Abstract
Due to the side effects of artificial preservatives in meat products, the use of natural preservatives has increased. The aim of this study was investigated the effect of coating and nano-coating of chitosan (C)- Cress (Lepidium sativum) seed gum (CSG) on the shelf life of beef during 18-day at cold temperature. For this purpose, composite coatings including 33% C:6% CSG, 50% C:50% CSG, 66% C:33% CSG were prepared. The nano-coating was also constructed using ultrasound method. Antioxidant and antibacterial properties were measured and the results showed that the antioxidant and antimicrobial activity of the coatings incremented with increasing C content (66% C:33% CSG) and the antimicrobial and antioxidant activity of nano-coating was significantly higher than the coating (p < 0.05). In order to study the effect of coatings on the shelf life, four treatments including control, coating (66% C:33% CSG), nano coating (66% C:33% CSG) and sodium nitrite were prepared and chemical (peroxide value and total volatile nitrogen) and microbial parameters (total viable count, psychrotrophic count, Pseudomonas aeruginosa and Staphylococcus aureus) were studied at − 2, 4, and 8 °C for 18 days. Synthetic and natural preservatives delayed lipid oxidation and improved the chemical properties in beef. Also, microbial spoilage was significantly reduced compared to the control (p < 0.05). The nano-coating performed better than other treatments in all experiments (p < 0.05), and reduced the growth of pathogenic bacteria from the third day to the end of the period to below the permissible limit. Therefore, it seems that the nano 66% C:33% CSG can be used as a safe preservative in the active packaging of beef during refrigerator and freezer storages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meat is a set of skeletal muscle tissues of slaughter animals that are associated with adipose and related connective tissues and are the most important protein sources and rich in essential amino acids, minerals (iron and zinc), vitamins as well as enough energy to make it one of the best and most complete foods. But the most important challenge for meat is its spoilage, which affects its health and quality [1,2,3]. Meat packaging technology has evolved over the past two decades. The purpose of packing of fresh meat is to delay spoilage, enhance the activity of enzymes to improve meat quality, reduce weight loss, and achieve to oxymyoglobin pigmen at retail or consumer level [1]. Concerns about packaging have led to different ideas about packaging and packaging wastes. Some packaging wastes can be reduced by edible coatings used in the food industry. Because these coatings are biodegradable and may be edible, they are not harmful to the environment. Accordingly, these coatings can reduce wastes disposal costs [1]. Edible coatings can also be used as a good barrier against the transfer of moisture, oxygen, carbon dioxide and other factors. These coatings are usually made from raw materials such as polysaccharides, lipids and proteins [4].
Lepidium sativum L. is an herbaceous, 1-year, aromatic, branched stem with a height of 10 to 45 cm [5]. The cress seed gum can provide a coating with a good appearance and satisfactory mechanical properties [6]. On the other hand, when compared to other polysaccharides, the gum has several advantages including low production cost, hydrophilicity and biocompatibility, as well as good rheological properties that indicate its potential to form suitable edible coatings [6]. Recently, the commercial value of chitin has increased due to its useful properties of soluble derivatives that are suitable in chemistry, biotechnology, agriculture, food processing, cosmetics, veterinary, medicine, dentistry, environmental protection and paper or textile production [7]. After cellulose, chitin is the most abundant natural polysaccharide that found in crustaceans, molluscs, marine diatoms, insects such as butterflies and shoemakers, the cell wall of algae, fungi and yeasts, as well as the outer skeleton of marine zooplankton species, corals and jellyfish. Waste obtained during the processing of crab, shrimp and oysters contains 75% of the total weight of the shellfish [7]. Perhaps the most important properties of chitosan are its anti-spoilage properties, as an antifungal preservative to extend storage, keep fresh produce, and prevent the growth of bacteria [4, 8, 9].
Scientists and industry owners have identified the potential uses of nanotechnology in almost all sectors of the food industry [2]. The two most important examples are food processing (improving texture of food, jelly production) and food packaging. In both cases, the technology has been more widely used in food packaging, most likely because nanoparticles are not added directly to food and the natural structure of the food is preserved, Nanotechnology offers great promise for achieving food packaging with greater safety and storage capabilities and ultimately healthier food. Nanoparticles increase the inhibitory properties (thermal mechanical, chemical, microbial) to improve mechanical properties and heat resistance, develop antimicrobial activity and biochemical changes [10].
Therefore, in this study, we compared the antimicrobial and antioxidant properties of coating and nano-coating of chitosan-cress seed gum composites and its effect on beef quality and shelf life.
