Abstract
Guar gum based coatings coupled with extracts derived from different condiments (Nigella sativa, Coriandrum sativum, Foeniculum vulgare miller and Laurus nobilis) have been found in this study to prolong the storage life and quality during postharvest life of tomato fruits. These coatings were successful to preserve the quality parameters of tomato fruits up to 60 days at 10 °C (85% relative humidity) and also maintained green maturity phase of tomatoes throughout the postharvest storage. The rate of change in total soluble solids, titratable acidity and pH of tomato fruits that were coated with guar gum + ethanolic extracts were found to be lower compared to both controls (uncoated and treated control) and coatings supplemented with methanolic extracts. In addition to physicochemical properties, percent rate of increment in bioactive compounds and bacterial load in terms of total viable counts were significantly reduced for tomatoes coated with guar gum incorporating ethanolic extracts. The application of the proposed edible coating could be used in the preservation of tomatoes specifically for retaining the same maturity phase of these fruits during storage without any spoilage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tomato (Solanum lycopersicum L.) is rich in important constituents that reduces the risk of cancer and cardiac ailments. The necessary constituents which contribute to the anticancer attributes are carotenoids mainly β-carotene and lycopene which gets deposited in tissues and extracellular matrix of cells on ingestion of tomato fruit. In addition to carotenoids, tomatoes are also a rich reservoir of other antioxidants which decelerates the process of lipid oxidation or generation of free radicals inside the body which are harmful to the cells. These antioxidants delay or inhibit the free radicals by terminating chain reactions of lipid oxidation [1]. Apart from these nutraceutical aspects, tomato is considered a very common ingredient of Asian culinary dishes. It is consumed in raw as well as cooked form depending on the necessity of the recipe. Therefore, tomato holds an important position among the fruits that are cultivated in South Asian countries [2]. As per statistical database of Food and Agriculture organization (FAOSTAT), since 2009 more than 150 million tons of tomatoes are harvested per annum [3]. Due to many biochemical and biological processes in the fruit, the tomato fruit has a reduced shelf life of 7 days. These processes include increased rate of maturity and senescence, loss of moisture content and contaminating fungus. Temperature plays an important role in delaying ripening of fruit. Increase in ethylene production and maturity is directly proportional to increase in temperature and fruits must be held below 0 °C to extend shelf life and reduce postharvest losses. As tomatoes are sensitive to temperatures below 10 °C therefore, the use of controlled or modified atmospheres is required to store such chill-sensitive fruits [4]. Hypobaric storage, modified atmosphere packaging (MAP) and controlled atmosphere packaging (CAP) can extend storage life of tomatoes but these processes lack economic feasibility. Therefore, it is of commercial interest to search for alternatives that simultaneously elevate the shelf life and are also cheaper.
Edible coatings create a modified atmosphere by generating a semi-permeable barrier against gaseous exchange (CO2 and O2) and solute diffusion, thus decreasing the rate of respiration, loss of moisture and prevents generation of reactive oxygen species. Proteins, lipids, polysaccharides have been commonly employed as edible coating materials. Apart from preventing the fruit decay, these edible coatings are derived from natural sources and are not artificially synthesized i.e. Generally regarded as safe (GRAS) [5]. Many researches have been conducted on the production of edible coatings on fruits and vegetables which includes, Gum Arabic, hydroxypropyl methyl cellulose [6], alginate or zein [6], rice starch [7], chitosan [8], beeswax [6] and aloe vera [9]. Guar gum also known as Guaran, clusterbean, Gum cyamposis, Guarina and Guyan is a natural non-ionic polysaccharide which is easily soluble in water at room temperature and is derived from the endosperm of cluster bean seeds. It is a galactomannan comprising of α (1,4)-linked β-d-galactose (i.e. 1,6-linked-α-d-galactopyranose) [10, 11]. Guar gum like other galactomannans is used in a variety of ways for human utilization. These are excellent stabilizers and stiffeners of emulsions, and due to their GRAS nature, they are consumed in therapeutics, textile, cosmetics, biomedical and food sector [12]. Edible coatings made from galactomannans possess benefits of having decreased gas transfer rates, good mechanical and optical properties and are non-toxic for human consumption. Galactomannans are used in synthesizing edible films/coatings for food applications [13]. In this work, coatings of guar gum incorporated with spices’ extracts on tomato fruit were evaluated. The suitability to coat tomatoes have also been assessed by various biochemical, physiological and microbiological aspects of different blends of edible coatings. The coatings were compared with the GG (guar gum) coating and with the extract rich coatings which have been supplemented with different antibacterial extracts. This research is novel in the sense that not only the use of guar gum to coat tomatoes is carried out for the first time but also the addition of spice extracts in galactomannan coatings is a novel aspect of this study.
