Abstract
The aim of this study was to investigate the effects of three different drying methods, freeze drying (FD), vacuum drying (VD) and oven drying (OD) on phenolic contents and antioxidant activities of litchi fruits. 20 polyphenols were exactly identified in the litchi fruits by UPLC-QqQ/MS. Significant losses were observed in the contents of total polyphenols and antioxidant activities in the dried litchi when compared with the fresh litchi. Principle component analysis indicated that there was significant difference of phenolic component between the use of thermal drying (VD and OD) and FD. Our results suggest that FD is the optimum drying method for litchi fruits considering the content of total polyphenols and antioxidant activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Litchi (Litchi chinensis Sonn.) is an important commercial crop which has been widely cultivated in Africa and Asia, especially in China for many years. Litchi fruits are well accepted by consumers worldwide due to their sweet, juicy and of great nutrition pulp (Jiang et al., 2004). Except for vitamins, minerals and dietary fiber, litchi is also known to have high levels of secondary compounds such as flavonoids and phenolic acids (Cabral et al., 2014; Su et al., 2014; Zhao et al., 2006). Several studies indicated the pericarp, pulp and flower of litchi are rich sources of phenolic compounds (Yang et al., 2012; Qiang et al., 2014). Litchi polyphenols show several bioactivities such as antioxidant, anticancer and hypoglycaemic activities (Man et al., 2016; Qiang et al., 2014). In traditional Chinese medicine, litchi has been used for the treatment of heart, spleen and liver related diseases (Zhang et al., 2013).
Fresh litchi fruit is easy to deteriorate since its high content of moisture, then the commercial value drops rapidly. Therefore, it is of high importance to study the processing method of litchi. Currently, drying process is widely used in the litchi processing industry since it reduces physiological, microbial, and enzymatic degradation of litchi (Zhao et al., 1998). For drying, oven drying (OD) method is commonly used since its relative cheap costing. However, conditions of high temperature and presence of oxygen may promote the activity of polyphenolic oxidase, resulting in browning (Takahiro et al., 2008). Freeze drying (FD) is usually considered as an efficient way to retain nutrients. However, it is an expensive and time-consuming process. In addition, vacuum drying (VD) has been a new drying process under the absence of oxygen which can enhance the quality of products, and its drying rate is faster than FD (Reis et al., 2017). OD and VD are effective thermal drying methods, and FD is one of the most used method due to it can produce high quality dried fruit. Nevertheless, some studies indicated FD might lead to much loss of bioactive compounds compared with OD (Aydin and Gocmen 2015; Nunes et al., 2016). Therefore, it is interesting to compare those drying methods on the quality of litchi fruits. To our knowledge, there are some studies comparing the moisture, reducing sugar and sensory evaluation under different drying process (Agarwal and Nath 1990; Song et al., 2015). However, very few studies are available on the variation of polyphenol composition and antioxidant activity of litchi fruits dried by FD, OD and VD.
In order to make full use of the potential benefits of litchi. The objective of this study was to compare three drying methods (FD, OD and VD) for the production of dried litchi with high concentration of polyphenols and high antioxidant activity.
Materials and methods
Reagents and standards
HPLC-grade acetonitrile was purchased from Adamas reagent, Ltd. (Shanghai, China). Standards used in this study for identification by UPLC-QqQ/MS were purchased from Shanghai Yuanye Biotechnology Co. Ltd. (Shanghai, China). Ultrapure water was obtained from Hangzhou Wahaha Group Co., Ltd. (Hangzhou, China). Vitamin C, 1, 1-diphenyl-2-picrylhydrazyl (DPPH), 2, 2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 2, 3, 5-Triphenyltetrazolium chloride (TPTZ) were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). All other reagents of analytical grade were bought from pengguang Chemical Reagent Co., Ltd. (Chongqing, China).