Materials and methods
Raw material
The beef were prepared after slaughter and kept at room temperature until the end of rigor mortis (12 h), then cut into 6 × 6 × 4 cm pieces. For extraction of gum, cress seed was obtained from medicinal herbs store located in Noor city (Mazandaran, Iran), and scientific name was confirmed by the Institute of Pharmacology. All chemicals used were prepared by Merck Company (Hamburg, Germany) and were of analytical grade. Medium molecular weight chitosan (C) (450 KDa) prepared from Sigma-Aldrich (St. Louis, USA).
Extraction of gum from cress seed
The cress seed was manually cleaned, and the gum was extracted by Karazhiyan et al. [11] method. Optimum extraction conditions for cress seed gum (51:1 v/w water to seed ratio, 25 °C temperature and pH = 5.5) In the process of gum extraction, the pH of deionized water was first adjusted with 0.1 M NaOH or HCl solution. It was adjusted and heated in the hot water bath to reach the desired temperature and then the seeds were added and again, to complete the water absorption process and stirred periodically, finally the hydrocolloid extract. It was extracted by a laboratory extractor and dried in an oven at 70 °C and then milled and sieved (with mesh 18) and the gum powder was kept in dry place for future experiments.
Coating preparation
The combination of C (was used 0.5% acetic acid for 24 h to increase the solubility) and cress seed gum at 1:2, 1:1 and 2:1 w/w ratio were used as wall coating. The mentioned concentrations of chitosan and CSG were mixed in deionized water to achieve 3% total solids. For better dissolve the compounds was used the magnetic stirrer for 15 min at ambient temperature. The solution was kept in the refrigerator for 24 h to complete the water absorption process. It was then homogenized using an ultrathorax homogenizer at 12,000 rpm at 10 °C for 5 min [12].
Nano coating preparation
The combination of C and CSG at 1:2, 1:1 and 2:1 w/w ratios was first prepared similar to free coating [12]. Then, to further reduce the particle size, a propeller type ultrasonic generator with 6 cycles, 30 s per cycle, and 15 s rest time between cycles was used. Samples were freeze-dried at a pressure of 0.07 mPa at − 57 °C for 48 h.
Antioxidant activity of composites
DPPH free radical scavenging assay
This test was performed according to the method described by Akkol et al. [13]. DPPH is a purple compound that is easily radicalized due to the presence of phenyl groups in its structure. This test was based on the percent inhibition of DPPH free radicals by adding antioxidant compounds. This compound changes color by taking the antioxidant electrons from purple to yellow. Free radicals present in DPPH absorb at 517 nm.
Ferric reducing antioxidant power assay (FRAP)
This test was performed according to the method of Akkol et al. [13]. In this method, antioxidants have a reductive role and cause the reduction of FeIII to FeII. Depending on the regenerative power of the extract, the yellow color of the test solution changes to green or blue.
Rheological properties of hydrocolloid solution
The rheological properties of the hydrocolloid suspensions were measured by rheometer in a constant temperature (25 °C) [14].
Antimicrobial properties of composites
The antimicrobial activity of soluble edible coatings on pathogenic and spoilage microorganisms including Staphylococcus aureus (S. aureus) (PTTC 1112), Listeria monocytogenes (L. monocytogenes) (PTTC 1304) and Escherichia coli (E.coli) (PTTC 1399) was examined using agar diffusion method. These microorganisms were purchased from Persian Type Culture Collection (PTCC). Microorganisms were cultured for 24 h in BHI agar at 37 °C. Discs with a diameter of 13.4 mm were prepared from edible films. Prior to placing the discs on the surface of the culture, surface culture was performed using 0.1 ml of liquid culture (approximately 106−107 log CFU/g) of each of the tested bacteria. The plates was be incubated at 37 °C for 48 h. The diameter of the inhibition zone was measured using a caliper [15].
Nano-coating experiments
The mean diameter, particle size distribution, and particle specific area were measured using a laser light refraction device (Zetasizer nano zs. Malvern Co., England) [16]. Zeta potential was measured using a Zetasizer apparatus (Zetasizer nano zs. Malvern Co., UK). The apparatus carries an electrochemical cell containing two electrodes. The samples were diluted with deionized water in a ratio of 5: 1 and immersed in cell. When the voltage was applied, the negatively charged particles moved toward the positive electrode and the particle movement velocity was measured [16].
Beef coating
The beef were immediately immersed in prepared hydrocolloid solutions (two stages) for 2 min at room temperature, and the coated beef was placed on a grid tray to remove additional coatings. The following experiments were performed on non-coated beef, coated beef (best C concentration: CSG), nano-coated beef sample (best C concentration: CSG), and beef containing 50 ppm sodium nitrite at three storage temperatures (− 2, 4 and 8 °C) and 7 time intervals (zero time, every 3 days).