Methodology
Chemicals
All chemicals used in this research were of analytical grade and were obtained from Sigma Aldrich (Sigma Aldrich GmbH, Sternheim, Germany). Tryptone soy agar was purchased from Thermo Scientific™ Oxoid™.
Preparation of coating solution
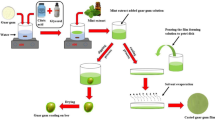
The coating solutions were prepared by dissolving 1.5 g guar gum (GG), 2 mL glycerol and 0.2 mL of different spice extracts in 97.8 mL of distilled water (20 °C) thus making final volume of coating solution equal to 100 mL. The resulting mixture was stirred at room temperature for 120 min and left undisturbed for 10 min. The extracts were extracted using the method of Cheikh-Rouhou et al. [14]. The extracts used in this research included methanolic and ethanolic extract of nigella seeds (MN and EN), methanolic and ethanolic extract of coriander seeds (MC and EC), methanolic and ethanolic extract of Bay leaf (MB and EB) and methanolic and ethanolic extract of fennel seeds (MF and EF) respectively.
Coating of tomato fruits
Green, un-ripe tomatoes having approximately same weight, maturity stage and free from any physical deformities were selected for this study. The fruits were washed with tap water to remove any dust particles from the surface and subsequently air dried. After drying, the tomatoes were tied with white cotton thread. The tomatoes were dipped in coating solutions thrice with the interval of 10 s, hung and left to dry at room temperature under continuous air flow. After complete drying, the tomatoes were packed in zip-lock bags and stored at 10 °C (80–85% relative humidity) in a cooling incubator (Heraeus BK 600). Each treatment was labeled according to the added extracts i.e. coating added with methanolic extract of Fennel seeds was labeled as MFEO. Two control sets were also run alongside. Treated control had the coating formulation as 1.5 g guar gum, 2 mL of glycerol and 98 mL of distilled water and untreated control/uncoated were the control sets. Treated control was labelled as GG whereas untreated control was labelled as UN. The control fruits were kept in zip-lock bag and at the same temperature (10 °C) and 85% relative humidity (RH).
Monitoring of maturity phase
Tomato fruits were graded according to the color grading mentioned in United States department of Agriculture (USDA) manual of tomato inspection. The following parameters were used to define color that corresponds to specific maturity stage of tomatoes [15]. The grading § 51.1860 are as follows:
-
1.
Green The color of tomato is green, and the shade of green may differ from lighter one to darker one.
-
2.
Breakers 10% of fruit has a transition in color ranging from green to yellow or even red in color
-
3.
Pink 30–60% of the fruit is pink or reddish in shade
-
4.
Light red > 60% and < 0% red in color or may be pinkish red in color
-
5.
Red > 90% of the fruit surface is in red shade
Preparation of tomato homogenate
One gram of diced tomato from treated and control samples was sliced and blended with 2 mL of distilled water as explained by Davis et al. [16]. The blend was filtered using Whatman filter paper and was collected in polypropylene centrifuge tubes. This tomato homogenate (1 mL) was further diluted with 4 mL of distilled water and was used to determine titratable acidity, pH and total soluble solids.
Titratable acidity
Titratable acidity was determined according to the method of Tiwari et al. [17]. Sodium hydroxide (0.1 N) was used to titrate this solution to the phenolphthalein end point (pH 8.2 ± 0.1). The titer volume of NaOH was converted into g citric acid/100 mL of tomato homogenate and titratable acidity values were calculated using the formula:
where, v is the volume of 0.1 N NaOH used and m is the mass of the tomato homogenate.
pH determination
The pH of tomato homogenates obtained from both controls and test samples was determined using digital pH meter [17].
Total soluble solids (Brix˚)
Total solids were determined using hand-held refractometer (ATAGO N-1α Japan) in terms of ˚Brix. The prism of refractometer was cleaned with distilled water after taking each reading [17].
