Abstract
The objective of this study was to develop an edible coating formulated with sage seed gum (SSG) and lemon verbena (Aloysia citrodora) essential oil (EO) nanoemulsions (NEOs) to extend the shelf life of turkey meat. Coatings were prepared with 1.5% and 3% EO and their corresponding NEO forms. Subsequently, the characteristics of the coating solutions (antioxidant and antimicrobial activity) and the physicochemical (pH, peroxide value (PV), thiobarbituric acid (TBA)), microbial, and sensory qualities of coated turkey were investigated during 16 days of refrigerated storage. Gas chromatography-mass spectrometry (GC-MS) identified caryophyllene oxide, spathulenol, and ar-curcumene as the major components of (A) citrodora EO. The NEO exhibited a droplet size of approximately 94.3 nm and a zeta potential of -46.2 mV. The coating solution containing SSG and 3% NEO demonstrated the highest antioxidant activity compared to other formulations. The control group exhibited a significant increase in total PV and TBA values during storage. Conversely, the SSG-3% NEO group displayed the lowest PV (0.43 meq O2/kg fat) and TBA (0.75 mg MDA/kg) values. Furthermore, the incorporation of EO and NEO exhibited a dose-dependent inhibition of bacterial growth and enhanced the antimicrobial activity of the SSG coating against (B) cereus, S. aureus, P. aeruginosa, and E. coli. Additionally, SSG coatings containing EO/NEO improved the sensory quality of turkey meat, with the SSG-3% NEO-coated group receiving the highest sensory scores after 16 days. These findings suggest that the SSG-NEO edible active coating possesses promising applications as an antimicrobial and antioxidant agent for meat products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poultry meat is a valuable source of high-quality animal protein. In particular, more consumers have shown interest in turkey meat because of its favorable amino acid composition. However, turkey meat has high contents of polyunsaturated fatty acids (PUFAs) and is often associated with lipid oxidation, thereby making it the subject of quality deterioration [1]. Several studies have been reported on prolonging the shelf life of the meat of poultry and its products. These studies are substantial because meat quality can be affected by both microbial spoilage and chemical reactions, including lipid oxidation [2].
Edible films and coatings are versatile materials that are commonly employed to prolong the shelf life of foods. Primarily derived from biodegradable polymers such as polysaccharides (gums), proteins, and lipids, these materials represent a promising alternative for preserving food by acting as a barrier against adverse chemical reactions and microbial growth. Furthermore, they can serve as carriers for antimicrobials, antioxidants, and other functional additives [3].
Sage seed gum (SSG), a water-soluble polysaccharide isolated from the seeds of Salvia macrosiphon, is a promising material for edible coatings. This biopolymer comprises galactomannan with a mannose/galactose ratio of 1.78–1.93:1 and also contains uronic acid (28.2–32.2%) [4]. This environmentally friendly and biodegradable material forms high-viscosity aqueous solutions exhibiting pseudoplastic characteristics and a gel-like behavior, resulting from its unique molecular conformation and chemical structure of the polymer network [5].
Essential oils (EOs) are volatile, aromatic liquid extracts derived from various plant materials. The majority of EOs are considered safe for human consumption and are generally recognized as safe (GRAS) by regulatory authorities. Owing to the presence of phenolic compounds, terpenes, and terpenoids, many EOs exhibit antimicrobial and antioxidant properties [6].
A promising approach to extending the shelf life of meat products involves the incorporation of EOs into coating formulations to create active films or coatings. These active films or coatings facilitate the controlled release of EOs onto the food surface [7]. Traditionally, EOs have been directly incorporated into foods or food packaging materials (films or coatings). However, their inherent volatility can limit their efficacy and activity. Encapsulation of EOs offers a viable strategy to overcome this limitation [6].
Lemon verbena (Aloysia citrodora) is an aromatic shrub indigenous to South America, but also cultivated in the Caribbean and South Africa. It has a history of traditional medicinal usage due to the presence of bioactive compounds with antioxidant and antimicrobial properties that offer potential health benefits [8, 9]. The essential oils (EOs) of lemon verbena (LVEOs) are primarily composed of geranial, neral, spathulenol, and limonene, and these EOs exhibit promising antimicrobial and antioxidant activity [10]. Several factors, including genotype, environmental conditions, and growth stages, can influence the composition of LV EOs [11].
To the best of our knowledge, this investigation represents the first application of sodium salt of carboxymethyl cellulose (SSG) coatings containing liquid-vapor low-energy oil nanoemulsions (LVLEOs) for the preservation of turkey meat. Therefore, the present study aimed to evaluate the effects of SSG coatings incorporating different concentrations of unencapsulated and nanoencapsulated LVLEO on the physicochemical, microbiological, and organoleptic characteristics of fresh turkey meat during refrigerated storage.
Materials and methods
Essential oils extraction from lemon verbena leaves
Approximately 60 g of lemon verbena (Aloysia citrodora) leaves were pulverized and subjected to hydrodistillation using a Clevenger-type apparatus. Deionized water (100 mL) was added to the apparatus, and the essential oil was extracted for 3 h at a condensation rate of 1 mL/min. Following extraction, the isolated essential oil was stored at 4 °C under darkness [8].
Gas chromatography/mass spectrometry (GC/MS) analysis
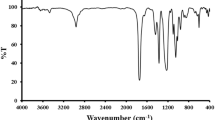
The analysis of extracted essential oil components was performed using gas chromatography (Agilent 6890) coupled to mass spectrometry (Agilent 5973 N) equipped with a BPX5 column. A separate analysis of the LVLEO was conducted using a Thermo Quest Finnigan gas chromatograph interfaced with a mass spectrometer (GC-MS) system operating in electron ionization (EI) mode. The column utilized was an HP-5MS fused silica capillary column with a stationary phase of 5% phenyl-methylsiloxane (30 m x 0.25 mm x 0.25 μm film thickness). Helium served as the carrier gas at a constant flow rate of 1.2 mL/min. The following parameters were used for the analysis: injector temperature, 290 °C; column temperature programmed from 50 °C to 265 °C at a ramp rate of 2.5 °C/min; injected volume, 1 µL with a solvent volume of 2 µL of dichloromethane; and split ratio, 1:10. The mass spectrometry acquisition parameters were: ionization mode, EI; ionization energy, 70 eV; scan range, 30–550 amu; and ion source temperature, 220 °C. The identification of chemical compounds within the LVLEO was achieved using the same Thermo Quest Finnigan GC system, employing the aforementioned capillary column and the identical analytical conditions described above.