Chemical factor
The peroxide value (PV) measures the amount of primary oxidation products (hydroperoxides). Peroxide value of samples was determined according to the method pearson [17]. Results were expressed in meq oxygen kg-1 lipids.
The total volatile basic nitrogen (TVB-N) of the beef was determined by the micro-diffusion method as described by Javadian et al. [18]. Results were expressed as mg N/100 g of samples.
Microbial factors
Ten grams of the beef sample was mixed and homogenized with 90 ml of sterile sodium chloride solution (0.85%) and the required dilutions were prepared. One ml of each dilution was used for culture of bacteria by pure plate method. Total viable counts and were counted on Plate Count agar at 37 °C for 2 days. The results were indicated as log CFU/g [18].
Staphylococcus aureus
Baird Parker agar was used to count of S. aureus. One ml of the desired dilution was cultured on the Baird Parker and incubated for 24 to 48 h at 37 °C. The formation of shiny black colonies with a thin white edge and a transparent zone around, it is characteristic of Staphylococcus. Two plates were considered for each dilution. Coagulase test with rabbit plasma and aerobic and anaerobic fermentation of mannitol in mannitol salt agar were used for confirmatory tests [19].
Pseudomonas aeruginosa (P. aeruginosa)
Specific culture media of Pseudomonas agar and its selective supplementation of cetirizine-fosidine-cephaloridine were used for enumeration of P. aeruginosa. One gram of sample beef was added to 9 ml of sodium chloride solution (0.85%) and then homogenized. Samples were diluted from 10− 2 to 10− 4. One tenth ml diluted sample was inoculated on Pseudomonas agar and incubation was done at 37 ° C for 48 h. Samples were tested as triplicates [20].
Statistical analysis
All experiments were performed in completely randomized design as triplicates and the result was reported as mean ± SD. Statistical analysis of treatments was performed by ANOVA using SPSS software. Significant mean differences were determined by Duncan test at 0.05 level and charts were plotted using Microsoft Excel software.
Results and discussion
Antioxidant activity
DPPH free radical scavenging assay
DPPH is a stable radical, with a maximum absorption of about 550 nm and can be rapidly reduced with an antioxidant. This method is widely used to measure the amount of free radical inhibition of various compounds [21]. Results of DPPH free radical scavenging showed the highest values in 2 C: CSG treatment and the lowest DPPH free radical inhibitory in C: 2 CSG (P < 0.05) (Fig. 1a). This is due to the higher antioxidant activity of C than CSG. In general, chitosan coating decrease the oxidation reaction by trapping free radicals and metal ions as well as barrier the oxygen contact with the product. Water-soluble derivatives of chitosan, obtained by the copolymerization of maleic acid sodium and hydroxypropyl chitosan and carboxymethyl chitosan sodium, are capable of trapping hydroxyl radicals [22]. As the concentration of chitosan increases, the antioxidant activities are increased too.
The use of nanoparticles also increased DPPH free radical scavenging. Overall, the highest DPPH free radical scavenging was observed in nano-coating of 2 C:CSG (85.89%) (P < 0.05). The use of nanoparticles protects hydrocolloids from environmental factors such as pH, oxygen, light, and so on. Volatile molecules also remain stable with this method, protecting them from oxidative, light, and volatile changes. Therefore, nanoparticles have greater potential to enhance bioavailability, improve release control, and aim to accurately incorporate biological constituents to improve antioxidant activity [23].
Ferric reducing antioxidant power assay
The reducing power assay in samples is due to reduction of iron III to iron II. The amount of iron complex can be measured by Prussian blue formation at 700 nm. The results of FRAP (Fig. 1b) were in agreement with the results of DPPH free radical scavenging and the highest values were observed in 2 C:CSG treatment and the lowest FRAP in C:2 CSG treatment (p < 0.05). Yen et al. [24] compared the antioxidant properties of fungal chitosan, ascorbic acid, BHA and alpha-tocopherol. The results showed that chitosan is suitable for antioxidant activity and trapping hydroxyl radicals and iron ions and can be a proper antioxidant source for use in the food and pharmaceutical industries. The use of nanoparticles also increased FRAP in all treatments. Overall, the highest amount of FRAP was observed in nano-2 C: CSG (p < 0.05) (789.59 µmol ferrous/g). This indicates an increase in the antioxidant property of chitosan after the nano-process.