Materials and drying process
Fresh litchi fruits (guiwei) at commercial maturity stage were collected from the garden in the Institute of China Southern Subtropical Crop Research (Zhanjiang, Guangdong). The fruits were manually peeled and enucleated. Then, the litchi pulps were dehydrated by OD, VD or FD. For OD, litchi was placed in a forced air circulation oven (101A-1, Shanghai Pudong Rong-Feng Scientific Instrument Company, Ltd., Shanghai, China) at 70 °C for 48 h. For VD, the drying temperature was also set at 70 °C, and litchi was dried in the vacuum chamber (DZF-1B, Shanghai Yuejin Medical Instrument Company, Ltd., Shanghai, China) for 48 h. Before FD, the litchi pulps were frozen at −60 °C for 12 h. Then, the frozen pulps were dried in an experimental vacuum lyophilizer (LGJ-10, Shengchao kechuang (Beijing) Biotechnology Co., Ltd., Beijing, China) for 72 h. The dried pulps were grinded rapidly and sieved thorough a 60-mesh screen. All the litchi powder was vacuum sealed and stored at −20 °C until use.
Determination of moisture
The moisture of fresh and dried litchi pulps were determined according to Aydin and Gocmen (2015). All the assays were performed in triplicate.
Extraction
The litchi flours were extracted as reported by Han et al. (2016). 50 g flours were ultrasonic extracted three times at 40 °C for 30 min using 80% aqueous methanol (w/v = 1:25). All the supernatants were combined, and then the extract solution was used for further analysis of total polyphenols and antioxidant activity, and the remaining extract concentrated by rotary evaporation at 40 °C was prepared for further utilization.
Identification and quantification of polyphenols by UPLC-QqQ-MS
The polyphenols of litchi extractions were analyzed with ultra high pressure liquid chromatography (UPLC, Agilent, California, USA), and triple quadruple mass spectrometry (6460QqQ-MS, Agilent, California, USA) equipped with an electrospray ionization source (ESI). ZORBAX Eclipse Plus C18 column (100 mm × 2.1 mm i.d., 1.8 µm, Agilent, Waldbronn, Germany) was used in this study. The mobile phase consisted of 0.1% aqueous formic acid (A) and acetonitrile (B) at a flow rate of 0.4 mL min−1. The gradient elution was set as follows: 0–0.5 min, 80% A; 0.5–15 min 80–10% A. Sample injection volume was 5 μL, and the column temperature was 30 °C. The ESI source was operated in negative ion mode, and the dynamic MRM (Multiple Reaction Monitoring) data were acquired. The drying gas flow and temperature were 10 L min−1 and 350 °C, respectively. All the compounds were exactly identified by comparing the dynamic MRM information including retention time, fragmentor voltages, collision energies and transitions with reference standards.
Total polyphenolic content assays
The total phenol content was analyzed by a modified Folin-Ciocalteu colorimetric method (Slinkard and Singleton, 1977). Briefly, 50 μL solution of polyphenolic extracts was mixed with 10 μL Folin-Ciocalteu Phenol reagent. Then, the mixture was incubated in dark for 6 min. After incubation, 100 μL 7% Na2CO3 and 80 μL distilled water were added, and the mixture was incubated at room temperature for 90 min. The absorbance was measured at 760 nm by microplate reader (PT3502PC, Beijing Potenov Technology Co., Ltd., Beijing, China). The results were expressed as milligram of gallic acid equivalent per gram of drying weight (mg GAE/g dw).
Determination of antioxidant activity
The antioxidant activities were determined with DPPH, ABTS and FRAP assays. For the assay of DPPH radical scavenging activity, 100 μL litchi polyphenol extraction solution was added into 100 μL of DPPH solution (0.5 mmo1 L−1). After incubation at room temperature for 30 min, the absorbance of mixture was measured at 517 nm. For the assay of ABTS+ radical scavenging activity, 100 μL litchi polyphenol extraction was added to 100 μL of ABTS+ solution (absorbance of 0.70 ± 0.02 at 734 nm), and the mixture was incubated at room temperature for 10 min. Then, the absorbance was measured at 734 nm. For the assay of ferric reducing antioxidant power (FRAP), 30 μL extraction was mixed with 100 μL FRAP solution (2.5 mL 20 mmol L−1 TPTZ, 2.5 mL 20 mmol/L FeCl3 and 25 mL 0.2 mol L−1 acetate buffer). The solution was incubated at 37 °C for 10 min, and absorbance was measured at 593 nm. In these studies, Vc was used as the standard and the antioxidant activity was expressed in μg Vc equivalents per gram of litchi fruit (dry weight, dw) (equivalent μg Vc/g dw).
