Abstract
The effects of different extraction solvents (water, EtOH, MeOH, acetone, DMSO, and their mixture) and various drying methods (air, oven, microwave, and freeze drying) were investigated on total polyphenol content (TPC), total flavonoid content (TFC) and antioxidant activity of peel, pulp, and seeds of yuzu fruit. Mixed solvents exhibited better results compared to the absolute solvents. 50 % acetone exhibited the highest FRAP and CUPRAC value in peel and pulp and TPC in seeds of yuzu fruits. 80 % acetone which also gave nearly similar results with 50 % acetone (p > 0.05) showed the highest TPC in peels and pulp and highest CUPRAC values in seeds. Absolute acetone was the least desirable solvent employed for extraction in most of the samples. Drying methods also significantly affected the total polyphenols content, total flavonoid content and antioxidant potential of yuzu fruits. Oven drying resulted in declines of the TPC, TFC, FRAP, DPPH, and CUPRAC from 0.2 to 33.0 % relative to fresh samples while air drying resulted a decrease in the range between 2.5 and 30.6 %. Freeze dried samples showed the highest percent retentions of polyphenols content, flavonoid content, and antioxidants (70.6–122.1 %). Drying methods caused the highest loss of TPC, TFC, FRAP, and CUPRAC in pulp samples except DPPH radical scavenging activity. In overall, the extraction of secondary metabolites and antioxidants was highly dependent on the extraction solvent, type of sample and sample processing methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nutraceutical properties of citrus fruit are related to their phytochemical components, among which phenolic compounds, flavonoids, limonoids, vitamins, carotenoids, organic acids, sugars, and aromatic compounds are reported in yuzu [1–4]. Recovery of phytochemicals from fruit samples is typically accomplished through different solvent extraction taking into account their chemistry and uneven tissue distribution. For example, phenolics, flavonoids, and vitamin C are present in higher concentrations in the rinds of fruits than in the inner tissues [5, 6]. The antioxidant capacity of citrus peel have also been reported to be superior other tissues [6, 7]. The seeds of citrus fruit are rich sources of limonoids and essential oils [3, 8–10]. Recovery of phytochemicals should also take account the nature of the target compounds to be extracted. Yuzu fruit contain phytochemicals from the highly polar (e.g., pectin) to less polar (e.g. terpenoids, essential oils, carotenoids) in nature. Hence, recovery of phytochemicals and antioxidant activity of the extracts are highly dependent on the solvent polarity [11]. The stability of different extracts from the same material depends on the extraction solvent. It is also apparent that different solvent extracts from the same plant material may vary widely with respect to their antioxidant concentrations and biological activities [12]. In a comparative study of four solvents, aqueous methanol, aqueous ethanol, aqueous acetone, and distilled water, Mohammedi and Atik [13] found that aqueous methanol was more effective than other solvents in recovering total polyphenols from Tamarix aphylla leaves. However, aqueous acetone extract was more potent free-radical inhibitor than other mixtures. In another study, Sultana et al. [14] found out that higher extract yields, polyphenolic contents and plant material antioxidant activity were obtained using aqueous organic solvents, as compared to the respective absolute organic solvents from medicinal plant extracts.
Different solvent systems have been used to extract antioxidants and phytochemicals from plant materials such as fruits, vegetables, legumes, and other foodstuffs. Water, ethyl acetate, DMF, DMSO, ethanol, methanol, acetone, and their combinations are commonly used to extract antioxidants from plant foods. For extractions of polyphenols and antioxidants from different parts of citrus fruits the solvent systems used include absolute ethanol [15], DMSO/MeOH [16, 17], ethyl acetate [18], 80 % methanol/water [19, 20], absolute methanol [21], water [7, 22], 70 % ethanol [3], 80 % acetone [5]. However, the reason for the selection of one solvent over another is not justified well. To understand the completion of the extraction and subsequent effect on the activities of the extracts, a comparative study is needed.
The cellular integrity is lost as cells die (the senescent process) causing the enzymes come in contact with substrates to which they are not normally exposed in living cells. Furthermore, the redox process, racemization, dimerization etc. also increases which in turn causes a problem to bioactive compounds like phenolic compounds [23]. The metabolic activities of the cells need to be curbed immediately after harvesting in order to control changes related to the enzyme activities. Depending on the nature of the sample, keeping on ice and dark conditions can be applied during storage and transportations [24, 25]. Various drying techniques at different temperature can also be performed [26–31]. However, it should be noted that drying processes could cause undesirable effect on the chemical constituents of plant samples [27]. Hence, planning and analyzing of research studies related to phytochemicals should be done with great caution.