Antibacterial activity
One of the methods for determining antibacterial activity is to determine the diameter of the bacterial growth inhibition zone. The values of the diameter of the inhibition zone in different treatments have been shown in Fig. 2a (S. aureus), Fig. 2b (E. coli) and Fig. 2c (L. monocytogenes). The best and the least of results were observed in 2C: CSG and C: 2 CSG treatments respectively (p < 0.05). Antimicrobial activity of chitosan was higher than CSG. The main mechanism of the antibacterial activity of chitosan is its ability to bind and disorder the function of the outer membrane of microorganisms. Chemical analysis and results of electrophoresis of chitosan-treated supernatants showed that the reaction of chitosan with some microorganisms releases lipopolysaccharides or other membrane lipids [25]. Sashiwa and Aiba [26] described two mechanisms for how chitosan acts as an antimicrobial agent: (1) binding of the cationic chitosan to membrane phospholipids prevents the transfer of microbial substances. (2) Penetration of the chitosan oligomers into the cells of the microorganism, inhibits DNA conversion to RNA subsequently cell growth.
Also, the highest of the inhibitory zone was observed S. aureus. Chitosan has a different effect against gram-positive and gram-negative bacteria. Many studies have shown that the antimicrobial effect of chitosan on gram-negative bacteria is stronger than gram-positive bacteria. While some studies have shown that the susceptibility of gram-positive bacteria to chitosan is greater than that of gram-negative bacteria. The main reason for the higher resistance of gram-negative bacteria is outer membrane existence in cell wall. The results studies also showed no difference between the resistance of gram-positive and gram-negative bacteria to chitosan. The differences in the results of the studies were due to differences in the experimental conditions [22]. The use of nanoparticles also increased the diameter of the inhibitory zone in all treatments. Overall, the highest inhibition zone diameter was observed in 2 nanoC:CSG (p < 0.05), indicating an increase in the antimicrobial activity of chitosan after the nano-process. Previous studies showed that chitosan nanoparticles had higher antibacterial activity against gram-negative or gram-positive bacteria than chitosan polymer. The better inhibitory effect of chitosan nanoparticles could be explained by the larger surface area of the nanoparticles for reaction with the bacterial cell wall. These nanoparticles can adsorb tightly onto the surface of cell wall, thereby damaging the cell membrane and killing the bacterium [9].
Nano-coating tests
One of the most important properties of nanocomposites is the size and distribution of the particles. Particle size was measured by calculating the mean diameter of the drop, which is represented by the symbol d43. According to the results (Table 1), the particle size in the nano-C:2 CSG was higher than the other treatments (p < 0.05). The reason for the smaller size of the particles prepared with higher concentration of nano-C than the CSG can be attributed to the higher viscosity of chitosan and decrease of the brownian motion of the oil droplets. In general, the size of droplets of an emulsion depends on several factors, the most important of which are the amount and type of emulsifier used the type of phases, the methods of emulsion preparation, and the environmental conditions such as pH and metal ions. In study by Najafi and Fazeli [27], the concentration of emulsion particles decreased with increasing concentration of CSG, but these changes were not significant. According to them, the most likely reason is the increase in viscosity and decrease of the brownian motion of the oil droplets. Samavati et al. [28] attribute this to the ability of hydrocolloids to cover a wider surface of droplets and to produce smaller droplets.
Electrostatic interactions play an important role in the biological processes that occur at the membrane surface. Factors such as stability, loading efficiency of the active substance, the binding strength of the active substance to the carrier and the rate of release of the active substance are greatly influenced by the electrostatic properties of the carrier [27]. The most common method for determining the electrostatic properties of nano-coatings is to calculate the zeta potential that is the total charge of a particle in a liquid medium or the potential difference between the moving ionic layer and the non-moving layer, and is the best index for determining the surface electrical condition of the dispersions. Because it indicates, the amount of charge accumulated in the non-moving layer and the intensity of the uptake of opposing ions to the particle surface [3]. Zeta potential is a method for predicting the stability of nanocomposites and is useful in the mass controlling and nano-extracts sedimentation. The latter factors are important in stability. According to zeta potential results (Table 1), the highest values were observed in 2nano-C:CSG (p < 0.05). Higher zeta potential in 2nano-C:CSG indicates more stability of nanoparticles produced by this carrier. According to previous studies, zeta potential increased with decreasing liposome size [27] which was similar to this study.