Statistical analysis
All the values were presented as mean ± SD (n = 3). Statistically significant differences among the results were analyzed by one-way analysis of variance (AVOVA), followed by Tukey test. The statistical analyses were performed using the IBM SPSS statistics (version 20.0). The correlation between polyphenol compounds and antioxidant activity were compared using a multivariate linear sparse partial least-squares analysis (sPLS). Principle component analysis and sPLS analysis were performed in R (× 64, 3.4.3) using the mixOmics package.
Results and discussion
Qualitative analysis of polyphenols
Methanol extracts of litchi were analyzed by UPLC-QqQ-MS system in negative ion modes. In this study, 7 phenolic acids and 13 flavonoids were exactly identified by comparing the retention time, molecular weight, characteristic fragments and transitions with the standards. The detail information including retention time, molecular weight, characteristic fragments, collision energy and fragmentor were shown in the Table 1. In this study, all the compounds were separated in 12 min. The representative chromatogram was shown in Fig. 1. Each peak in different color represents different compounds. All those compounds were found both in the fresh and the dried litchi pulps (Table 2). Some flavonoids such as rutin, naringenin, quercetin, isoquercitrin, phloridzin and phloretin have already been reported in litchi fruits (Lyu et al., 2019; Su et al., 2014). To the best of our knowledge, this study is the first to report the presence of narirutin, naringin, quercetin-7-O-β-d-glucopyranoside, hesperidin, neohesperidin, methyl hesperidin and didymin in litchi fruits. Most of these compounds have been reported in the citrus fruits and the leaves (Yoichi et al., 2006). In addition, except chlorogenic acid, ferulic acid and p-hydroxybenzoic acid which had been reported by Su et al. (2014) and Zhang et al. (2013), quinic acid, 4-hydroxycinnamic acid, p-coumaric acid and rosmarinic acid were also detected in this study. However, some flavonoids such as kaempferol, isorhamnetin and their glucosides which had been isolated from litchi (feizixiao) fruits were not detected in this study (Lyu et al., 2019). This result may be because different species of litchi (guiwei) was used in our study.
Representative chromatograms of the identified compounds in litchi fruits. The numbers are consistent with those in the Table 1. 1, Quinic acid; 2, Chlorogenic acid; 3, 4-Hydroxycinnamic acid; 4, p-Coumaric acid; 5, Fumalic acid; 6, p-Hydroxybenzoic acid; 7, Rosmarinic acid; 8, Narirutin; 9, Naringin; 10, Rutin; 11, Quercetin-7-O-β-d-glucopyranoside; 12, Isoquercitrin; 13, Hesperidin; 14, Neohesperidin; 15, Phloridzin; 16, Methyl hesperidin; 17, Didymin; 18, Quercetin; 19, Naringenin; 20, Phloretin
Quantitive analysis of identified polyphenols in the extracts of litchi pulps
As shown in Table 2, quinic acid, ferulic acid, rutin and methyl hesperidin were main polyphenols in the fresh litchi pulps in this study. Rutin was the highest compound among all the 20 polyphenols in fresh litchi, and this result was consist with Zhang’s study which indicated rutin was the highest phenolic compound in the litchi pulps of different species cultivated in Southern China (Zhang et al., 2013).