Encapsulation of EOs
Nanoemulsions were prepared based on the method reported by Gholamhosseinpour and Hashemi [12], with slight modifications. Essential oil (EO) (2.5% w/v) and Tween 20 (at a concentration of 7.5% w/v relative to EO) were dissolved together under magnetic stirring until complete solubilization was achieved. The mixture was then subjected to high-energy ultrasonication (Vcleaner, Iran) for a duration of 7 min to achieve homogenization.
Characterization of nanoparticles
Droplet size, PDI and zeta potential
The mean droplet size, polydispersity index (PDI), and zeta potential of the nanoparticles were characterized using dynamic light scattering (DLS) (SZ-100, Horiba, Japan) at 25 °C.
Antioxidant activity
Free radical scavenging activity of DPPH
The radical scavenging capacity of LVLEO leaves and nanoemulsions was assessed using 2,2-diphenyl-1-picrylhydrazyl (DPPH), a stable free lipophilic radical. This experiment investigated the hydrogen or electron donation ability of EO and nanoemulsions, which can convert DPPH to its reduced form, DPPH-H.
The DPPH free radical scavenging assay was performed as follows. One milliliter (1 mL) of a 0.1 mM DPPH solution in 95% methanol was mixed with 3.0 mL of EO or nanoemulsion at various concentrations ranging from 0.05 to 10 µg/mL. The mixtures were then incubated in the dark at room temperature for 2 h. Absorbance (A) at 517 nm was measured using a UV-vis spectrophotometer. A negative control group, containing neither EO nor nanoemulsion, was included. Gallic acid served as a positive control, and its IC50 value was determined. The IC50, defined as the half-maximal inhibitory concentration, represents the concentration of a substance required to scavenge 50% of the DPPH free radicals. The following formula was used to calculate the percentage of antioxidant activity:
Antioxidant activity (Inhibition%) = [(Acontrol- Agroup)/ Acontrol) × 100] [13]. (Eq. 1)
Ferric reducing antioxidant power (FRAP) assay
The ferric reducing antioxidant power (FRAP) assay was conducted in accordance with the methodology delineated by Azizkhani et al. [13]. The FRAP reagent stock solution was prepared by amalgamating 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) (10 mM) dissolved in 40 mM HCl (10:1 v/v), 0.3 M acetate buffer (pH 3.6), and 20 mM FeCl3 in H2O (1:1 v/v). Subsequently, 900 µL of this solution was preheated to 37 °C and combined with 100 µL of EO or nanoemulsion at various concentrations (0.2–10 µg/mL). Following incubation for 30 min at 37 °C, the absorbance was quantified at 595 nm. The reduction of the ferric tripyridyltriazine (Fe³⁺-TPTZ) complex to its ferrous (Fe²⁺) form results in the formation of an intense blue color. A calibration curve was constructed utilizing FeSO4 solutions ranging from 100 to 1000 µM. The experiment was conducted in triplicate, and FRAP values were computed using Eq. 2.
FRAP = (∆Agroup/ ∆Astandard) × FRAP value of the standard (µM) (Eq. 2).
Preparation of sage seed gum coating solution
To prepare the coating solution, a 1.5% (w/w) sodium silicate gel (SSG) was dissolved in 100 mL of deionized water under magnetic stirring (D500, ALFA, Iran) for 30 min, as previously described [4]. Subsequently, different concentrations (1.5% and 3%) of essential oil (EO) and nanoemulsion (NEO) were incorporated into the coating solution mixture. Finally, 30 wt% (based on dry matter) of glycerol was added to the mixture with continued stirring for 25 min. A control SSG coating was prepared following the same procedure, but without the incorporation of EO or NEO [14].
Antimicrobial activity of coating solutions
The antibacterial activity of coating solutions incorporated with different concentrations of EOs and NEO was evaluated utilizing the disc diffusion method. Briefly, different suspensions of gram-positive (B. cereus and S. aureus) and gram-negative bacteria (P. aeruginosa and E. coli) were diluted and spread onto PCA. Subsequently, filter paper discs (6 mm) were impregnated with EOs/NEOs and placed on the surface of petri dishes. Then, all plates were incubated at 37 °C for 24 h. The antibacterial activity was expressed as the diameter of the inhibition zone (mm) [8].
Coating of Turkey
The turkey samples were prepared and divided into six groups, including a control group (non-coated), a group coated with SSG, a group coated with SSG-EO 1.5%, a group coated with SSG-EO3%, a group coated with SSG-1.5% NEO, and a group coated with SSG-NEO3%. For the coating process, the turkeys were immersed in the coating solution at ambient temperature for 3 min. Subsequently, each treatment was individually packaged in a LDPET bag and stored under refrigerated conditions at 4 ± 1 °C for 16 days [7].
Chemical properties
pH
Approximately 10 g of each meat group sample was combined with 90 mL of distilled water and homogenized at 13,000 rpm for 30 s. Subsequently, the pH value was determined utilizing a pH meter (3510 pH meter, GERMANY) at room temperature [15].
Peroxide value (PV) determination
Twenty-five mL of a chloroform acetic acid solution (with a 3:2 ratio of chloroform to acetic acid) were added to the oil extracted from the turkey meat samples in an Erlenmeyer flask. Subsequently, 0.5 mL of a saturated potassium iodide solution, 30 mL of distilled water, and 0.5 mL of a 1% starch solution were incorporated into the mixture, and the amount of released iodine was titrated against a sodium thiosulfate solution (0.1 N). The peroxide value was calculated utilizing the following Eq. 3 [16, 17]:
PV= (volume proportion × regularity × 100)/ (weight group proportion) (Eq. 3).