Fresh fruits need costly preservation method and have also short shelf life. To reduce the cost of preservation and extend their life, citrus fruits can be used in dry form. The nutritional quality (phytochemical content) and bioactivity largely affected by the type of drying method employed [32]. Some novel drying techniques have been used to dry citrus by-products. In practice, sun-drying, hot air-drying, freeze drying, oven drying, and microwave drying are the major drying methods which are relatively inexpensive. Dried yuzu rind is used to flavor various dishes such as vegetables, fish, or noodles. Dried, powdered yuzu is tangy and sweet and is also used in desserts.
The objective of this study is to examine the effect of different extraction solvents and various drying methods on total polyphenol, total flavonoid and antioxidant activity of peel, pulp, and seeds of yuzu. The results of these study has a significant importance in developing protocols for producing flavoring additives with comparable or superior nutritional quality to those of commercial ones.
Materials and methods
Chemicals and reagents
Ethanol, acetone, and methanol, were supplied by DAEJUNG (Gyonggi do, South Korea). Ammonium acetate, ferric trichloride hexahydrate and 2,4,6-tripyridyl-s-triazine (TPTZ) were from JUNSEI (Tokyo, Japan). Sodium acetate and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were from Fluka (Buchs, Switzerland). Folin–Ciocalteu reagent, and sodium carbonate were from Sigma-Aldrich (Buchs, Switzerland). Dimethyl sulfoxide (DMSO), gallic acid, neocuproine, 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (TROLOX), and quercetin were purchased from Sigma-Aldrich (St. Louis, MO, USA). All reagents used for analysis were of analytical or high purity grade.
Plant material and drying process
Fresh fruits of yuzu (Citrus junos Sieb ex Tanaka) were obtained from a farm in Jeju province, South Korea. The peel, pulp, and seeds are separated manually and composite samples were prepared. Each part was divided in two five; one fresh and others to be subjected to various drying conditions. The same procedure repeated two more times. Samples were subject to four different drying methods, i.e., microwave-, oven-, freeze-, and air-drying. For each drying method, 10 g of sample was used. In microwave-drying, samples were dried in a domestic digital microwave oven (Samsung M1719N, Samsung Electronics, Seoul, South Korea) with technical features of ~230 V, 50 Hz, and 1150 W, a frequency of 2450 MHz (a wavelength of 12.24 cm). Oven-drying involved drying in a convection oven (Vision scientific, South Korea) at 50 °C. Air drying was conducted in the shade under normal air at daylight and ambient mean temperature of 25 °C. For each of the above drying methods, samples were spread out evenly on a Petri dish. In freeze drying, samples were lyophilized for 3 days in a vacuum flask at 0.125 mbar and −70 °C in a freeze-dryer.
Extraction of polyphenols and antioxidants
1 g for powdered (oven-, microwave-, air-, freeze- dried) and 5 g for fresh sample of peel, pulp, and seed of yuzu fruit were used for extraction. To study the effect of sample preservation methods on the content of selected metabolites and antioxidants, 80 % ethanol was used as an extraction solvent. For the study of the effect of extraction solvent, a freeze dried ground plant material was extracted with each of the solvents–water, 50 % EtOH (50, 80, and 100 %) MeOH (50, 80, and 100 %), acetone (50, 80, and 100 %), MeOH:DMSO, and EtOH:DMSO. The extraction was conducted in a 100 mL beaker by 30 mL of the solvent. The mixture of the sample powder and solvent was sonicated for 30 min, and the solution was then centrifuged at 10,000×g for 10 min at 4 °C. The residue was re-extracted by repeating the above steps under the same conditions two times. The supernatants were combined and transferred to a 250 mL round bottom flask. The extract was evaporated under reduced pressure at 40 °C using a rotary evaporator (EYELA, SB-651, Rikakikai Co. Ltd. Tokyo, Japan). The crude extract was re-dissolved by the respective solvent and made to a final volume of 25 mL. The final extract was stored at −18 °C until analysis.