Except p-hydroxybenzoic acid and rutin, all the phenolic compounds identified in the fresh litchi pulps were much higher than those in the dried litchi pulps. This result indicated drying processing led to the degradation of polyphenols since the structure of phenolic compounds were easy to be destroyed under the condition of heating, oxygen or freezing (Amarowicz et al., 2009). Actually, FD has been regarded as an effective drying method to obtain high-quality fruit and vegetables (Huang et al., 2009). However, our results showed freeze drying led to much lower contents of phenolic compounds compared with fresh litchi fruits. Similar results have been reported in the study of pumpkin and guava (Aydin and Gocmen 2015; Nunes et al., 2016). The decreased phenolic compounds may be because rapid change of pressure during freeze drying led to the degradation of polyphenols. Interestingly, rutin was increased in FD compared with the fresh pulps. This result might be because freeze-drying may lead to the development of ice crystals within the matrix. Ice crystals can result in rupturing of the cell structure which may benefit for the release of bound compounds from the matrix (Keinanen and Julkunen-Tiitto, 1996). In addition, high temperature (140 °C) also could cause the disruption of the achene structure, and thereby supported higher rutin extraction yields from the inner parts of the achenes (Kalinova et al., 2018). However, the concentration of rutin in OD and VD were much lower than that in fresh litchi pulps which could be due to the loss of glycosyl and splitting under heating process since high temperature may induce oxidative condensation or decomposition of thermolabile polyphenols (Aneta et al., 2009; Wojdyło et al., 2014). The increasement of p-hydroxybenzoic acid in OD and VD may be explained by transformation from its conjugates during heating and oxidation, which have been reported in the study of guava (Nunes et al., 2016).
In this study, principal component analysis was developed according to the phenolic compounds identified in this work (Fig. 2). As shown in Fig. 2, fresh fruit was separated from OD, VD and FD by PC1. FD was also separated from OD and VD by PC2. However, VD and OD clustered together. This result also indicated that all the drying methods had significant effect on the phenolic compounds. OD and VD showed the similar influence on the polyphenol component.
Total polyphenols
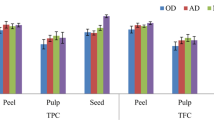
Folin-Ciocalteu method was employed to detect the total polyphenols of litchi in this study (Fig. 3A). Compared with the fresh fruits, FD, VD and OD significantly decreased the total polyphenols. However, our results showed dried litchi was also a good source of phenolic compounds. The total polyphenols in the FD, VD and OD of litchi fruits were 10.35, 9.85, 10.29 mg GAE/g dw, respectively, which are higher than those in the tomato and ginger flour dried by sun, oven or vacuum oven (Gumusay et al., 2015). The correlation analysis indicated that quercetin-7-O-β-d-glucopyranoside, hesperidin and neohesperidin were the main compounds that led to the difference of total polyphenols in different treatments (Fig. 4).
Total polyphenols (A) and antioxidant activities evaluated by DPPH (B), ABTS (C) and FRAP (D) assays in fresh litchi and dried litchi by oven drying (OD), vacuum drying (VD) and freeze drying (FD). Values are presented as mean ± SD of three replicates. Different letters in each species indicate significantly difference as determined by ANOVA and Tukey’s post hoc test (p < 0.05)
In this study, there were no significant difference of total polyphenols among FD, VD and OD. Studies on tomato and ginger showed that FD led to higher contents of phenolic compounds compared with OD and VD. OD and VD led to similar contents of polyphenols in tomato and ginger (Gumusay et al., 2015). In strawberry and corn, freeze-drying also preserved higher levels of total polyphenols in comparison with air-drying (Asami et al., 2003). However, OD of pumpkin led to higher contents of polyphenols than FD (Aydin and Gocmen 2015). Therefore, those studies indicated that it is difficult to choice the suitable drying method aiming at phenolic compounds stability only based on the data from other different foods.