Thiobarbituric acid (TBA) measurement
The quantification of thiobarbituric acid reactive substances (TBARS) was conducted according to the method described by Smith and Alfawaz [18] with some modifications. Briefly, 5 g of ground turkey meat and 50 mL of distilled water were added to a distillation flask and stirred for two minutes. Subsequently, 47.5 mL of boiling water and 2.5 mL of 4 N hydrochloric acid were combined, and 5 mL of this mixture was transferred to a test tube containing 5 mL of distilled water and 5 mL of thiobarbituric acid reagent. A control sample was prepared in the same manner, substituting the meat sample with water. After being heated to 100 °C for 35 min in a water bath, the test tubes were cooled for 10 min. Following this, the absorbance at 538 nm was measured utilizing a spectrophotometer (UV 2150, Unico, China).
Microbial properties
Microbial analysis was conducted on days 0, 4, 8, 12, and 16 of refrigerated storage. Approximately 5 g of each meat group sample were combined with 45 mL of sterile water in a stomacher bag. The mixture was homogenized utilizing a homogenizer (Wiggins Company, D130 model, Germany) for 2 min, and the homogenate was subsequently diluted up to 105 mL with sterile diluent. Following this, 0.1 mL of each diluted homogenate was utilized for microbial analysis. The total bacterial count was determined by surface plating on plate count agar (PCA) after incubation at 37 °C for 48 h. For the enumeration of psychrotrophic bacteria, the same medium was utilized after incubation at 7 °C for 10 days [7]. The Enterobacteriaceae population was enumerated by surface plating on Violet Red Bile Glucose Agar and incubation at 37 °C for 24 h. Yeasts and molds were also enumerated by surface plating on Rose Bengal chloramphenicol selective agar and incubation at 25 °C for 3 days [1]. Microbiological counts were expressed as log colony-forming units per gram (log CFU/g) of the respective meat group samples.
Sensory properties
The coated turkey samples were subjected to grilling, and the sensory quality of the turkey coated with SSG films was evaluated by a panel of ten individuals in terms of sensorial attributes such as color, odor, and overall acceptability. A hedonic scale ranging from 1 to 9 points was utilized for scoring (1, dislike extremely to 9, like extremely) [19].
Statistical analysis
The statistical analysis of the data was conducted through one-way analysis of variance (ANOVA) utilizing SPSS software (version 16, SPSS Inc., Chicago, IL, USA). Multiple comparisons among means were performed employing Tukey’s test, and a p-value less than 0.05 was considered statistically significant. All data were presented as mean ± standard deviation of three replicates.
Results and discussion
Essential oil composition
The results of the essential oil analysis conducted by the gas chromatography-mass spectrometry (GC-MS) are presented in Table 1. The GC-mass analysis of the essential oil from lemon verbena (Aloysia citrodora) identified a total of 17 components. The major constituent of the essential oil derived from lemon verbena leaves was caryophyllene oxide (15.68%). Furthermore, other significant components comprised spathulenol (10.53%), ar-curcumene (8.49%), limonene (7.20%), and eucalyptol (7.09%) as the main components. Other components such as neral (4.29%) and E-caryophyllene (2.72%) were also identified. Additionally, the essential oils from lemon verbena leaves contained lower concentrations of carvotanacetone (1.85%), α-cadinol (1.81%), α-terpineol (1.79%), and α-copaene (1.09%).
These results were consistent with a study conducted by Sprea et al. [20], which identified citral isomers geranial, neral, and spathulenol, caryophyllene oxide, limonene, and ar-curcumene as major components of lemon verbena leaf essential oils (LVLEOs) obtained through hydrodistillation. As reported by Rashid et al. [11], monoterpenes (60.48%) were the predominant constituents of LVLEO, and D, L-limonene (18.80%) was identified as the major component, followed by the sesquiterpene hydrocarbon muurolene (14.13%). Moreover, other components such as transchrysanthenyl acetate (10.27%), verbenyl acetate (9.10%), 1,8-cineole (8.14%), β-caryophyllene (5.09%), and spathulenol (3.75%) were also present. Hosseini and Jamshidi [8] additionally claimed that eugenol, D-limonene, trans-citral, β-spathulenol, z-citral, eucalyptol, sulcatone, and caryophyllene were the principal compounds of the lemon verbena (leaves and buds) essential oil. A similar profile of components was also reported by Ayran and Çelik [9] for LVLEO from Ordu. The differences in compound quantities are attributed to the extraction method, growing season and harvest period, and different regions of the plant [20].
Nanoemulsion properties
Particle size and PDI
The average droplet size, polydispersity index (PDI), and zeta potential of the nanoemulsion are presented in Table 2. According to the results, the nanoemulsion droplet size was 94.3 nm with a zeta potential of -46.2 mV. The application of ultrasound emulsification reduced the droplet size of the emulsions to the nanometer range, resulting in higher dispersibility of the essential oils [21]. According to the results, the PDI of the nanoemulsion was approximately 0.185, indicating a narrow droplet size distribution, homogeneity, and high stability for emulsions with a PDI below 0.3 [22]. Our results were consistent with those of Gholamhosseinpour, Hashemi [12], who prepared an edible coating with a nanoemulsion of Echinophora cinerea essential oil.
Antioxidant activity
The free radical scavenging capabilities of the sodium salt of γ-carrageenan (SSG), SSG-1.5% essential oil (EO), SSG-3% EO, SSG-1.5% nanoemulsion (NEO), and SSG-3% NEO were evaluated by examining their ability to scavenge the stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical. Upon reaction with a free radical scavenger, the prominent absorption band of DPPH at 517 nm gradually diminishes [23].