Ascorbic acid content
Ascorbic acid content was determined according to the method of Barros et al. [18]. One hundred milligrams of tomato homogenate were extracted with 10 mL of 1% meta-phosphoric acid (w/v) at ambient temperature. One milliliter of this solution was mixed with 9 mL of DCPIP (2,6-Dichlorophenolindophenol) and absorbance was measured at 515 nm against a blank. The results were reported on the basis of a standard curve of l-ascorbic acid and expressed as mg of ascorbic acid/g tomato homogenate.
Antioxidant activity
Antioxidant activity of tomato homogenates was determined according to the method of Han et al. [19]. Two hundred microliters of tomato homogenate were mixed with 2.7 mL of 0.06 mM methanolic solution of DPPH (2,2-diphenyl-1-picrylhydrazyl) and incubated at room temperature for 30 min. The absorbance of the resulting mixture was measured at 515 nm using UV–Vis spectrophotometer (JascoV-670 UV–VIS-NIR Spectrophotometer Tokyo, Japan) and expressed as percent scavenging activity using the formula:
where, S is the sample, SB is the absorbance of blank sample (2000 µL methanol + 200 µL tomato homogenate), C is the absorbance of the control (2000 µL DPPH + 200 µL methanol) and, CB is the absorbance of the blank control i.e. methanol.
Total phenolic content
Total phenolic content was evaluated using the method of Waterhouse [20] and calibrated against the standard curve of gallic acid. Twenty microliters of tomato homogenate were added to a mixture containing 1.58 mL of distilled water and 100 µL of Folin–Ciocalteu reagent. After incubation for 8 min, 0.3 mL of aqueous 15% (w/v) sodium bicarbonate was added. The reaction mixture was left at room temperature for 2 h and subsequent measurement of absorbance was made at 765 nm using UV–Vis spectrophotometer (JascoV-670 UV–Vis-NIR Spectrophotometer Tokyo, Japan).
β-carotene, lycopene, chlorophyll ‘a’ and chlorophyll ‘b’ contents
Pigments were extracted by the method of Bhumsaidon et al. [21] using 1 g of tomato fruit with 4:6 (v/v) Acetone: n-hexane using Ultra-Turrax homogenizer for 1 min. After filtration, the absorbance of the supernatant was determined at 663 nm, 645 nm, 505 nm and 453 nm using UV–Vis spectrophotometer (JascoV-670 UV–Vis-NIR Spectrophotometer Tokyo, Japan). The pigment contents were calculated using following equations in mg/100 mL:
(A663, A645, A505 and A453 are absorbance at 663 nm, 645 nm, 505 nm and 453 nm respectively.)
Microbiological count
Microbial load in tomato samples at zero and at the end of the storage period (60th day) was determined in terms of log CFU/mL [7]. One gram of tomato fruit was aseptically sliced and homogenized in 10 mL of peptone water and vortexed for 2 min. 100 µL of 10-fold serial dilutions were spread on the surface of tryptone soy agar and the plates were incubated at 37 ± 2 °C for 18 h (n = 3). Total viable counts were made and converted into log CFU/mL.
Statistical analysis
Analysis of variance was employed to compute significant differences between the means, and Duncan’s test at P ˂ 0.05 was used to separate means using SPSS software (version 24, SPSS Inc., USA).