Antioxidant activity
The antioxidant capacity of fresh, OD, VD and FD litchis were evaluated by DPPH, ABTS and FRAP assays (Fig. 3B–D). Generally, fresh and dried litchi flours showed good antioxidant activity. It was higher than that reported for fresh peach and fresh plums even though the data in the reported literature was converted into dry mass (75% water) (Gil et al., 2002). The DPPH, ABTS and FRAP values in the fresh litchis were significantly higher than those in dried litchi. This maybe because the total polyphenols in the fresh litchi were higher than those in the dried litchi flours. Our results showed that the correlation coefficients between total polyphenols and DPPH, ABTS and FRAP were 0.83,0.88 and 0.88 respectively. Therefore, it may be concluded that the polyphenols content is largely responsible for their antioxidant activity. The positive correlation between total phenolics contents and the antioxidant activities was also observed in previous studies (Nunes et al., 2016). In addition, the antioxidant activities in FD were higher than those in the OD and VD even though the total polyphenols among the three groups had no difference. This maybe because the flavonoids identified in this study in the FD was higher, compared to those in the VD and OD since previous study indicated flavonoids but not non-flavonoids contributed to the antioxidant activity (Nunes et al., 2016). In this study, the correlation analysis showed the main compounds that influenced the antioxidant activity were naringin, methyl hesperidin and p-hydroxybenzoic acid (Fig. 4), which had been reported to have strong antioxidant activity (Farhoosh et al., 2016; Ioannou et al., 2018). Those three methods were commonly used for measuring antioxidant activities of fruits and vegetables, but we do not know which method is suitable for measuring antioxidant activity of polyphenol components in litchi.
In all, polyphenols and antioxidant capacities of litchi fruits were significantly affected by all of the drying methods. Heating drying techniques (VD and OD) caused more losses of most of the polyphenols and antioxidant activities when compared to FD. Even though the FD is a costly procedure, it is superior for litchi drying considering the total polyphenols, especially rutin, and the maximum antioxidant capacity.
References
Agarwal N, Nath N. Effect of pretreatments on quality characteristics and water activity of air-dried litchis (Litchi chinensis Sonn.) cv. Rose Scented. Die Nahrung 34: 255-263 (1990)
Amarowicz R, Carle R, Dongowski G, Durazzo A, Galensa R, Kammerer D, Maiani G, Piskula MK. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 53: S151-S183 (2009)
Aneta W, Adam F, Jan O. Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. J. Agric. Food Chem. 57: 1337-1343 (2009)
Asami DK, Hong YJ, Barrett DM, Mitchell AE. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 51: 1237-1241 (2003)
Aydin E, Gocmen D. The influences of drying method and metabisulfite pre-treatment on the color, functional properties and phenolic acids contents and bioaccessibility of pumpkin flour. LWT Food Sci. Technol. 60: 385-392 (2015)
Cabral TA, Cardoso LDM, Pinheiro-Sant’ Ana HM. Chemical composition, vitamins and minerals of a new cultivar of lychee (Litchi chinensis cv. Tailandes) grown in Brazil. Fruits 69: 425-434 (2014)
Farhoosh R, Johnny S, Asnaashari M, Molaahmadibahraseman N, Sharif A. Structure-antioxidant activity relationships of o-hydroxyl, o-methoxy, and alkyl ester derivatives of p-hydroxybenzoic acid. Food Chem. 194: 128-134 (2016)
Gil MI, Tomásbarberán FA, Hesspierce B, Kader AA. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from california. J. Agric. Food Chem. 50: 4976-82 (2002)
Gumusay OA, Borazan AA, Ercal N, Demirkol O. Drying effects on the antioxidant properties of tomatoes and ginger. Food Chem. 173: 156-162 (2015)
Han X, Li W, Huang D, Yang X. Polyphenols from hawthorn peels and fleshes differently mitigate dyslipidemia, inflammation and oxidative stress in association with modulation of liver injury in high fructose diet-fed mice. Chem. Biol. Interact. 257: 132-140 (2016)
Huang LL, Zhang M, Yan WQ, Mujumdar AS, Sun DF. Effect of coating on post-drying of freeze-dried strawberry pieces. J. Food Eng. 92: 107-111 (2009)