Antioxidants are considered to employ free radical scavenging as a key mechanism for delaying oxidative processes. According to Wu and Zhou [24], DPPH is a stable free radical that can accept an electron or a hydrogen radical to form a stable diamagnetic molecule. Figure 1a illustrates that the radical scavenging activity differed significantly among all groups. This study revealed that the groups containing essential oil (EO) and nanoemulsion (NEO) exhibited superior free radical scavenging capacity compared to the control and sodium salt of γ-carrageenan (SSG) groups. The groups with EO and NEO demonstrated superior free radical scavenging ability when compared to the control and SSG groups. It is evident that the groups’ ability to scavenge free radicals increased as the concentration of EO increased. The results indicate that EO has a greater potential to scavenge free radicals at the nanoscale than at the macroscale. SSG-3% NEO exhibited the highest DPPH inhibition percentage (72.33%). Due to differences in the units of measurement, the DPPH and ABTS values reported in this study cannot be directly compared to those in other studies. The DPPH inhibition percentage of lemon verbena leaf essential oil (LVLEO) was found to be higher in the study by Golmakani and Farahmand [25], likely because they used higher concentrations of essential oils compared to this investigation.
a and b. Antioxidant activity of different sage seed gum (SSG) coatings incorporated with leaves of lemon verbena essential oil (EO) and nanoemulsion of EO (NEO). control: turkey meat, SSG: turkey meat covered with sage seed gum, SSG-EO 1.5%: turkey meat covered with sage seed gum containing 1.5% lemon verbena essential oil, SSG-EO3%: turkey meat covered with sage seed gum containing 3% lemon verbena essential oil, SSG-1.5% NEO: turkey meat covered with sage seed gum containing 1.5% nanoemulsion of lemon verbena EO, and SSG-NEO3%: turkey meat covered with sage seed gum containing 3% nanoemulsion of lemon verbena EO
The reducing power assay is employed to evaluate the electron-donating capacity and may serve as a useful indicator of antioxidant activity. To assess the antioxidant activity of the essential oils (EOs), the ferric ion reducing antioxidant power (FRAP) was determined in this investigation. Antioxidants can reduce Fe3+-tripyridyltriazine (Fe3+-TPTZ) to Fe2+-TPTZ in acidic solutions [26].
Figure 1b depicts the experimental outcomes. The ferric reducing antioxidant power (FRAP) of each group differed significantly. It is clear from the provided statement that there was a significant difference in the ferric reducing antioxidant power (FRAP) among each group. The rise in EO and NEO concentrations had an impact on the outcomes. A high value of the FRAP was observed in SSG-3% NEO (59.22%). The results showed that EO at the nanoscale exhibits more free radical scavenging capacity than EO and is comparable to the free radical scavenging activity observed in the DPPH test. One popular technique for determining the antioxidant capacity of different biological samples is the FRAP assay. In a redox-linked colorimetric reaction, it utilizes antioxidants as reductants to convert Fe3+ to Fe2+. By providing free radicals with an electron to stabilize them and reduce the damage they cause to DNA, cells, and organ systems, antioxidant molecules act as reducing agents. Examples of antioxidant compounds include polyphenols, flavonoids, vitamins, and enzymes such as superoxide dismutase and glutathione peroxidase. The antioxidant activity of LVLEO has been demonstrated by Golmakani, Farahmand [25] and Jalal [27].
Our results indicate that nanoscale electrocatalytic oxidation (EO) exhibits an enhanced capacity for free radical scavenging compared to bulk EO. This finding is of significant interest due to its potential implications for the field of antioxidant research. However, further studies are required to validate these observations and elucidate the underlying mechanisms responsible for this phenomenon.
Antimicrobial activity
The antimicrobial inhibition zones of different treatments against gram-positive (B. cereus and S. aureus) and gram-negative (P. aeruginosa and E. coli) bacteria are presented in Table 3. According to the results, SSG exhibited reduced effectiveness in inhibiting P. aeruginosa and E. coli, with inhibition zones of 2.43 ± 0.03 and 3.55 ± 0.04 mm for B. cereus and S. aureus, respectively. In contrast, the incorporation of EO into the edible film enhanced its antimicrobial activity. Furthermore, all bacteria were strongly inhibited upon the addition of EO into the SSG, and the growth of the investigated pathogens was completely inhibited in a dose-dependent manner (p < 0.05). Comparisons between the effectiveness of SSG edible coating incorporated with NEO and the free form of EO have shown that NEO demonstrated superior inhibition of bacterial growth.
Our findings demonstrate that SSG-NEO coatings exhibit superior antimicrobial activity compared to SSG-EO coatings. This activity is further significantly enhanced with increasing NEO concentration (p < 0.05). This phenomenon can be attributed to the combined effects of encapsulation and the sustained release of EO from the coating matrix, leading to potentiated antimicrobial efficacy [28]. Nanoemulsions, owing to their small droplet size and high surface area, offer superior efficacy compared to conventional emulsions. Consequently, the gradual release of essential oils from nanoemulsion-based edible coatings contributes to their enhanced antimicrobial performance [29].
Additionally, the difference between the inhibition zone diameter of the SGG-NEO coating is remarkably dependent on the NEO content. The strongest antimicrobial activity was observed for SSG-3%NEO against the investigated gram-positive (B. cereus) and gram-negative (P. aeruginosa) bacteria. The sensitivity profile of microorganisms against the SGG-3%NEO is as follows: B. cereus > S. aureus > P. aeruginosa > E. coli. Moreover, gram-positive bacteria were more susceptible to SSG coating incorporated with LVLEOs, which was in agreement with Hosseini, Jamshidi [8]. The high resistance of gram-negative bacteria is attributed to the high hydrophobicity of their lipopolysaccharide (LPS) outer membrane, which serves as a permeability barrier [8]. In agreement with our findings, Yekrang et al. [5] confirmed the antibacterial activity of SSG nanofibers against S. aureus. The antimicrobial activity of SSG might be related to their structure and ability to cause cell damage and leakage of vital contents, ultimately leading to cell death [5]. Similarly, Sprea et al. [20] reported the antibacterial effect of EO from (A) citrodora against (B) cereus and gram-negative bacteria such as Yersinia enterocolitica and S. aureus. Oukerrou et al. [30] also confirmed the antibacterial activity of hydrodistillated EOs from lemon verbena against E. coli and S. aureus. EOs can disrupt the cell membrane and increase membrane permeability, resulting in the leakage of cellular contents. Therefore, they inhibit bacterial growth [8].