Results and discussion
Shelf life of tomatoes
The coating solution prepared using 1.5% of guar gum and 2% glycerol had good coating capability as this formulation yielded a smooth and homogenous surface after being applied to fruits and hence was opted for the coating applications. Antimicrobial compounds rich extracts were supplemented at the concentration of 0.2% (v/v). It is remarkable to mention that the extracts preserved tomatoes in such lower concentration. All biological coatings applied on tomato fruits to date comprise of starch, chitosan and gum Arabic, compounded with glycerol. In the present study, a novel blend of guar gum, glycerol and spice extracts were used to enhance the shelf life of perishable food. As this blend is comprised of extracts derived from spices and herbs such as Nigella, fennel, coriander seeds and bay leaf it is a safer and a healthier alternative to synthetic preservatives. Ghannadi et al. [22] elucidated the antioxidant potential of Nigella seeds polyphenols and elaborated their analgesic and anti-inflammatory responses when administered orally. Ahmad et al. [23] reported the presence of biologically active compounds such as trans-anethole and petroselinic acid in Fennel seed extracts. Magnesium, calcium, boron and copper was reported in highest contents in coriander seeds (Gamze CV.) [24] and the bay leaf was found to be the highest source of antioxidant activities i.e. IC50 111.5 ± 0.62 µg/mL [25]. On contrary, artificial preservatives exert a harmful effect not only on humans but also on the environment when these chemicals are discharged into environment. These herbal decoctions have also exhibited antibacterial activity against food borne pathogens in vitro but also were inhibitory when tested in vivo conditions even at lowest tested concentrations i.e. 62.5 µg/mL [26]. Furthermore, their addition in food systems exert no harmful effect on the food safety. The diffusion of the active compound in the food matrix results in the preservation of the coated or preserved food enhancing the storage stability but also the antioxidant content of the product during its shelf life [27]. This blend successfully increased the shelf life of tomatoes up to 60 days during cold storage. However, the guar gum coated (treated control) had a shelf life of 30 days and uncoated fruits decayed on the 15th day of storage. Therefore, the shelf life of coated tomatoes was successfully improved on contrary to both controls. All the tested fruits survived up to green maturity phase. USDA Manual was used as a guide to detect the maturity phase of tomatoes using color grading (§ 51.1860 Color classification number 1) [15].
pH, titratable acidity (TA) and total soluble solids (Brix˚)
The values obtained for titratable acidity, pH and ˚Brix of treated and untreated tomato fruits are represented in Table 1a–c. On a general basis, increase in total soluble solids content was observed during the entire storage period of tomato fruits. The TSS content was significantly higher (P ˂ 0.05) in uncoated and guar gum (control) compared to treated tomato fruits. Similar observations were made by Mejía-Torres et al. [28]. EFEO and ENEO had no effect on TSS of fruit while TSS was increased in a multiple of 3 and 4 in GG and UN respectively. This is mainly because of the excellent semi-permeable envelop around the treated fruit, changing the internal atmosphere by increasing CO2 and inhibiting the ethylene generation. This deceleration in respiration rates simultaneously decreased the synthesis and consumption of metabolites thus lowering TSC [29].
The titratable acidity (TA) values of coated and uncoated fruits during storage was decreased with increase in storage days and the values were significantly highest (P ˂ 0.05) in fruits coated with EBEO (i.e. 6.70 g citric acid/100 mL on 60th day of storage). However, both the controls had higher TA values within a shorter duration of time i.e. 6.04 g citric acid/100 mL and 12.06 g citric acid/100 mL on 30th and 15th day of storage in GG and UN respectively. The results were congruent to observations made by Mejía-Torres et al. [28]. The low level of titratable acidity in control fruit in contrast to treated/coated fruit samples suggested that these coating solutions served as a semi-permeable layer around the fruit hence delay in ripening was observed. Since organic acids such as citric acid and malic acid are the basic elements for respiration process, a decrease in acidity may occur in a fruit with elevated levels of respiration [30]. The synthetic edible coatings are known to decrease the rate of respiration and subsequently delayed utilization of organic acids [29]. Edible coatings and films have been reported to preserve the titratable acidity of fruits [31].
The pH values of coated as well as uncoated fruits increased during the storage and a statistically significant difference was observed in the readings (P ˂ 0.05). The increase in pH was due to the breakdown of organic acids into neutral compounds. The coating solution that comprised of ECEO had little effect on TSS (pH 4.39 on 60th day of storage) of treated fruits whereas GG and UN had 4.38 and 4.36 pH on 30th and 15th day of storage (Table 1a). The present investigation showed that lower pH values were emphatically associated with slower rate of respiration and an effective quality preservation of tomato fruits. An inverse relationship was also observed between pH and titratable acidity and are used as a measure for acidity index of tomato fruits.
