible coating and its effect on cold storage of fresh pork. Food Control 110, 107018 (2020)
G.B. Reddy, A.R. Sen, P.N. Nair, K.S. Reddy, K.K. Reddy, N. Kondaiah, Effects of grape seed extract on the oxidative and microbial stability of restructured mutton slices. Meat Sci. 95(2), 288–294 (2013)
J. Libera, A. Latoch, K.M. Wójciak, Utilization of grape seed extract as a natural antioxidant in the technology of meat products inoculated with a probiotic strain of LAB. Foods 9(1), 103 (2020)
I. Karabagias, A. Badeka, M. Kontominas, Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 88(1), 109–116 (2011)
W. Guan, X. Ren, Y. Li, L. Mao, The beneficial effects of grape seed, sage and oregano extracts on the quality and volatile flavor component of hairtail fish balls during cold storage at 4 °C. LWT 101, 25–31 (2019)
K. Vatavali, L. Karakosta, C. Nathanailides, D. Georgantelis, M.G. Kontominas, Combined effect of chitosan and oregano essential oil dip on the microbiological, chemical, and sensory attributes of red porgy (Pagrus pagrus) stored in ice. Food Bioprocess Technol. 6(12), 3510–3521 (2013)
L. Karre, K. Lopez, K.J. Getty, Natural antioxidants in meat and poultry products. Meat Sci. 94(2), 220–227 (2013)
K.G. Zinoviadou, K.P. Koutsoumanis, C.G. Biliaderis, Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 82(3), 338–345 (2009)
M. Llana-Ruiz-Cabello, S. Pichardo, J.M. Bermudez, A. Baños, J.J. Ariza, E. Guillamón, S. Aucejo, A.M. Cameán, Characterisation and antimicrobial activity of active polypropylene films containing oregano essential oil and Allium extract to be used in packaging for meat products. Food Addit. Contam. A. 35(4), 783–792 (2018)
V.L. Alves, B.P.M. Rico, R.M.S. Cruz, A.A. Vicente, I. Khmelinskii, M.C. Vieira, Preparation and characterization of a chitosan film with grape seed extract-carvacrol microcapsules and its effect on the shelf-life of refrigerated Salmon (Salmo salar). LWT 89, 525–534 (2018)
F. Karimnezhad, V. Razavilar, A.A. Anvar, S. Dashtgol, A. Pilehvar Zavareh, Combined effect of chitosan-based edible film containing oregano essential oil on the shelf-life extension of fresh chicken meat. J. Nutr. Food Secur. 4(4), 236–242 (2019)
P. Hassanzadeh, M. Moradi, N. Vaezi, M.H. Moosavy, R. Mahmoudi, Effects of chitosan edible coating containing grape seed extract on the shelf-life of refrigerated rainbow trout fillet. in Veterinary Research Forum (Faculty of Veterinary Medicine, Urmia University, Urmia, 2018)
P.J. Marriott, R. Shellie, C. Cornwell, Gas chromatographic technologies for the analysis of essential oils. J. Chromatogr. A. 936(1–2), 1–22 (2001)
A.M. Langroodi, H. Tajik, T. Mehdizadeh, Preservative effects of sumac hydro-alcoholic extract and chitosan coating enriched along with Zataria multiflora Boiss. essential oil on the quality of beef during storage. in Veterinary Research Forum (Faculty of Veterinary Medicine, Urmia University, Urmia, 2018)
A.E. Goulas, M.G. Kontominas, Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): biochemical and sensory attributes. Food Chem. 93(3), 511–520 (2005)
Y.-J. Jeon, J.Y. Kamil, F. Shahidi, Chitosan as an edible invisible film for quality preservation of herring and Atlantic cod. J. Agric. Food Chem. 50(18), 5167–5178 (2002)
J. Pikul, D.E. Leszczynski, F.A. Kummerow, Evaluation of three modified TBA methods for measuring lipid oxidation in chicken meat. J. Agric. Food Chem. 37(5), 1309–1313 (1989)