Characterization of coated turkeys
pH
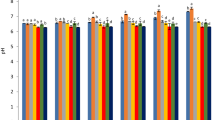
Five grams of each treated meat sample were homogenized in 25 mL of distilled water for 1–2 min. The homogenate pH was then measured using a digital pH meter at 25 °C [31]. Figure 2a depicts the impact of sage seed gum coating containing LLEO on the pH of fresh turkey meat during storage. A statistically significant effect (P < 0.05) of the coating on the pH of all groups was observed throughout the storage period. The initial pH values for the control and SSG-3%NEO groups were 5.82 and 5.76, respectively, increasing to 7.95 and 6.22 after 14 days of storage. All groups exhibited a significant (P < 0.05) increase in pH during storage. These findings align with those of Ivonkovic et al. [32], Konuk Takma and Korel [33], Hassanzadeh et al. [34], and Zhao et al. [35], who reported a rise in pH of coated samples over storage time. The observed increase in pH during storage is likely attributable to the release of protein metabolites, primarily basic amines, due to microbial activity associated with meat product storage [36, 37]. As illustrated in Fig. 2a, the presence of EO and NEO significantly reduces pH changes during storage. The lower pH values observed in the EO-containing groups may be attributed to the suppression of microbial growth and the inhibition of endogenous protease activity [38].
Peroxide value (PV) determination
The peroxide value (meq O2/kg fat) of various groups at different storage durations is presented in Fig. 2b. The peroxide value serves as an indicator of the extent of lipid oxidation in food products and is a crucial factor in determining the food products’ quality and shelf life. According to the data, the peroxide value of all groups increased sharply with extended storage up to the 12th day, after which it decreased. Among all treatments, the control group exhibited the highest peroxide value (3.6 meq O2/kg fat) after 12 days of storage. This reduction in peroxide value after 12 days of storage could be attributed to the breakdown of peroxides and the formation of secondary lipid oxidation products [39]. A similar trend was observed by Khanjari et al. [40]. After 16 days of storage, the SSG-3%NEO group exhibited the lowest peroxide value (0.43 meq O2/kg fat). The results indicate that the inclusion of EOs and NEOs in the groups provided protection against lipid oxidation, as evidenced by the lower peroxide values in the EO and NEO-containing groups compared to the control group. The SSG-3% NEO group, which displayed the lowest peroxide value across all storage durations, exhibited the most protective effect. It is crucial to note that while the peroxide value is one factor used to evaluate the quality and shelf life of food products, additional factors, such as the thiobarbituric acid (TBA) value, can also be employed to determine the extent of lipid oxidation in food products. Similarly, Taheri et al. [2] found that the peroxide value (PV) was significantly lower in all treatments involving a solution comprising 2% chitosan, 1% essential oils from cumin seeds, and 1% acetic acid applied for coating, compared to the control.
TBA
The thiobarbituric acid (TBA) values (mg MDA/kg) of various groups at different storage durations are displayed in Fig. 2c. The TBA value is used to determine the level of lipid oxidation in food products. The findings showed that the TBA values of all groups increased over the storage period. The increase in TBA values revealed protein degradation by spoilage bacteria and endogenous enzymes [41]. However, the addition of LVLEO/NEO to the SSG coating had a significant impact on the TBA value, and higher concentrations exhibited a better protective effect against lipid oxidation (p < 0.05). According to the results, after 16 days of storage, the control group (3.3 mg MDA/kg) and the SSG-3%NEO group (0.75 mg MDA/kg) exhibited the highest and lowest TBA values, respectively. Moreover, the encapsulation of EO was more effective compared to the free form of EO, which might be related to the protective effect of the carrier and emulsion system, resulting in better activity of the EO. Therefore, the SSG-3% NEO group significantly inhibited MDA production and exhibited the lowest TBA value (0.75 mg MDA/kg) throughout storage. The lower levels of TBA value observed for the EO/NEO-loaded coatings could be related to the antimicrobial activity of the EO compositions and the ability of phenolic compounds to inhibit spoilage bacteria, consequently retarding lipid oxidation and protein breakdown [14]. Moreover, edible coatings prolong the shelf life of meat products by decreasing the ability of bacteria to perform oxidative deamination reactions on non-protein nitrogenous compounds [42]. Sayadi et al. [1] and Mojaddar Langroodi et al. [19] reported similar findings in turkey meat. Furthermore, according to Tural and Turhan [43], anchovy by-product protein coatings containing thyme essential oil (TEO) have a positive effect on the shelf life of anchovy (Engraulis encrasicolus L.) fillets stored at 4 ± 1◦C and effectively delay lipid oxidation in the fillets. Studies have shown that essential oils can mitigate the production of free radicals by donating electrons [31].
Microbiological analysis
The changes in the total bacterial counts (TBC), psychrotrophilic bacteria, Enterobacteriaceae, and mold and yeast count of turkeys treated with SSG –based coating incorporated with EO/NEO during 16 days of storage are illustrated in Fig. 3. According to the results, the synergistic effect of SSG coating and LVLEO on the shelf life of turkeys was observed. As can be seen (Fig. 3a), the initial TBC of all groups was about 3.7–3.9 log CFU/g. Mojaddar Langroodi et al. [19], reported similar counts of 3.68 log CFU/g for turkeys. The microbial quality decreased rapidly after 16 days and the TBC of control (uncoated) reached to 8.72 log CFU/g. While, the TBC of SSG coated turkeys increased gradually and reached to 7.35 log CFU/g, 5.59 log CFU/g and 4.25 log CFU/g in SSG, SSG-3%EO and SSG-3%NEO, respectively. The recommended acceptable TBC limit for fresh poultry meat is 7 log CFU/g [19], therefore, the turkeys treated with SSG containing 1.5 and 3% EO or NEO had good microbial quality.