Determination of total polyphenol content (TPC)
The TPC was determined by the Folin–Ciocalteu method according to the protocol of Singlton and Rossi [33] with slight modifications. Briefly, 250 μL of the extract stock solution was mixed with 250 μL of the Folin–Ciocalteu reagent and 2000 μL of water. The mixture was kept at room temperature for 7 min and then 500 μL of 20 % sodium carbonate (w/v) was added. The reaction was allowed to continue for 60 more minutes. Finally, the absorbance was measured at 765 nm using a Shimadzu UV-2550 spectrophotometer. Each measurement was repeated three times and total polyphenolic content was expressed as mg gallic acid equivalents (GAE)/g dry weight (DW) of sample.
Determination of total flavonoid content (TFC)
The TFC was determined by the colorimetric aluminum chloride method [34]. Briefly, 0.5 mL solutions of appropriately diluted sample solutions were separately mixed with 1.5 mL ethanol, 0.1 mL of 10 % aluminum chloride, 0.1 mL of 1 M potassium acetate, and 2.8 mL of deionized water and incubated at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm using a Shimadzu UV-2550 spectrophotometer. Results were reported in mg of quercetin equivalents (QE)/g dry weight (DW) of sample.
Ferric reducing antioxidant power (FRAP) assay
The ferric reducing antioxidant potential (FRAP) of yuzu extracts was estimated by the method of Benzie and Strain [35]. Briefly, FRAP reagent was prepared from 25 mL of 300 mM acetate buffer (pH 3.6), 2.5 mL of 10 mM TPTZ solution in 40 mM HCl and 2.5 mL of 20 mM FeCl3·6H2O solution. The reagent was prepared immediately before use as required. The assay procedure consisted of mixing 3000 µL of FRAP reagent, 300 µL of water and 100 µL of the test sample or standard TROLOX solution. The reaction mixture was kept at 37 °C for 30 min, and then the absorbance was recorded at 593 nm using a Shimadzu UV-2550 spectrophotometer. Results were reported as mg of TROLOX equivalents (TE)/g dry weight (DW) of sample.
DPPH free radical scavenging assay
The protocol of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity test was adapted from Brand-Williams [36], with some changes. In brief, 0.1 mL of the extract stock solution were mixed with 2.5 mL of DPPH radical solution (0.1 mM) and was made up to 3 mL with 60 % ethanol/methanol. The DPPH solution mixed with 60 % ethanol/methanol/acetone instead of the extract was used as a control. The mixture was shaken vigorously and left to stand for 80 min in the dark. Preliminary experiments have shown that such a long interval is required for the reaction to go to completion. The absorbance was recorded at 517 nm using a Shimadzu UV-2550 spectrophotometer. 60 % ethanol/methanol/acetone was used as a reference accordingly. TROLOX solution was used to construct calibration curves and results were reported as mg of TROLOX equivalents (TE)/g dry weight (DW) of sample.
DPPH radical scavenging activity was calculated according to the equation:
where A0 is the absorbance in the absence of samples, and A1 is the absorbance in the presence of samples.
Cupric reducing antioxidant capacity (CUPRAC)
The protocol of the CUPRAC test was adapted from Apak et al. [37] with a little modification. Briefly, to a test tube was added 1 mL of 10 mM CuCl2 solution, 1 mL of ammonium acetate buffer (pH 7), and 1 mL of 7.5 mM neocuproine solution were mixed together in a test tube. To this mixture were added 100 µL of the extracted sample (standard) solution and 1 mL of water so as to make the final volume 4.1 mL. The tubes were stoppered and after 30 min, the absorbance at 450 nm was recorded against a reagent blank. The assay was calibrated with standard solutions of TROLOX to express results in mg of TE per gram of dried sample.
Weight, size, soluble solids, and moisture content
Twenty fruits of each replicate were randomly selected and the average fruit weights were recorded. The seed–pulp–peel content, the individual weights of seeds, pulp, and peel present in yuzu fruits, were determined by separating manually. The mean weight of each part of the fruits was calculated. The size of the fruit was determined using a vernier caliper (Digimatic caliper, Mitutoyo Corp., Japan) to values at least 0.01 mm. The diameter was measured along the equatorial section of the fruit and the height was measured along the longitudinal (stem to blossom end) section.
Triplicate samples of 10 fruits per treatment were homogenized in a laboratory blender at high speed for 1 min and then subjected to analysis. The refractive index was determined using a pocket refractometer (PAL-1, Atago, Japan). The homogenates were filtered and the total soluble solids of the resulting clear juice samples was determined by placing 2–3 drops of the undiluted juice in the refractometer. Analyses were carried out in triplicate. Moisture content of the fruits was determined by keeping the samples in a thermostatically controlled electric oven at 105 ± 1 °C for 3 h (AOAC, 1990). The percentage of the moisture was calculated as:
where W1 is the initial weight of empty dish; W2 is the weight of dish + fresh sample; W3 is the weight of dish + dried sample.