Ascorbic acid content
The levels of ascorbic acid in treated and control fruits showed a rise during the storage period (Fig. 1). The lowest concentration of ascorbic acid was found in fruits coated with ENEO i.e. 53.3 mg ascorbic acid/g of tomato homogenate. The highest content of ascorbic acid was found in fruit coated with MFEO i.e. 83 mg ascorbic acid/g of tomato homogenate. The increase in ascorbic acid content peaked during the 45th day to 60th day at 10 °C. On contrary, the ascorbic acid content on 30th and 15th of storage in GG and UN were 16 mg ascorbic acid/g and 19 mg ascorbic acid/g respectively. The rate of ascorbic acid increase was significantly decreased by the application of these edible coatings. Increase in ascorbic acid contents likewise other parameters is linked to the process of ripening [32]. The lowering in the rate of increase in ascorbic acid content showed that edible coating slowed down the synthesis of Vitamin C but could not halt its synthesis [33].
Effect of different edible coatings on ascorbic acid content of tomato during storage (10 °C). The vertical bars represent the standard error of means of three replicates. MFEO; MNEO; MBEO; MCEO guar gum coating added with methanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, EFEO, ENEO, EBEO, ECEO guar gum coating added with ethanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, GG guar gum coated control, UN uncoated control
Total phenolic content (TPC)
The quantification of phenols was carried out in this study which is a major indicator of maturation process going inside the fruit. The phenols are generated when the fruit is undergoing ripening process. As the proposed edible coatings decreased the production rate of polyphenols therefore, the maturation process was also decelerated in coated fruits. Other source of phenols included the extracts that were added to the coatings. But the presence of these phenols has been nullified by keeping the control sets. The phenolic content of tomato homogenate obtained from treated tomatoes is nearly the same as in the control sets. This excludes the quantification of phenols that have been diffused from the coatings to the pulp of the fruits. The highest TPC was observed in fruits coated with MCEO (Fig. 2) at 60th day of storage. On contrary, both untreated (UN) and treated control (GG) showed elevated levels of TPC within shorter period i.e. 15 and 30 days i.e. 0.25 mg/L and 0.5 mg/L respectively. The fruits treated with ENEO showed a gradual increase in TPC during 60 days of storage and the lowest TPC values i.e. 0.546 GAE mg/L.
Effect of different edible coatings on total phenolic content of tomato during storage (10 °C). The vertical bars represent the standard error of means of three replicates. MFEO; MNEO; MBEO; MCEO guar gum coating added with methanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, EFEO, ENEO, EBEO, ECEO guar gum coating added with ethanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, GG guar gum coated control, UN uncoated control
TPC of the fruits under study increase with the increase in the antioxidant capacities [34]. All the treated tomato fruits showed increment in TPC due to ripening process but the decline in TPC was not observed as the fruits decayed before reaching the maximum maturity stage.
Pigments content
β-carotene and lycopene contents of treated control, untreated and treated fruits should increase over the period of time (Figs. 3, 4). However, chlorophyll ‘a’ and chlorophyll ‘b’ contents decreased with storage (Figs. 5, 6). The levels of β-carotene and lycopene were lowest (0.056 mg/100 mL β-carotene and 0.14 mg/100 mL lycopene) in EFEO coated fruits whereas the highest values of chlorophyll ‘a’ and chlorophyll ‘b’ (0.2 mg/100 mL chlorophyll a and 0.3 mg/100 mL chlorophyll b) were observed for EFEO coated fruits. Treated and untreated control fruits had a greater rate of increase in pigment contents within lesser period of time. β-carotene content was 0.9 mg/100 mL and 0.5 mg/100 mL, lycopene content was 0.5 mg/100 mL and 0.3 mg/100 mL, chlorophyll ‘a’ content was 0.2 mg/100 mL and 0.1 mg/100 mL and Chlorophyll ‘b’ content was 0.2 mg/100 mL 0.16 mg/100 mL in GG and UN during 30th and 15th day of storage. The fruits when undergoing ripening process, the levels of β-carotene and lycopene increases along with a decrease in chlorophyll pigments (‘a’ and ‘b’) [1]. The pigments production and breakdown are merely controlled by the rate of respiration and temperature of storage. Parallel observations were obtained when tomato fruit was stored at 4 °C [1].