Furthermore, SSG coatings incorporating LVLEO/NEO effectively retarded the growth of both psychrotrophic bacteria and Enterobacteriaceae, exhibiting a similar trend (Fig. 3b). The initial psychrotrophic count of the control (uncoated) group was 1.6 log CFU/g and steadily increased throughout storage, reaching 7.87 log CFU/g on day 16. While the SSG coating alone significantly improved the microbial quality of the turkey meat, the addition of EO/NEO to the SSG matrix further enhanced its antimicrobial activity. Consequently, SSG coatings containing higher EO/NEO concentrations exhibited superior antimicrobial properties. Groups coated with SSG-1.5%EO (6.44 log CFU/g) and SSG-3%EO (5.52 log CFU/g) exhibited lower psychrotrophic bacterial counts compared to the SSG group (7.07 log CFU/g). Notably, the SSG-NEO treatment demonstrated even greater antimicrobial efficacy, with the psychrotrophic bacteria count in the SSG-3%NEO group reaching only 3.86 log CFU/g by the end of storage.
As illustrated in Fig. 3c, the initial Enterobacteriaceae counts in the control and SSG-treated groups were 3.9 log CFU/g and 3.86 log CFU/g, respectively. These counts increased to 7.94 log CFU/g and 7.23 log CFU/g after 16 days of storage. In contrast, the SSG-1.5%NEO and SSG-3%NEO groups exhibited lower initial counts of 4.98 log CFU/g and 4.61 log CFU/g, respectively, and these counts remained lower throughout the storage period.
Moreover, mold and yeast counts increased significantly throughout the storage period, as shown in Fig. 3d. The SSG-3%NEO coated group exhibited the lowest mold and yeast count (3.56 log CFU/g) at the end of storage, while the control (uncoated) group displayed a significantly higher count of 7.92 log CFU/g. This enhanced antifungal activity of SSG-3%NEO coatings may be attributed to the synergistic interaction between SSG and LVLEO. This finding is in accordance with the observations of [14], who reported similar antifungal properties of chitosan coatings containing LVLEO.
Sensory evaluation
Table 4 presents the sensory evaluation of turkey samples wrapped with SSG containing EO/NEO after 16 days of storage, based on texture, odor, color, and overall acceptability. As shown in Table 4, the overall quality of all groups significantly declined (p < 0.05) during storage. Notably, the decrease in overall quality was more pronounced in the control group compared to the SSG-treated groups (p < 0.05). Conversely, the incorporation of EO/NEO with the SSG coating resulted in a further increase in sensory scores. SSG-EO and SSG-NEO treatments yielded significantly higher scores (p < 0.05) compared to the control group. This difference was particularly evident between the control and the EO/NEO-treated groups (p < 0.05). All groups coated with SSG-EO or SSG-NEO exhibited superior sensory attributes compared to the control group. However, the SSG coating alone did not significantly improve all sensory characteristics compared to the uncoated groups (p < 0.05).
Initially, panelists exhibited a preference for the sensory attributes (texture, color, and overall acceptability) of uncoated control turkeys. However, these scores declined throughout storage. Notably, no significant difference in texture scores was observed between the SSG and SSG-1.5%EO groups (p > 0.05). Odor and color are particularly important factors for consumer acceptance of food products with edible coatings due to their direct impact on sensory perception. Our results indicate that the incorporation of EO/NEO into the coating solution positively influenced the panelists’ perception. Specifically, groups coated with SSG-NEO received significantly higher odor and color scores compared to other groups starting on day 8 (p < 0.05). Notably, the SSG-3%NEO group received the highest odor score, suggesting a protective effect arising from encapsulation and the slow release of EO. It is well-established that off-odor and flavor deterioration in meat products are linked to lipid oxidation and the generation of volatile compounds [44]. Conversely, edible coatings containing natural antioxidants, such as essential oils, can extend the shelf life of meat products by hindering lipid oxidation.
Sensory evaluation revealed that turkey meat treated with a coating supplemented with 3% NEO achieved the highest scores (odor, color, texture, and overall acceptability) after 16 days of storage (p < 0.05). These superior organoleptic properties are likely attributable to the encapsulation of EO and its gradual release during storage. Encapsulation within the edible coating protects the EO, improves its stability, and enhances its effectiveness through controlled release [16, 45, 46]. Similar findings were reported by Taheri et al. (2018) for chitosan-cumin EO coated turkey breast. The control group in their study exhibited quality loss due to lipid oxidation, microbial growth, and the associated production of off-odors and discoloration after only 6 days of storage. In contrast, coating the turkey with chitosan-cumin EO extended the shelf life by up to 15 days, likely due to the coating’s combined antioxidant and antimicrobial activities [2]. Another study demonstrated that combining EOs with modified atmosphere packaging (MAP) can enhance the organoleptic properties of turkey breast and prevent undesirable chemical changes during storage [47].
Conclusion
GC-MS analysis identified caryophyllene oxide as the primary component of the essential oil, followed by spathulenol and ar-curcumene. Notably, the use of sage seed gum (SSG) as the base coating material for supplementation with EO and NEO not only enhanced the edible film’s antioxidant activity but also improved its antimicrobial properties. However, the SSG film alone exhibited only a slight antimicrobial effect. Furthermore, the active edible coatings were effective in mitigating sensory quality deterioration in turkeys, particularly at higher NEO concentrations. The investigated SSG coating containing EO/NEO also displayed lower peroxide (PV) and thiobarbituric acid (TBA) values, along with a reduced pH. These findings suggest that the SSG-based active edible coating, incorporating the lemon verbena EO nanoemulsion, significantly extended the shelf life of refrigerated turkey and presents a promising approach to preserving the quality of meat products.