Calculation of percentage true retention
The loss of nutrients during drying was estimated by calculating percent true retention (%TR) according to the following formula [38]:
Statistical analysis
All analyses were carried out in triplicate, and data were reported as a mean ± SD. The data were analyzed by the SPSS 17.0 software using one-way ANOVA and homogenous subsets were determined to separate the mean values of the different treatments. The statistical significance level was set up at p < 0.05.
Results and discussion
Physical properties of different parts of yuzu fruit
The weight, size, moisture content and soluble solids of yuzu fruit is presented in Table 1. The average fruit has a mean weight per fruit of 112.30 ± 10.64 g; fruit index of 0.84 at its matured stage. The percent relative weight of the peel generally decreases as it get matures. The mean juice weight per fruit is 53.29 ± 12.42 g. The average value of soluble solids of matured yuzu fruit was 15.5 ± 2.3 °Brix.
Effect of extraction solvent on the antioxidant activity
Amount (mg/100g of freeze dried plant material) of the antioxidant capacity (using FRAP, CUPARC, and DPPH assays) were determined for the peel, pulp, and seeds of yuzu fruit using 12 different solvents expressed as TROLOX equivalents are shown in Table 2. As depicted in the table, the ferric reducing antioxidant power, DPPH radical scavenging activity, and cupric reducing antioxidant power of different parts of yuzu fruit were affected by the type of extraction solvents employed.
FRAP assay depends on the reduction of ferric tripyridyltriazine (Fe(III)–TPTZ) complex to the ferrous tripyridyltriazine (Fe(II)–TPTZ) by a reductant (antioxidants or other reducing agents) at low pH. Fe(II)–TPTZ has an intensive blue color and can be monitored at 593 nm [35]. Extracts of peel, pulp, and seeds of yuzu fruit from different extraction solvent differ significantly (p < 0.05) in their FRAP. The FRAP of peel of yuzu fruit ranges from 469.4 to 1751.9 mgTE/100g dw; pulp from 261.9 to 776.8 mgTE/100g dw; and seeds from 292.3 to 490.0 mgTE/100g dw. The FRAP value was affected significantly by the extraction solvents. For example, the best solvents were 80 % acetone for peel, MeOH:DMSO (1:1) for pulp and seeds. In general pure solvents were less effective. Organic solvents mixed with water or DMSO mixed with either ethanol or methanol is good solvent for extraction of antioxidant compounds active towards the reduction of ferric tripyridyltriazine to ferrous tripyridyltriazine.
The CUPRAC assay utilizes the copper(II)–neocuproine [Cu(II)–Nc] reagent as the chromogenic oxidizing agent and involves faster kinetics as opposed to that of ferric reducing antioxidant power (FRAP) method [37]. The Cu(II) ion reducing ability of the extracts of the different parts of yuzu fruit were varied significantly (p < 0.05). The CUPRAC of the peel of yuzu fruits ranged from 702.4 to 2195.2 mgTE/100g dw; pulp from 348.7 to 1067.1 mgTE/100g dw; and seeds from 338.9 to 785.1 mgTE/100g dw. The CUPRAC value was affected by the extraction solvent significantly. For example, the best solvents were 50 % acetone for peel and pulp; while 80 % acetone gave the best results for seeds. In general, the mixture of water and organic solvents (methanol, ethanol, and acetone) were found best solvents for extraction antioxidants active towards cupric ion reduction. Pure solvents were found least effective. Similar to FRAP assay, lowest CUPRAC values were observed using absolute acetone for peel and pulp, while the lowest value for seeds was obtained using water extract. This might be attributed to the relatively less polar compounds such as limonoids accumulated in the seeds of yuzu [9] are less soluble in water.