Peroxide value (PV)
The peroxide value shows the total amount of hydroperoxides and is one of the most commonly used quality assessment index of fats and oils during production and storage [18]. The results of peroxide value (Fig. 3a–c) at all three storage temperatures showed a significant increase over time in all treatments especially in the control sample compared to the other treatments. Various storage periods indicated that the natural and synthetic antioxidants (nitrite) slowed the peroxide value compared to the control treatment. The slow increase of the peroxide value in the antioxidant treatment indicates that they have antioxidant activity. Bingol et al. [29] stated that chitosan has antioxidant power to fat preservation in food. Hydrocolloid gums, such as CSG, prevents the penetration of oxygen into the tissue [27], thereby the rate of initial oxidation of fats and subsequent formation of hydroperoxides decreases.
Some studies carried out by Khan et al. [30] and Khazaei et al. [6] have shown that adding chitosan and seed gum to meat products slowed the peroxide value rate.
Overall, the peroxide value was lower in the nanocomposite treatments than in the other treatments. The nano-process increases the antioxidant activity, protecting the hydrocolloids from environmental factors such as pH, oxygen, light, and so on. Volatile molecules also remain stable with this method, protecting them from oxidative, light, and volatile changes. Thus nano-coating has the potential to enhance bioavailability, improve emission control, and target precisely the biological constituents resulting in improved antioxidant activity [23]. The permitted level of peroxide value in meat products for human consumption is 5 [31]. Accordingly, all treatments kept at − 2 °C had acceptable range until the end of the storage period. Samples stored at 4 °C: control treatment up to 9 days, coating treatment up to 12 days and nano-coating and nitrite treatments up to 15 days had acceptable level. Samples stored at 8 °C, control treatment up to 6 days, coating treatment up to 9 days, and nano-coating and nitrite treatments up to 12 days had acceptable level.
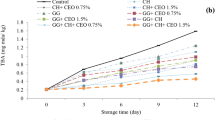
Total volatile base nitrogen (TVB-N)
TVB-N is mainly produced by bacterial and enzymatic degradation of meat proteins and non-protein nitrogen compounds. TVB-N is a general term that includes trimethylamine (caused by bacterial spoilage), dimethylamine (produced by autolytic enzymes during storage), ammonia (caused by the deamination of amino acids and nucleotide catabolites) and other volatile nitrogenous compounds [4]. According to the results, the highest values of TVB-N (Fig. 3d–f) were observed in control treatment on most days. TVB-N value in nano-coatings and nitrite treatments were lower than other treatments and in most of the time had acceptable level. Presence of bacteria in the meat leads to the autolysis and degradation of the proteins and production of various compounds such as trimethylamine oxides, peptides, amino acids etc. The higher bacterial loads in the control treatment can be justified to increase the amount nitrogen bases in them [4]. Edible coatings act as antimicrobial agents and affect the amount of volatile nitrogenous bases. Lopez-Caballero et al. [32] reported the protective effect of chitosan-gelatin composites on reducing TVB-N and consequently microbial spoilage. TVB-N values in the nano-coating treatments were lower than the other treatments. This is due to the increased antibacterial activity of the coatings after the nano process or the maintenance of the antibacterial properties for a longer period after the coating.
The desirable limit of TVB-N in meat and its products is reported to be 20 mg per 100 g of meat [30]. Accordingly, all treatments kept at − 2 °C until the end of the storage period had acceptable range. Treatments kept at 4 °C: control treatment up to 3 days, coating treatment up to 6 days and nano-coating and nitrite treatments up to 9 days had acceptable limit. Treatments kept at 8 °C: control treatment up to 3 days, coating treatment up to 5 days and nano-coating and nitrite treatments up to 6 days had acceptable range.
Total viable count (TVC)
Because of the chemical components of meat, it is a good place for growth and proliferation of many microorganisms especially bacteria. The surface of the meat is usually contaminated with various species of saprophytic organisms, especially coccobacillus sp, bacillus sp, and micrococcus sp. Bacteria in the natural condition secrete enzymes that cause tissue changes and eventually spoilage. After bacterial growth in the meat are occurred different changes such as unpleasant taste, odor and texture. The extension changes in taste and odor gradually becomes bitter or sulphide and as a result, the meat becomes unusable [30].
Over time at all three storage temperatures, TVC (Fig. 4) increased in all treatments and these changes were greater in control treatment. According to the results of statistical analysis, the highest values were observed in control treatment on most days. In most of the time, the lowest total bacterial counts in nano-coating and nitrite treatments were lower than the control, and these two treatments did not differ significantly at all times. Some studies carried out by Khan et al. [33] and Khazaei et al. [6] have shown that adding chitosan and seed gum to meat products inhabited the growth of TVC.