Effect of different edible coatings on β-carotene of tomato during storage (10 °C). The vertical bars represent the standard error of means of three replicates. MFEO; MNEO; MBEO; MCEO guar gum coating added with methanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, EFEO, ENEO, EBEO, ECEO guar gum coating added with ethanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, GG guar gum coated control, UN uncoated control
Effect of different edible coatings on lycopene content of tomato during storage (10 °C). The vertical bars represent the standard error of means of three replicates. MFEO; MNEO; MBEO; MCEO guar gum coating added with methanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, EFEO, ENEO, EBEO, ECEO guar gum coating added with ethanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, GG guar gum coated control, UN uncoated control
Effect of different edible coatings on chlorophyll ‘a’ content of tomato during storage (10 °C). The vertical bars represent the standard error of means of three replicates. MFEO; MNEO; MBEO; MCEO guar gum coating added with methanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, EFEO, ENEO, EBEO, ECEO guar gum coating added with ethanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, GG guar gum coated control, UN uncoated control
Effect of different edible coatings on chlorophyll ‘b’ content of tomato during storage (10 °C). The vertical bars represent the standard error of means of three replicates. MFEO; MNEO; MBEO; MCEO guar gum coating added with methanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, EFEO, ENEO, EBEO, ECEO guar gum coating added with ethanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, GG guar gum coated control, UN uncoated control
Total antioxidant capacities
The results of antioxidant content were represented as radical scavenging abilities (%). The lowest antioxidant capacities were observed in fruits coated with supplementation of EFEO i.e. 72% scavenging abilities on 60th day of storage (Fig. 7). However, the rate of increase in antioxidant activity was elevated in both controls within lesser duration of time i.e. 64% and 39% for GG and UN on 30th and 15th day of storage. This indicates of their incapability to reduce the rate of biochemical reactions occurring inside the fruit and hence the increase in antioxidant contents of fruits was observed.
Effect of different edible coatings on total antioxidant activity (radical scavenging abilities in percent) of tomato during storage (10 °C). The vertical bars represent the standard error of means of three replicates. MFEO; MNEO; MBEO; MCEO guar gum coating added with methanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, EFEO, ENEO, EBEO, ECEO guar gum coating added with ethanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively. GG guar gum coated control, UN uncoated control
The tomato fruits comprise of antioxidants that are carotenoids, phenolic compounds and ascorbic acid [1]. The antioxidant capacities depend on factors such as soil type, date of harvest, post-harvest events, genetics and environmental attributes [35]. On a general basis, the levels of TPC are directly linked to antioxidant capacities [34]. Likewise, other bioactive compounds, antioxidant capacities also fluctuate with ripening process. Due to alterations in lipophilic antioxidant abilities, the antioxidant activity of the fruits also increased [36]. The antioxidant activities are also dependent on the concentrations of γ-tocopherol, ascorbic acid and β-carotene [35].
Microbiological analysis
The microbiological analysis of the tomato fruits at zero and final day of storage was carried out to have a comparison of the total viable count of bacteria after treatments (Fig. 8). From the Duncan’s test applied on readings of zero day, it could be concluded that only the MFEO coated fruits found to be significantly different while on 60th day, all the treatments were found to be statistically different (P ˂ 0.05). On contrary, both the control sets had an increase in the total viable count on the last day of storage (30 and 15 days for GG and UN respectively). The edible coatings supplemented with EFEO not only prevented the bacterial contamination but also reduced the microbial counts that were present initially in the fruits due to their antibacterial activities. A detailed study was conducted to explain that these extracts exert bactericidal action when tested in vitro [37]. The study showed the activity of these extracts against foodborne pathogens such as i.e. Escherichia coli ATCC 8739, Listeria monocytogenes ATCC 13932, Vibrio parahaemolyticus ATCC 17802, Bacillus cereus ATCC 11778 and Vibrio alginolyticus ATCC 17749 and also elucidated that the prolonged incubation with these extracts were inhibitory to the tested pathogens via Scanning electron microscopy (SEM) images. However, parallel results were not obtained for treated and untreated control fruits. After 60 days of incubation, the fruits were covered with white fungal mycelia indicating the antifungal potency of these coatings protected the fruits up to 60 days. On contrary, commercially available antifungal compounds could not decrease the fungal colonies when tomatoes were stored for 30 days [38]. The microbial counts increased in both the control fruits showing the lack of these coatings to prevent contaminating bacteria from invading the fruits during storage while Workneh et al. [38] and Ahmed et al. [39] reported increase in bacterial counts during storage. The edible coatings supplemented with extracts significantly enhance the antimicrobial activity of coatings.