DPPH is a free radical and stable at room temperature, which produces a violet solution in ethanol/methanol. Reduction of DPPH by antioxidants results in a loss of absorbance. Thus, the degree of discoloration of the solution indicates the scavenging efficiency of the added substances [36]. The DPPH values of the antioxidant extracts from different extraction solvents presented in Table 2 differed significantly (p < 0.05). The DPPH values of the peel of yuzu fruit ranged from 295.1 to 479.3 mgTE/100g dw; pulp from 57.8 to 265.5 mgTE/100g dw; seeds from 81.5 to 232.8 mgTE/100g dw. The DPPH values were affected by extraction solvent significantly. For example, the best solvents for DPPH values were 80 % acetone for peel, 50 % EtOH for pulp; and 80 % MeOH for seeds. Pure solvents were least effective. The results suggested that water mixed with acetone, methanol, and ethanol are good solvents for extraction of DPPH radical scavengers from peel, pulp and seeds of yuzu fruit. A similar trend with FRAP and CUPRAC assays was noticed for DPPH assay in that the antioxidants from seeds were better extracted by acetone than water. However, water was better than absolute acetone in extracting antioxidants from the peel and pulp. It should also be noted that both water and absolute acetone were the least performing solvents in most of the experiments conducted. The results of this study are in agreement with previously reported investigations [11, 14, 39]. The results from the three assays suggested that antioxidant activity of extracts is strongly dependent on the solvent. This is due to the different antioxidant potentials of compounds with different polarity.
Effects of extraction solvent on the TPC and TFC
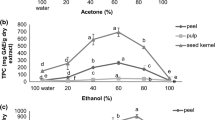
The polyphenol and flavonoid contents of the peel, pulp and seeds of yuzu were examined and presented in Table 3. Polyphenol content of the peel, pulp and seeds of yuzu fruit ranged from 1407.7 to 2271.7, 408.9 to 1039.4, and 167.8 to 654.8 mgGAE/100g dw respectively. An aqueous acetone was the best extraction solvent for polyphenols from yuzu fruit. There was no significant difference between the polyphenols content obtained with 50 and 80 % acetone (p > 0.05). Absolute acetone was found undesirable for extraction of polyphenols from yuzu fruit relative to other solvents considered in the study. As shown in the table, polyphenol contents of yuzu extracts were strongly dependent on the solvents at different concentrations and solvent types. The TPC extracted by the selected solvents were affected significantly. 80 % acetone was found the best solvent composition for peel and pulp. Whereas, 50 % acetone gave the highest TPC for seeds. Like the antioxidant activity assays, pure solvents were the least effective for phenolic compound extraction. Considering acetone, methanol and ethanol only, 80 % of each organic solvent extracted the highest amount of polyphenols followed by 50 % for peel and pulp. However, 50 % of each solvent extracted relatively higher content of polyphenols followed by 80 % of them for seeds.
Flavonoids are widespread plant secondary metabolites, including flavones, flavanols, and condensed tannins [39]. In addition to the TPC, the TFC of the extracts were analyzed and the results are presented in Table 3. Yuzu extracts from different extraction solvents differed significantly (p < 0.05). The TFC of peel, pulp, and seeds of yuzu fruit extracts ranged from 102.8 to 177.4, 49.9 to 84.4, and 3.2 to 5.4 mgQE/100g dw respectively. 80 % methanol and 80 % ethanol (p > 0.05) were the best among the 12 selected solvents for extracting flavonoids from peels of yuzu. Whereas, 50 % ethanol and 50 % methanol were the best extraction solvents for extraction of yuzu pulp flavonoids with TFC values differed insignificantly (p > 0.05). The TFC of seeds of yuzu was better extracted by 80 % methanol (p < 0.05) followed by 50 % acetone, 50 % MeOH, 80 % acetone, and 80 % EtOH (p > 0.05). The least desirable solvents among the solvents employed for extraction of flavonoids from yuzu peels, pulp and seed were the following order from low to high: absolute acetone < water < absolute ethanol.
Effect of drying methods on the TPC and TFC
Different drying methods of fresh yuzu fruits (air, oven, freeze, and microwave drying) had an impact on TPC and TFC. Results are presented in Table 4. Quantitative evaluation of total polyphenols in extracts of the differently dried peel, pulp, and seeds of yuzu as estimated by the method of Folin–Ciocalteu revealed that the peel, pulp, and seeds of yuzu fruit exhibited variable contents ranging from 1964.1 to 2112.8, 809.3 to 915.5, and 42.4 to 55.4 mg of GAE/100g of dw respectively. The amounts of total polyphenols in fresh peel, pulp and seeds of yuzu fruit were 437.9 ± 11.9, 150.5 ± 15.3, and 23.8 ± 2.7 mgGAE/100g FW respectively. The highest TPC content was recorded in freeze dried samples followed by oven dried and microwave dried samples. Air dried samples exhibited the least TPC in all the three parts of fruit samples. On the other hand, total flavonoid content as measured according to the method described by Chang et al. [34] were of 89.6 ± 1.0, 27.5 ± 0.4, and 2.6 ± 0.1 mgGAE/100g FW in peel, pulp and seeds respectively. The TFC content of the dried fruit extract was affected significantly (p < 0.05) by the drying method used and the TFC content was ranked in the order from high to low: Freeze drying > oven drying, microwave drying (p > 0.05) > air drying.