Various factors affect the antibacterial activity of chitosan. Although its exact mechanism has not been elucidated, different opinions have been presented. The theory has attributed this effect of chitosan to the presence of positively charged amino groups, which bind to the larger negatively charged molecules on the surface of the microbial cell, leading to bacterial cell membrane breakdown, intracellular components leakage, and ultimately death. In addition, chitosan can be effect on the lipopolysaccharide layer of the outer membrane [34, 35].
The results of TVC were also lower in the nano-coating treatments than in the other treatments. This is due to the increased antimicrobial activity of the nanoparticles. The use of nanoparticles improves the preservation of bioactive compounds, thereby protecting food from oxidation as well as pathogenic and spoilage microorganisms [18]. Increased antimicrobial activity of natural preservatives after nano-processing by different carriers has also been reported by other researchers [17, 18].
A total bacterial count of 7 log CFU/g meat has been suggested [36]. Accordingly, all treatments kept at − 2 °C until the end of the storage period had acceptable range. Treatments kept at 4 °C: control treatment up to 9 days, coating treatment up to 12 days, and nano-coating and nitrite treatments up to 15 days had acceptable limit. Treatments kept at 8 °C: control treatment up to 6 days coating treatment up to 9 days, and nano-coating and nitrite treatments up to 12 days had acceptable limit.
Pseudomonas aeruginosa
Pseudomonas is a gram-negative bacterium that is one of the main spoilage microorganisms in meat and meat products. As gram-negative bacteria (especially pseudomonas species) grow more rapidly under aerobic and cold conditions, they constitute the dominant microbial population in refrigerated and air-exposed meats [8, 37].
At all three storage temperatures, the amount of P. aeruginosa (Fig. 5a–c) increased in the control and coating treatments. This change was more than the control treatment. In fact, the addition of C and CSG composites decreased the amount of p. aeruginosa, and that represents the antimicrobial activity of chitosan. Various mechanisms have been reported for the antimicrobial activity of chitosan: (1) chitosan as a chelating agent of metal ions and nutrients inhibits bacterial growth, (2) chitosan is capable of binding to ionic groups in the surface of bacterial cells and the formation of polyelectrolyte complexes with these compounds This process inhibits the transport of nutrients into the cells and ultimately inhibits cell growth [9].
At − 2 °C: Nano-coating and nitrite treatments decreased to zero on day 6.
At 4 °C: Nano-coating and nitrite treatments decreased to zero on day 9.
At 8 °C: Nano-coating and nitrite treatments decreased to zero on day 12.
Overall, the highest values were observed in the control treatment for most days and the lowest values were observed in nano-coating and nitrite treatments. In fact, nano-coating increased the antimicrobial properties of the coatings. Donsi et al. [38] reported that bioactive nanoparticles may increase antimicrobial activity due to increased dispersal of antimicrobial agents in the aqueous phase and activation of the cellular uptake. In fact, the use of nano-coatings has been able to improve the physical and chemical stability of bioactive materials.
Staphylococcus aureus
Staphylococcus is a gram-positive round-shaped, beta hemolytic that is catalase and coagulase-positive and ferments mannitol. This bacterium is usually the cause of many human infections, and every human is infected with it at least once in their life. Staphylococcal food poisoning is one of the most important food poisoning so that from 24 million cases of food poisoning reported in the United States of America, 8.9 million were S. aureus [37, 39].
At all 3 storage temperatures, S. aureus (Fig. 5d–f) increased in the control treatment. In fact, the addition of chitosan coating with CSG decreased the bacterium (similar to P. aeruginosa). At all times, bacterial levels in the nano-coating treatment were lower than that of chitosan treatment. This is due to the increased antibacterial activity of the coatings after nano-coating.
According to the microbial permissible limits stated by the Department of Food and Drug Administration, the permissible limit for S. aureus in meat is log CFU/g:
At − 2 °C: In coating treatment and nano-coating and nitrite treatments were lower than permissible on day 6 and 3 respectively.
At 4 °C: In coating treatment and nano-coating and nitrite treatments were lower than permissible on day 9 and 3 respectively.
At 8 °C: In coating treatment and nano-coating and nitrite treatments were lower than permissible on day 12 and 3 respectively.
Conclusions
The results of the effect of different coatings on the quality and shelf life of beef also showed that the coating has antimicrobial and antioxidant properties and nano-coating composites increased its antimicrobial and antioxidant properties. The nanocomposites significantly delayed the microbial and oxidative spoilage process of the beef fillets and increased the shelf life of the beef at all three storage temperatures and had a similar effect to the synthetic nitrite preservative. Therefore, C and CSG nano composites can be used for extending shelf life meat product instead of composite coating in terms of low oxidative stability and microbial spoilage.