References
M. Sayadi, A.M. Langroodi, K. Pourmohammadi, Combined effects of chitosan coating incorporated with Berberis vulgaris extract and Mentha pulegium essential oil and MAP in the shelf life of Turkey meat. J. Food Meas. Charact. 15, 5159–5169 (2021)
T. Taheri, A. Fazlara, L. Roomiani, S. Taheri, Effect of chitosan coating enriched with cumin (Cuminum cyminum L.) essential oil on the quality of refrigerated Turkey breast meat. Ital. J. Food Sci., 30, 2018
F. Esmaeili, M. Mehrabi, H. Babapour, B. Hassani, A. Abedinia, Active coating based on carboxymethyl cellulose and flaxseed mucilage, containing burdock extract, for fresh-cut and fried potatoes, LWT, vol. 192, p. 115726, 2024
S.M.A. Razavi, A.M. Amini, Y. Zahedi, Characterisation of a new biodegradable edible film based on sage seed gum: influence of plasticiser type and concentration. Food Hydrocoll. 43, 290–298 (2015)
J. Yekrang, R. Saghafi, A. Yousefi, F. Ghaffari, Sage seed gum as a novel source for polysaccharide-based antibacterial nanofibers: Physical, chemical, and rheological characterization, Journal of Industrial Textiles, vol. 51, pp. 7796S-7819S, 2022
A. Gholamhosseinpour, S.M.B. Hashemi, D. Jafarpour, Nanoemulsion of Satureja Sahendica bornm essential oil: antibacterial and antioxidant activities. J. Food Meas. Charact. 17, 317–323 (2023)
M. Majdinasab, M. Niakousari, S. Shaghaghian, H. Dehghani, Antimicrobial and antioxidant coating based on basil seed gum incorporated with Shirazi thyme and summer savory essential oils emulsions for shelf-life extension of refrigerated chicken fillets. Food Hydrocoll. 108, 106011 (2020)
M. Hosseini, A. Jamshidi, M. Raeisi, M. Azizzadeh, The antibacterial and antioxidant effects of clove (Syzygium aromaticum) and lemon Verbena (Aloysia citriodora) essential oils. J. Hum. Environ. Health Promotion. 5, 86–93 (2019)
İ. Ayran, S.A. Çelik, M.M. Özcan, A. Kırlı, Ö. Dede, C. Çiçek et al., The essential oil yield and compositions of Lemon Verbena (Lippia citriodora Kunth.) Cultivated in Ordu ecological conditions. Akademik Ziraat Dergisi. 10, 365–370 (2021)
A. Hematian Sourki, A. Ghani, F. Kiani, A. Alipour, Phytochemical profiles of lemon verbena (Lippia citriodora HBK) and its potential application to cookie enrichment. Food Sci. Nutr. 9, 3100–3113 (2021)
H.M. Rashid, A.I. Mahmod, F.U. Afifi, W.H. Talib, Antioxidant and Antiproliferation Activities of Lemon Verbena (Aloysia citrodora): An in vitro and in vivo study, Plants, vol. 11, p. 785, 2022
A. Gholamhosseinpour, S.M.B. Hashemi, K. Ghaffari, Physicochemical and microbial qualities of Citrus reticulata cv. Bakraei coated with Lepidium sativum gum containing nanoemulsified Echinophora Cinerea essential oil during cold storage. Postharvest Biol. Technol. 199, 112275 (2023)
M. Azizkhani, F. Jafari Kiasari, F. Tooryan, M.H. Shahavi, R. Partovi, Preparation and evaluation of food-grade nanoemulsion of tarragon (Artemisia dracunculus L.) essential oil: antioxidant and antibacterial properties. J. Food Sci. Technol. 58, 1341–1348 (2021)
M. Rezaeifar, T. Mehdizadeh, A. Mojaddar Langroodi, F. Rezaei, Effect of chitosan edible coating enriched with lemon verbena extract and essential oil on the shelf life of vacuum rainbow trout (Oncorhynchus mykiss). J. Food Saf. 40, e12781 (2020)
Q. Wang, Q. Guo, W. Niu, L. Wu, W. Gong, S. Yan et al., The pH-responsive phase separation of type-A gelatin and dextran characterized with static multiple light scattering (S-MLS). Food Hydrocoll. 127, 107503 (2022)
B.A. Behbahani, M. Noshad, H. Jooyandeh, Improving oxidative and microbial stability of beef using Shahri Balangu seed mucilage loaded with cumin essential oil as a bioactive edible coating. Biocatal. Agric. Biotechnol. 24, 101563 (2020)
M. Xu, Q. Liu, X. Ni, C. Chen, X. Deng, Y. Fang et al., ,., Lipidomics reveals the effect of hot-air drying on the quality characteristics and lipid oxidation of Tai Lake whitebait (Neosalanx taihuensis Chen), LWT, vol. 197, p. 115942, 2024
J.S. SMITH, M. ALFAWAZ, Antioxidative activity of Maillard reaction products in cooked ground beef, sensory and TBA values. J. Food Sci. 60, 234–236 (1995)
A. Mojaddar Langroodi, A. Nematollahi, M. Sayadi, Chitosan coating incorporated with grape seed extract and Origanum vulgare essential oil: an active packaging for Turkey meat preservation. J. Food Meas. Charact. 15, 2790–2804 (2021)
R.M. Sprea, L.H. Fernandes, T.C. Pires, R.C. Calhelha, P.J. Rodrigues, J.S. Amaral, Volatile compounds and biological activity of the essential oil of Aloysia citrodora Paláu: Comparison of hydrodistillation and microwave-assisted hydrodistillation, Molecules, vol. 28, p. 4528, 2023
Y. Ozogul, E.K. Boğa, I. Akyol, M. Durmus, Y. Ucar, J.M. Regenstein et al., Antimicrobial activity of thyme essential oil nanoemulsions on spoilage bacteria of fish and food-borne pathogens. Food Bioscience. 36, 100635 (2020)
Q. He, M. Guo, T.Z. Jin, S.A. Arabi, D. Liu, Ultrasound improves the decontamination effect of thyme essential oil nanoemulsions against Escherichia coli O157: H7 on cherry tomatoes. Int. J. Food Microbiol. 337, 108936 (2021)
L. Ramezanzade, S.F. Hosseini, M. Nikkhah, Biopolymer-coated nanoliposomes as carriers of rainbow trout skin-derived antioxidant peptides. Food Chem. 234, 220–229 (2017)
Z. Wu, W. Zhou, C. Pang, W. Deng, C. Xu, X. Wang, Multifunctional chitosan-based coating with liposomes containing laurel essential oils and nanosilver for pork preservation. Food Chem. 295, 16–25 (2019)