Peel and seed samples dried by lyophilisation were found best suitable to retain the maximum content of TPC (106 % for peel and 120.4 % for seeds), TFC (109.7 % for peel and 97.8 % for seeds). TPC and TFC in pulp sample were best preserved in microwave drying (90.7 % for TPC and 86.6 % for TFC). The effect of drying methods on retention of TPC and TFC is presented in Fig. 1.
Drying of plant material could result in a decrease or in an increase of the TPC and TFC. Microwave, oven, air, and sun drying resulted in sharp declines in TPC in dried leaves of Alpinia zerumbet, Etlingera elatior, Curcuma longa, and Kaempferia galangal [29]. These authors also noted that freeze-drying resulted in significant gains in TPC. In another report, the TPC increased considerably in oregano and peppermint leaves when they were dried at ambient air [40]. Capecka et al. [40] explained that the drying process may result in high or low levels of TPC depending on the type of phenolic compounds present in the plant material and their location in the cell. According to Dorta et al. [31], freeze and oven drying in peel and seeds of mango fruit increased the TPC using aqueous extraction solvents of ethanol and acetone. However, the TPC from peel of mango using absolute ethanol as extraction solvent was highest in fresh samples even though there was no decrease in oven and freeze dried sample relative to the aqueous solvents.
Microwave treatment increased the content of individual flavonoid compounds in citrus mandarin peels [41]. These Authors noted that the contents of flavonoid compounds (FCs) were increased with microwave power. The increase in contents of polyphenols in microwave dried plants could be explained by the fact that the intense heat generated from the microwaves creates a high vapor pressure and temperature inside plant tissue, resulting in the disruption of plant cell wall polymers. Consequently, in certain cases, cell wall phenolics or bond phenolics could be released, thus causing more phenolics to be extracted [42]. On the other hand, simple heat treatments increased the TPC and TFC of methanol extract of citrus fruit (Citrus sinensis (L.) Osbeck) peels [30]. This indicated that phenolic compounds of plants should be linked with different type of bonds depending on plant species. In this study freeze drying appears to be a good method preserving TPC and TFC in peels and seeds while microwave was superior for the juice (pulp) samples of yuzu fruit. Polyphenols are important medicinal compounds. The potential effect of freeze drying on these constituents should be considered when planning and analyzing research studies on the medicinal properties of plants.
Effect of sample preservation techniques on the antioxidant activity (AOA)
The antioxidant activity of the peel, pulp, and seeds of matured yuzu fruits as affected by different drying methods is presented in Table 5. Quantitative evaluation of the antioxidant activity in extracts of the differently dried (oven, microwave, air, freeze) peel, pulp, and seeds of yuzu as estimated by FRAP, CUPRAC, and DPPH assays revealed that the peel, pulp, and seeds of yuzu fruit exhibited variable contents. The FRAP values were ranged from 1373.6 to 1642.3, 589.2 to 712.1, and 393.3 to 490.3 mgTE/100g DW; CUPRAC from 1776.4 to 1998.3, 763.2 to 868.3, and 395.5 to 512.2 mgTE/100g DW; DPPH from 406.2 to 468.2, 163.1 to 227.5, and 200.4 to 246.9 mgTE/100g DW in peel, pulp and seeds respectively. The antioxidant activities of all the samples tested with the FRAP, CUPRAC, and DPPH assays were highest in freeze dried samples and lowest in air dried samples. The AOA of microwave and oven dried samples didn’t show a significant difference with the exception of FRAP values of seed extract.
Impacts of different drying methods on percent true retentions of antioxidant activities of peel, pulp, and seeds of yuzu are illustrated in Fig. 2. Freeze dried peel and seed samples exhibited a maximum retention of FRAP (105.6 % for peel and 122.1 % for seeds), DPPH (108.9 % for peel and 124.9 % for seeds), and CUPRAC (108.9 % for peel and 124.9 % for seeds). AOA in pulp sample was best preserved by MWD with retention of 84.0, 115.6, and 78.2 % for FRAP, DPPH, and CUPRAC assays respectively. For peels and seeds, freeze drying resulted in gains of antioxidant activities with the exception of CUPRAC values of seed samples.