References
J.H. Han, Edible films and coatings: a review, in Innovations in food packaging, 2nd edn., ed. by J.H. Han (Acadmic Press, San Diego, 2014), pp. 213–255
A. Mohammadi Nafchi, A. Olfat, M. Bagheri et al., Preparation and characterization of a novel edible film based on Alyssum homolocarpum seed gum. J. Food Sci. Technol. 54, 1703–1710 (2017)
S.S. Rashidaie Abandansarie, P. Ariaii, M. Charmchian Langerodi, Effects of encapsulated rosemaryextract on oxidative and microbiological stability of beefmeat during refrigerated storage. Food Sci. Nutr. 7, 3969–3978 (2019)
F. Valipour Kootenaie, P. Ariaii, D. Khademi Shurmasti, M. Nemati, Effect of chitosan edible coating enriched with eucalyptus essential oil and α-tocopherol on silver carp fillets quality during refrigerated storage. J. Food Saf. 37(1), e12295 (2017)
A.S. Zargari, Medicinal Plants, vol. 4, 6th edn. (University of Tehran Publications, Tehran, 1997)
K.M. Khazaei, S. Jafari, M. Ghorbani, A.H. Kakhki, Application of maltodextrin and gum Arabic in microencapsulation of saffron petal’s anthocyanins and evaluating their storage stability and color. Carbohydr. Polym. 105, 57–62 (2014)
A. Ashrafi, M. Jokar, Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 108, 444–454 (2018)
V. Mahdavi, E. Hosseini, A. Sharifian, Effect of edible chitosan film enriched with anise (Pimpinella anisum L.) essential oil on shelf life and quality of the chicken burger. Food Sci. Nutr. 6(2), 269–279 (2018)
L. Qi, Z. Xu, X. Jiang, C. Hu, X. Zou, Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 339, 2693–2700 (2004)
P. Sanguansri, Nanoscale material development, a food industry perspective. Trend Food Sci. Technol. 175, 1447–1455 (2008)
H. Karazhiyan, S.M.A. Razavi, G.O. Phillips, Y. Fang, S. Al-Assaf, K. Nishinari, R. Farhoosh, Rheological properties of Lepidium Sativum seed extract as a function of concentration, temperature and time. Food Hydrocoll. 23, 2062–2068 (2009)
H.C. Carneiro, R.V. Tonon, C.R. Grosso, M.D. Hubinger, Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 115, 443–451 (2013)
E.K. Akkol, I.E. Orhan, E. Yeşilada, Anticholinesterase and antioxidant effects of the ethanol extract, ethanol fractions and isolated flavonoids from Cistus laurifolius L. leaves. Food Chem. 131, 626–631 (2012)
C. Silva, M.D. Torres, F. Chenlo, R. Moreira, Rheology of aqueous mixtures of tragacanth and guar gums: effects of temperature and polymer ratio. Food Hydrocoll. 69, 293–300 (2017)
A. Broumand, Z. Emam-Djomeh, M. Hamedi, S.H. Razavi, Antimicrobial, water vapour permeability, mechanical and thermal properties of casein based Zataraia multiflora Boiss. Extract containing film. LWT-Food Sci. Technol. 44(10), 2316–2323 (2011)
I.J. Joye, G. Davidov-Pardo, D.J. McClements, Encapsulation of resveratrol in biopolymer particles produced using liquid antisolvent precipitation. Part 2: Stability and functionality. Food Hydrocoll. 49, 127–134 (2015)
R. Bagheri, R. Izadi Amoli, N. Tabari Shahndash, S.R. Shahosseini, Comparing the effect of encapsulated and unencapsulated fennel extracts on the shelf life of minced common kilka (Clupeonella cultriventris caspia) and Pseudomonas aeruginosa inoculated in the mince. Food Sci. Nutr. 4(2), 216–222 (2016)
S.R. Javadian, S.R. Shahoseini, P. Ariaii, The effects of liposomal encapsulated thyme extract on the quality of fish mince and Escherichia coli O157: H7 inhibition during refrigerated storage. J. Aquat. Food Prod. Technol. 26(1), 115–123 (2017)
Iranian National Standard organization. (ISRI) 2001. Detection and enumeration method of Staphylococcus aureus coacolase (+) in food. No. 1194
A. Pavelková, M. Kačániová, E. Horská, K. Rovná, L. Hleba, J. Petrová, The effect of vacuum packaging, EDTA, oregano and thyme oils on the microbiological quality of chicken’s breast. Anaerobe 29, 128–133 (2013)