Effect of different edible coatings on microbial load of tomato during storage (10 °C). Values are mean of three replicates. Lower case letters on black bars indicate significant differences in log CFU/mL at 0th day whereas numbers on white bars indicate significant differences in log CFU/mL values on last day of storage (P < .05). The vertical bars represent the standard error of means of three replicates. MFEO; MNEO; MBEO; MCEO guar gum coating added with methanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, EFEO, ENEO, EBEO, ECEO guar gum coating added with ethanolic extract of fennel seeds, nigella seeds, bay leaf and coriander seeds respectively, GG guar gum coated control, UN uncoated control
The results reported in this study showed that seed oils of ethanolic origin were comparatively more potent in slowing down the rate of biochemical reactions occurring inside the coated fruit. Together with biochemical processes, these edible coatings formulations also delayed physicochemical changes in tomato pulp. Due to higher contents of total flavonoid, total flavonol, phenolic and antioxidant contents may contribute to higher preservative effects [40]. The abundance in antioxidant contents rendered these edible coatings to scavenge reactive oxygen species at a higher rate [41]. Supplementation of seed oils to edible coatings may affect the gaseous exchange through the packaging material by contributing as oxygen scavengers.
Conclusions
In a nutshell, all edible coatings with added extracts served as a semi-permeable membrane to slow down the rate of respiration in coated fruits. The results were remarkable when compared to both controls which failed to either increase the shelf life or to decrease the rate of biochemical reactions that causes senescence. Extracts of both the methanolic and ethanolic origin extended the shelf life up to 60 days during cold storage but coatings that contained ethanolic extracts slowed down the process of ripening as compared to its counterpart. The results justify the use of proposed edible coatings where immature tomato fruits are required for consumption.
References
A. Ali, M. Maqbool, P.G. Alderson, N. Zahid, Postharvest Biol. Technol. 76, 119–124 (2013)
A. Pieroni, L. Houlihan, N. Ansari, B. Hussain, S. Aslam, J. Ethnopharmacol. 113(1), 100–110 (2007)
Y. Hou, J. Gao, L. Gu, S. Wang, R. Zeng, J. Food Process. Preserv. 39(6), 949–955 (2015)
P. Gómez, M. Ferrer, J.P. Fernández-Trujillo, A. Calderón, F. Artés, M. Egea-Cortines, J. Weiss, J. Sci. Food Agric. 89(9), 1543–1551 (2009)
A. Ali, M. Maqbool, S. Ramachandran, P.G. Alderson, Postharvest Biol. Technol. 58(1), 42–47 (2010)
C. Fagundes, L. Palou, A.R. Monteiro, M.B. Pérez-Gago, Postharvest Biol. Technol. 92, 1–8 (2014)
D.K. Das, H. Dutta, C.L. Mahanta, LWT-Food Sci. Technol. 50(1)), 272–278 (2013)
P. Di Pierro, A. Sorrentino, L. Mariniello, C.V.L. Giosafatto, R. Porta, LWT-Food Sci. Technol. 44(10), 2324–2327 (2011)
K. Athmaselvi, P. Sumitha, B. Revathy, Int. Agrophys. 27(4), 369 (2013)
A. Biswas, P. Mourya, D. Mondal, S. Pal, G. Udayabhanu, J. Mol. Liq. 251, 470–479 (2018)
J.L.M. Silveira, T.M.B. Bresolin, Quim. Nova 34(2)), 292–299 (2011)
M.A. Cerqueira, ÁM. Lima, J.A. Teixeira, R.A. Moreira, A.A. Vicente, J. Food Eng. 94(3), 372–378 (2009)
M. Cerqueira, A. Bourbon, A. Pinheiro, J. Martins, B. Souza, J. Teixeira, A. Vicente, Trends Food Sci. Technol. 22(12), 662–671 (2011)