Processing methods are reported to have variable effects on AOA of plant samples. Impacts include little or no change, significant losses, or enhancement in AOP [29]. An increase in antioxidant activity of thermal treatment have been reported in orange peel extracts [30], citrus mandarin peels [41], citrus mandarin pomace [43], red pepper [44], and Citrus unshiu peels [45]. Increase in AOA following thermal treatment is attributed to the release of bound phenolic compounds brought about by the breakdown of cellular constituents and the formation of new compounds with enhanced antioxidant potential [29]. Losses in AOA due to thermal treatments were also reported in other studies [46, 47].
Results of this study showed that drying methods had variable effects on the antioxidant potential. Gains of FRAP and DPPH in seeds sample were exhibited by all the drying methods considered. Freeze drying caused a significant gain of AOA in peels of yuzu fruits. It would be presumptuous to infer that drying methods resulted in gains or losses in AOA without analyzing a wide range of antioxidant potential and testing a variety of samples. A single treatment applied on a different sample could have variable effects on its antioxidant potential.
Conclusion
The present study has clearly demonstrated that extraction solvent mixtures greatly affected yuzu antioxidant activity evaluation including DPPH radical, FRAP, and CUPRAC. The total polyphenol content and flavonoid content were also significantly affected by the extraction solvent types. Aqueous solvent (80 % methanol, 80 % ethanol, 80 % acetone, 50 % methanol, 50 % ethanol, and 50 % acetone) extracts of yuzu peel, pulp, and seeds exhibited better antioxidant activities and higher phenolic and flavonoid contents. Drying methods resulted in significant quantitative variation of the chemical composition of the extracts. Freeze drying seems to enhance TPC, total flavonoids, and antioxidant potential as assessed by the DPPH stable radical, CUPRAC, and FRAP methods. The highest decline (1.7–33 %) was observed in pulp samples relative to peel and seeds in all methods excepting DPPH scavenging activity of freeze and microwave dried samples. In general, the efficiency solvent type and drying method to give better results was dependent up on the methods of determination as well as the type of sample. The present data would certainly help to ascertain the potency of the tested samples as potential source of natural antioxidants to be used for nutraceutical and functional food applications.
References
M. Sawamura, Expr. Multidiscip. Flavour Sci. 431 (2008)
S. Lee, J. Shin, N. Sung, J. Agric. Life Sci. 44, 81 (2010)
I. Choi, S. Choi, B. Nam, Y. Kim, H. Choi, Food Sci. Biotechnol. 17, 373 (2008)
T. Suetsugu, H. Iwai, M. Tanaka, M. Hoshino, A. Quitain, M. Sasaki, M. Goto, Chem. Eng. Sci. 1, 87 (2013)
Y. Shin, Food Sci. Biotechnol. 21, 1477 (2012)
K.M. Yoo, K.W. Lee, J.B. Park, H.J. Lee, I.K. Hwang, J. Agric. Food Chem. 52, 5907 (2004)
C. Guo, J. Yang, J. Wei, Y. Li, J. Xu, Y. Jiang, Nutr. Res. 23, 1719 (2003)
H. Ueno, M. Tanaka, S. Machmudah, M. Sasaki, M. Goto, Food Bioprocess Technol. 1, 357 (2008)
M. Minamisawa, S. Yoshida, A. Uzawa, Food Funct. 5, 330 (2014)
S.Y. Kim, K.S. Shin, Prev. Nutr. Food Sci. 18, 196 (2013)