M.T. Golmakani, M. Farahmand, A. Ghassemi, M.H. Eskandari, M. Niakousari, Enrichment of citral isomers in different microwave-assisted extraction of essential oil from fresh and dried lemon verbena (Aloysia citridora) leaves. J. Food Process. Preserv. 41, e13215 (2017)
B. Zhang, X.-M. Zhang, W. Wang, Z.-Q. Liu, Y.-G. Zheng, Metabolic engineering of Escherichia coli for d-pantothenic acid production. Food Chem. 294, 267–275 (2019)
Z. Jalal, Chemical composition and antibacterial activity of the essential oil from Aloysia citriodora leaves (Verbenaceae) cultivated in Morocco. J. Pharm. Sci. Res. 12, 1227–1232 (2020)
W. Zhang, H. Jiang, J.-W. Rhim, J. Cao, W. Jiang, Effective strategies of sustained release and retention enhancement of essential oils in active food packaging films/coatings. Food Chem. 367, 130671 (2022)
M. Huang, H. Wang, X. Xu, X. Lu, X. Song, G. Zhou, Effects of nanoemulsion-based edible coatings with composite mixture of rosemary extract and ε-poly-L-lysine on the shelf life of ready-to-eat carbonado chicken. Food Hydrocoll. 102, 105576 (2020)
M.A. Oukerrou, M. Tilaoui, H.A. Mouse, I. Leouifoudi, A. Jaafari, A. Zyad, Chemical composition and cytotoxic and antibacterial activities of the essential oil of Aloysia citriodora palau grown in Morocco, Advances in Pharmacological and Pharmaceutical Sciences, vol. 2017, 2017
M. Sayadi, A.M. Langroodi, D. Jafarpour, Impact of zein coating impregnated with ginger extract and Pimpinella anisum essential oil on the shelf life of bovine meat packaged in modified atmosphere. J. Food Meas. Charact. 15, 5231–5244 (2021)
A. Ivonkovic, K. Zeljko, S. Talic, M. Lasic, Biodegradable packaging in the food industry. J. Food Saf. Food Qual. 68, 26–38 (2017)
D.K. Takma, F. Korel, Active packaging films as a carrier of black cumin essential oil: development and effect on quality and shelf-life of chicken breast meat. Food Packaging Shelf Life. 19, 210–217 (2019)
P. Hassanzadeh, H. Tajik, S.M.R. Rohani, M. Moradi, M. Hashemi, J. Aliakbarlu, Effect of functional chitosan coating and gamma irradiation on the shelf-life of chicken meat during refrigerated storage. Radiat. Phys. Chem. 141, 103–109 (2017)
C.-C. Zhao, S. Benjakul, J.-B. Eun, Changes in protein compositions and textural properties of the muscle of skate fermented at 10 C. Int. J. Food Prop. 22, 173–185 (2019)
M. Xu, X. Ni, Q. Liu, C. Chen, X. Deng, X. Wang et al., Ultra-high pressure improved gelation and digestive properties of Tai Lake whitebait myofibrillar protein. Food Chemistry: X. 21, 101061 (2024)
F.T. Saricaoglu, S. Turhan, Performance of mechanically deboned chicken meat protein coatings containing thyme or clove essential oil for storage quality improvement of beef sucuks. Meat Sci. 158, 107912 (2019)
B.K. Tokur, F. Sert, E.T. Aksun, F. Özoğul, The effect of whey protein isolate coating enriched with thyme essential oils on trout quality at refrigerated storage (4 ± 2 C). J. Aquat. Food Prod. Technol. 25, 585–596 (2016)
Y. Li, Y.B. Li, C.J. Liu, Changes in lipid oxidation and fatty acids in altay sheep fat during a long time of low temperature storage. J. Oleo Sci. 66, 321–327 (2017)
A. Khanjari, A. Bahonar, N. Noori, M.R. Siahkalmahaleh, M. Rezaeigolestani, Z. Asgarian et al., In vitro antibacterial activity of Pimpinella anisum essential oil and its influence on microbial, chemical, and sensorial properties of minced beef during refrigerated storage. J. Food Saf. 39, e12626 (2019)
E.S. Abdou, G.F. Galhoum, E.N. Mohamed, Curcumin loaded nanoemulsions/pectin coatings for refrigerated chicken fillets. Food Hydrocoll. 83, 445–453 (2018)
S.M.T. Gharibzahedi, S. Mohammadnabi, Effect of novel bioactive edible coatings based on jujube gum and nettle oil-loaded nanoemulsions on the shelf-life of Beluga sturgeon fillets. Int. J. Biol. Macromol. 95, 769–777 (2017)
S. Tural, S. Turhan, Effect of anchovy by-product protein coating incorporated with thyme essential oil on the shelf life of anchovy (Engraulis encrasicolus L.) fillets. Food Sci. Biotechnol. 26, 1291–1299 (2017)
S. Gautam, L. Lapčík, B. Lapčíková, R. Gál, Emulsion-Based Coatings for Preservation of Meat and Related Products, Foods, vol. 12, p. 832, 2023
Z. Azarashkan, S. Farahani, A. Abedinia, M. Akbarmivehie, A. Motamedzadegan, J. Heidarbeigi et al., Co-encapsulation of broccoli sprout extract nanoliposomes into basil seed gum: effects on in vitro antioxidant, antibacterial and anti-listeria activities in ricotta cheese. Int. J. Food Microbiol. 376, 109761 (2022)
S.M.B. Hashemi, D. Jafarpour, The efficacy of edible film from Konjac Glucomannan and saffron petal extract to improve shelf life of fresh-cut cucumber. Food Sci. Nutr. 8, 3128–3137 (2020)
M. Sayadi, S. Amiri, M. Radi, Active packaging nanocomposite gelatin-based films as a carrier of nano TiO 2 and cumin essential oil: the effect on quality parameters of fresh chicken. J. Food Meas. Charact., pp. 1–11, 2021
Acknowledgements
This research was financially supported by Fasa University of Medical Sciences under project number IR.FUMS.REC.1401.206.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sayadi, M., Eskandari, Z., Jafarpour, D. et al. Effect of sage seed gum edible coating incorporated with leaves of lemon verbena (Aloysia citrodora) essential oil nanoemulsion on chemical, microbial and sensory properties of fresh Turkey meat. Food Measure 18, 6816–6828 (2024). https://doi.org/10.1007/s11694-024-02695-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-024-02695-4