K.I. Sallam, Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control 18(5), 566–575 (2007)
M. Raeisi, H. Tajik, J. Aliakbarlu, S.H. Mirhosseini, S.M.H. Hosseini, Effect of carboxymethyl cellulose-based coatings incorporated with Zataria multiflora Boiss. essential oil and grape seed extract on the shelf life of rainbow trout fillets. LWT-Food Sci. Technol. 64(2), 898–904 (2015)
S. Van Haute, K. Raes, P. Van der Meeren, I. Sampers, The effect of cinnamon, oregano and thyme essential oils in marinade on the microbial shelf life of fish and meat products. Food Control 68, 30–39 (2016)
A. Govaris, N. Solomakos, A. Pexara, P.S. Chatzopoulou, The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. Int. J Food Microbiol. 137(2–3), 175–180 (2010)
M. Raeisi, M. Hashemi, M. Aminzare, F. Ghorbani Bidkorpeh, M. Ebrahimi, B. Jannat, B. Tepe, S.M.A. Noori, Effects of sodium alginate and chitosan coating combined with three different essential oils on microbial and chemical attributes of rainbow trout fillets. J. Aquat. Food Prod. Technol. 29(3), 253–263 (2020)
A. Paparella, G. Mazzarrino, C. Chaves-López, C. Rossi, G. Sacchetti, O. Guerrieri, A. Serio, Chitosan boosts the antimicrobial activity of Origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol. 59, 23–31 (2016)
E. Sogut, A.C. Seydim, The effects of Chitosan and grape seed extract-based edible films on the quality of vacuum packaged chicken breast fillets. Food Packag. Shelf Life 18, 13–20 (2018)
E. Cagdas, S. Kumcuoglu, Effect of grape seed powder on oxidative stability of precooked chicken nuggets during frozen storage. J. Food Sci. Technol. 52(5), 2918–2925 (2015)
S. Banon, P. Díaz, M. Rodríguez, M.D. Garrido, A. Price, Ascorbate, green tea and grape seed extracts increase the shelf life of low sulphite beef patties. Meat Sci. 77(4), 626–633 (2007)
G. Yuan, X. Chen, D. Li, Chitosan films and coatings containing essential oils: the antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 89, 117–128 (2016)
T. Mehdizadeh, H. Tajik, A. Mojaddar Langroodi, R. Molaei, A. Mahmoudian, Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 163, 108073 (2020)
M. Mayeli, T. Mehdizadeh, H. Tajik, F. Esmaeli, A. Mojaddar Langroodi, Combined impacts of zein coating enriched with methanolic and ethanolic extracts of sour orange peel and vacuum packing on the shelf life of refrigerated rainbow trout. Flavour Fragr. J. 34(6), 460–470 (2019)
S.S. Shekarforoush, S. Basiri, H. Ebrahimnejad, S. Hosseinzadeh, Effect of chitosan on spoilage bacteria, Escherichia coliand Listeria monocytogenes in cured chicken meat. Int. J. Biol. Macromol. 76, 303–309 (2015)
K. Ünal, A.S. Babaoglu, M. Karakaya, Effect of oregano, sage and rosemary essential oils on lipid oxidation and color properties of minced beef during refrigerated storage. J. Essent. Oil-Bear. Plants 17(5), 797–805 (2014)
Acknowledgements
The authors would like to thank the Fasa University of Medical Sciences for the financial grants of this study (IR. FUMS. REC. 1399. 114).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest was stated by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mojaddar Langroodi, A., Nematollahi, A. & Sayadi, M. Chitosan coating incorporated with grape seed extract and Origanum vulgare essential oil: an active packaging for turkey meat preservation. Food Measure 15, 2790–2804 (2021). https://doi.org/10.1007/s11694-021-00867-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-021-00867-0