N. Turkmen, F. Sari, Y.S. Velioglu, Food Chem. 99, 835 (2006)
G.A. Akowuah, Z. Ismail, I. Norhayati, A. Sadikun, Food Chem. 93, 311 (2005)
Z. Mohammedi, F. Atik, Int. J. Pharma Biosci. 2, 609 (2011)
B. Sultana, F. Anwar, M. Ashraf, Molecules 14, 2167 (2009)
B. Tao, F. Ye, H. Li, Q. Hu, S. Xue, G. Zhao, J. Agric. Food Chem. 7166 (2014)
A. Cano, A. Medina, A. Bermejo, J. Food Compos. Anal. 21, 377 (2008)
S. Kawaii, Y. Tomono, E. Katase, K. Ogawa, M. Yano, J. Agric. Food Chem. 47, 3565 (1999)
V. Goulas, G.A. Manganaris, Food Chem. 131, 39 (2012)
D. Ramful, T. Bahorun, E. Bourdon, E. Tarnus, O.I. Aruoma, Toxicology 278, 75 (2010)
D.C. Abeysinghe, X. Li, C. Sun, W. Zhang, C. Zhou, K. Chen, Food Chem. 104, 1338 (2007)
A. Bocco, M. Cuvelier, H. Richard, C. Berset, J. Agric. Food Chem. 8561, 2123 (1998)
G.H. Xu, J.C. Chen, D.H. Liu, Y.H. Zhang, P. Jiang, X.Q. Ye, J. Food Sci. 73, C11 (2008)
J.C. Rickman, D.M. Barrett, C.M. Bruhn, J. Sci. 87, 930 (2007)
P. Rapisarda, M. Lo Bianco, P. Pannuzzo, N. Timpanaro, Postharvest Biol. Technol. 49, 348 (2008)
M.I. Gil, E. Aguayo, A.A. Kader, J. Agric. Food Chem. 54, 4284 (2006)
C. Beaudry, G.S.V. Raghavan, C. Ratti, T.J. Rennie, Dry. Technol. 22, 521 (2004)
K. Abascal, L. Ganora, E. Yarnell, Phyther. Res. 19, 655 (2005)
C. Butryee, P. Sungpuag, C. Chitchumroonchokchai, Int. J. Food Sci. Nutr. 60(Suppl 2), 162 (2009)
E.W.C. Chan, Y.Y. Lim, S.K. Wong, K.K. Lim, S.P. Tan, F.S. Lianto, M.Y. Yong, Food Chem. 113, 166 (2009)
M.L. Chen, D.J. Yang, S.C. Liu, Int. J. Food Sci. Technol. 46, 1179 (2011)
E. Dorta, M. G. Lobo, M. González, LWT Food Sci. Technol. 45, 261 (2012)
Y. Sun, Y. Shen, D. Liu, X. Ye, LWT Food Sci. Technol. 60, 1269 (2015)
V.L. Singleton, J.A. Rossi, Am. J. Enol. Vitic. 16, 144 (1965)
C.C. Chang, M.H. Yang, H.M. Wen, J.C. Chern, J. Food Drug Anal. 10, 178 (2002)
I.F. Benzie, J.J. Strain, Anal. Biochem. 239, 70 (1996)
W. Brand-Williams, M. E. Cuvelier, C. Berset, LWT Food Sci. Technol. 28, 25 (1995)
R. Apak, K. Güçlü, M. Ozyürek, S.E. Karademir, J. Agric. Food Chem. 52, 7970 (2004)
R. K. Saini, N. P. Shetty, M. Prakash, P. Giridhar, J. Food Sci. Technol. 51, 2176 (2014)
S.K.C. Xu, B.J. Chang, J. Food Sci. 72, 159 (2005)
E. Capecka, A. Mareczek, M. Leja, Food Chem. 93, 223 (2005)
K. Hayat, X. Zhang, H. Chen, S. Xia, C. Jia, F. Zhong, Sep. Purif. Technol. 73, 371 (2010)
S. Inchuen, W. Narkrugsa, P. Pornchaloempong, Kasetsart J. Nat. Sci. 44, 142 (2010)
K. Hayat, X. Zhang, U. Farooq, S. Abbas, S. Xia, C. Jia, Food Chem. 123, 423 (2010)
L. Hungarian, A. Vega-gálvez, K. Di, K. Rodríguez, R. Lemus-mondaca, M. Miranda, J. López, M. Perez-won, Food Chem. 117, 647 (2009)
S.-M. Jeong, S.-Y. Kim, D.-R. Kim, S.-C. Jo, K.C. Nam, D.U. Ahn, S.-C. Lee, J. Agric. Food Chem. 52, 3389 (2004)
A. Ismail, Z.M. Marjan, C.W. Foong, Food Chem. 87, 581 (2004)
M.K. Roy, M. Takenaka, S. Isobe, T. Tsushida, Food Chem. 103, 106 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Assefa, A.D., Keum, Y.S. Effect of extraction solvent and various drying methods on polyphenol content and antioxidant activities of yuzu (Citrus junos Sieb ex Tanaka). Food Measure 11, 576–585 (2017). https://doi.org/10.1007/s11694-016-9425-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-016-9425-x