S. Jafarzadeh, S.M. Jafari, A. Salehabadi, A.M. Nafchi, U.S. Uthaya Kumar, H.P.S.A. Khalil, Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 100, 262–277 (2020)
M. Tajik, H. Rohani, M. Uromiei, A. Malekinejad, S. Dehkordi, Evaluation of antioxidant characteristics, color and antibacterial effects of chitosan edible film containing essential oils against listeria monocytogenes. J. Armagane danesh 16, 60 (2008)
P. Ezhilarasi, P. Karthik, N. Chhanwal, Nanoencapsulation techniques for food bioactive components: a review. Food Bioprocess. Technol. 6, 628–647 (2013)
M.T. Yen, Y.H. Tseng, R.C. Li, J.L. Mau, Antioxidant properties of fungal chitosan from shiitake stipes. LWT–Food Sci. Technol. 40, 255–261 (2007)
G. Biliaderis, D.S. Marta, P. Izydorczyk, Functional Food Carbohydrates (CRC Press, Boca Raton, 2007), pp. 215–238
H. Sashiwa, S. Aiba, Chemically modified chitin and chitosan as biomaterials. Progress Polym. Sci. 29, 887–908 (2004)
M. Najaf_Najafi, A. Fazeli, Evaluation of Lepidium sativum seed gum effect on physical stability and flow properties of oil-in-water emulsion prepared by high-speed dispersing. FSCT 14(64), 126–116 (2016)
V. Samavati, Z. Emam-Djomeh, M.A. Mohammadifar, M. Omid, A. Mehdinia, Influence of tragacanth gum exudates from specie of Astragalusgossypinus on rheological and physical properties of whey protein isolate stabilized emulsions. Int. J. Food Sci. Technol. 46(8), 1636–1645 (2011)
B.B. Bostan., K. Varlık., C. Uran, H. Üçok, A.D. Sivri, Effects of chitosan treatment on the quality parameters of shrimp (Parapenaeus longirostris) during chilled storage. Turk. J. Fish. Aquat. Sci. 15, 821–831 (2015)
M.M.S. Ashour, R.K. Moawad, G.F. Bareh, Quality enhancement and shelf-life extension of raw beef patties formulated with lactate/thyme essential oil during refrigerated storage. J. Appl. Sci. Res. 9(13), 6699–6709 (2013)
Y. Yanar, Quality changes of hot smoked catfish (Clarias Gariepinus) during refrigerated storage. J. Muscle Foods 18, 391–400 (2007)
M.E. López-Caballero, O. Martínez‐Alvarez, M.D.C. Gómez‐Guillén, P. Montero, Quality of thawed deepwater pink shrimp (Parapenaeus longirostris) treated with melanosis‐inhibiting formulations during chilled storage. Int. J. Food Sci. Technol. 42(9), 1029–1038 (2007)
I. Khan, C. Nkufi Tango, O. Deog-Hwa, Development and evaluation of chitosan and its derivative for the shelf life extension of beef meat under refrigeration storage. Int. J. Food Sci. Technol. 52, 1111–1121 (2017)
C.O. Jeon, Y.V.A. Kamil, F. Shahidi, Chitosan as an edible invisible film for quality preservation of Herring and Atlantic Cod. J. Agric. Food Chem. 50, 5167–5178 (2002)
F. Dalvandi, H. Almasi, B. Ghanbarzadeh, H. Hosseini, K. Khosroshahi, Effect of vacuum packaging and edible coating containing black pepper seeds and turmeric extracts on shelf life extension of chicken breast fillets. J. Food Bioprocess Eng. 3(1), 69–78 (2020)
ICMSF, Microorganisms in Foods 6: Microbial Ecology of Food Commodities, 2nd edn. (Plenum Publishers, New York, 2005) (1st edn published 1998)
J.M. Jay, M.J. Loessner, D.A. Golden, Modern Food Microbiology, 7th edn. (Springer, New York, 2005)
F. Donsì, M. Annunziata, M. Vincensi, G. Ferrari, Design of nanoemulsion-based delivery systems of natural antimicrobials: effect of the emulsifier. J. Biotechnol. 159, 342–350 (2012)
Z. Kiarsi, M. Hojjati, B.A. Behbahani, M. Noshad, In vitro antimicrobial effects of Myristica fragrans essential oil on foodborne pathogens and its influence on beef quality during refrigerated storage. J. Food Saf. 40(3), e12782 (2020)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Esmaeili, M., Ariaii, P., Nasiraie, L.R. et al. Comparison of coating and nano-coating of chitosan- Lepidium sativum seed gum composites on quality and shelf life of beef. Food Measure 15, 341–352 (2021). https://doi.org/10.1007/s11694-020-00643-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-020-00643-6