Ioannou I, M’Hiri N, Chaaban H, Boudhrioua NM, Ghoul M. Effect of the process, temperature, light and oxygen on naringin extraction and the evolution of its antioxidant activity. Int. J. Food Sci. Technol. 53: 2754-2760 (2018)
Jiang Y, Duan X, Joyce D, Zhang Z, Li J. Advances in understanding of enzymatic browning in harvested litchi fruit. Food Chem. 88: 443-446 (2004)
Kalinova JP, Vrchotova N, Triska J. Contribution to the study of rutin stability in the achenes of Tartary buckwheat (Fagopyrum tataricum). Food Chem. 258: 314-320 (2018)
Keinanen M, Julkunen-Tiitto R. Effect of sample preparation method on birch (Betala pendula Roth) leaf phenolics. J. Agric. Food Chem. 44: 2724-2727 (1996)
Lyu Q, Kuo TH, Sun CD, Chen KS, Hsu CC, Li X. Comprehensive structural characterization of phenolics in litchi pulp using tandem mass spectral molecular networking. Food Chem. 282: 9-17 (2019)
Man S, Jiang M, Wang C, Yu L, Gao W, Lu F. Chemical composition and hypoglycaemic effect of polyphenol extracts from Litchi chinensis seeds. J. Funct. Food. 22: 313-324 (2016)
Nunes JC, Lago MG, Castelo-Branco VN, Oliveira FR, Torres AG, Perrone D, Monteiro M. Effect of drying method on volatile compounds, phenolic profile and antioxidant capacity of guava powders. Food Chem. 197: 881-890 (2016)
Qiang L, Si M, Yan Y, Luo F, Hu G, Wu H, Sun C, Xian L, Chen K. Effects of phenolic-rich litchi (Litchi chinensis Sonn.) pulp extracts on glucose consumption in human HepG2 cells. J. Funct. Food. 7: 621-629 (2014)
Reis FR, Ivahashi MM, Rosa AHG. Effect of vacuum drying temperature on drying kinetics, effective moisture diffusivity and quality of peeled litchi (Litchi chinensis Sonn.). J. Food Process Eng. 40(2): 1-9 (2017)
Slinkard K, Singleton V. Total phenol analysis: automation and comparison with manual methods. Am. J. Enol. Vitic. 28: 49-55 (1977)
Song CF, Cui ZW, Jin GY, Mujumdar AS, Yu JF. Effects of four different drying methods on the quality characteristics of peeled litchis (Litchi chinensis Sonn.). Dry. Technol. 33: 583-590 (2015)
Su DX, Zhang RF, Hou FL, Zhang MW, Guo JX, Huang F, Deng YY, Wei ZC. Comparison of the free and bound phenolic profiles and cellular antioxidant activities of litchi pulp extracts from different solvents. BMC Complement. Altern. Med. 14: 10 (2014)
Takahiro O, Long W, Takeo S, Akio T. Drying characteristics of kiwifruit during hot air drying. J. Food Eng. 85: 303-308 (2008)
Wojdyło A, Figiel A, Lech K, Nowicka P, Oszmiański J. Effect of convective and vacuum–microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioprocess Technol. 7: 829-841 (2014)
Yang DJ, Chang YZ, Chen YC, Liu SC, Hsu CH, Lin JT. Antioxidant effect and active components of litchi (Litchi chinensis Sonn.) flower. Food Chem. Toxicol. 50: 3056-3061 (2012)
Yoichi N, Koji S, Hiroyuki S, Toshinao I, Masamichi Y, Hideaki O. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 70: 178-192 (2006)
Zhang, RF, Zeng QS, Deng YY, Zhang MW, Wei Z., Zhang Y, Tang XJ. Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chem. 136: 1169-1176 (2013)
Zhao M, Yang B, Wang J, Li B, Jiang Y. Identification of the major flavonoids from pericarp tissues of lychee fruit in relation to their antioxidant activities. Food Chem. 98: 539-544 (2006)
Zhao H, Li C, Guan Z. Experimental research on drying characteristics of litchi. Dry. Technol. 17: 1915-1925 (1998)
Acknowledgements
This research is funded by Chongqing Natural Science Foundation. The project No. is cstc2018jcyjAX0687. The authors also gratefully thanks the China Litchi and Longan Industry Technology Research System (CARS-32-02), the research projects of commission of science and technology in Chongqing (KJQN201801437) and research projects of School of Advanced Agriculture and Bioengineering in Yangtze Normal University (CSZKY1816) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tan, S., Tang, J., Shi, W. et al. Effects of three drying methods on polyphenol composition and antioxidant activities of Litchi chinensis Sonn.. Food Sci Biotechnol 29, 351–358 (2020). https://doi.org/10.1007/s10068-019-00674-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-019-00674-w