S. Cheikh-Rouhou, S. Besbes, B. Hentati, C. Blecker, C. Deroanne, H. Attia, Food Chem. 101(2), 673–681 (2007)
U.D.O. Agriculture, United States Standards for Grades of Fresh Tomatoes, (U.D.O. Agriculture, Washington, DC, 1997)
L. Ciaccheri, L. Tuccio, A.A. Mencaglia, A.G. Mignani, E. Hallmann, K. Sikorska-Zimny, S. Kaniszewski, M.J. Verheul, G. Agati, J. Food Compos. Anal. 71, 65–71 (2018)
B. Tiwari, K. Muthukumarappan, C. O’donnell, P. Cullen, LWT-Food Sci. Technol. 41(10), 1876–1883 (2008)
L. Barros, M.-J. Ferreira, B. Queiros, I.C. Ferreira, P. Baptista, Food Chem. 103(2), 413–419 (2007)
J. Han, X. Weng, K. Bi, Food Chem. 106(1), 2–10 (2008)
A.L. Waterhouse, Determination of total phenolics. In: Current Protocols in Food Analytical Chemistry, (Wiley, New York, 2003)
A. Bhumsaidon, M. Chamchong, Agric. Nat. Resour. 50(4), 257–263 (2016)
M.S. Butt, M.T. Sultan, Crit. Rev. Food Sci. Nutr. 50(7), 654–665 (2010)
B.S. Ahmad, T. Talou, Z. Saad, A. Hijazi, M. Cerny, H. Kanaan, A. Chokr, O. Merah, Ind. Crops Prod. 111, 92–98 (2018)
E. Beyzi, K. Karaman, A. Gunes, S.B. Beyzi, Ind. Crops Prod 109, 74–78 (2017)
R. Sudan, M. Bhagat, S. Gupta, T. Devi, Free Radicals Antioxid. 3, S70–S73 (2013)
A. Naeem, T. Abbas, T.M. Ali, Hasnain, Arab. Gulf J. Sci. Res. 34(1/2), (2016)
B. Shan, Y.Z. Cai, J.D. Brooks, H. Corke, Int. J. Food Microbiol. 117(1), 112–119 (2007)
S.I. Mejía-Torres, M.I. Vega-García, J.A. Valverde-Juárez, J.O. López-Valenzuela, J.O. Caro-Corrales, J. Food Qual. 32(6), 735–746 (2009)
C. Mannozzi, U. Tylewicz, F. Chinnici, L. Siroli, P. Rocculi, M. Dalla Rosa, S. Romani, Food Chem. 251, 18–24 (2018)
A. El-Anany, G. Hassan, F.R. Ali, J. Food Technol. 7(1), 5–11 (2009)
B. Saberi, J.B. Golding, J.R. Marques, P. Pristijono, S. Chockchaisawasdee, C.J. Scarlett, C.E. Stathopoulos, Postharvest Biol. Technol. 137, 9–20 (2018)
M. Tigist, T.S. Workneh, K. Woldetsadik, J. Food Sci. Technol. 50(3), 477–486 (2013)
S. Masood, M.A. Randhawa, M.S. Butt, M. Asghar, J. Food Process. Preserv. 40(1), 3–13 (2016)
X. Li, M. Li, J. Wang, L. Wang, C. Han, P. Jin, Y. Zheng, Postharvest Biol. Technol. 137, 106–112 (2018)
E. Azzini, G. Maiani, A. Turrini, F. Intorre, G. Lo Feudo, R. Capone, F. Bottalico, H. El Bilali, A. Polito, J. Sci. Food Agric. 2018, 1097 (2018)
L. Ávila-Juárez, H. Miranda-Rodríguez, J. Chem. 2018, 1–9 (2018)
A. Naeem, T. Abbas, T.M. Ali, Hasnain, Food Technol. Biotechnol. 56(2), (2018) https://doi.org/10.17113/ftb.56.02.18.5561
T.S. Workneh, G. Osthoff, M. Steyn, J. Food Sci. Technol. 49(6), 685–694 (2012)
L. Ahmed, A.B. Martin-Diana, D. Rico, C. Barry-Ryan, J. Food Process. Preserv. 36(2), 141–151 (2012)
A. Naeem, T. Abbas, T.M. Ali, A. Hasnain, Microbiol. Res. (2018). https://doi.org/10.4081/mr.2018.7465
L. Atarés, A. Chiralt, Trends Food Sci. Technol. 48, 51–62 (2016)
Acknowledgements
I would like to thank Dr. Tahira Mohsin Ali for basically perusing the manuscript and worthwhile discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Naeem, A., Abbas, T., Ali, T.M. et al. Effect of antioxidant and antibacterial properties of guar gum coating containing spice extracts and its application on tomatoes (Solanum lycopersicum L.). Food Measure 12, 2725–2734 (2018). https://doi.org/10.1007/s11694-018-9890-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9890-5