Abstract
Biosynthesis and accumulation of jasmonate (JA) can regulate plant defense responses and organ development under insect herbivore stress conditions. One of these responses, the inhibition of root growth, is a common phenomenon. However, the physiological and molecular mechanisms of how JA affects root growth are not completely understood. In this study, the influence of MeJA treatment on auxin and proton flux rates in the root apex region of Col-0, coi1-1, pin2, and aux1-7 mutant lines was examined using a non-invasive micro-test technique. The auxin and H+ flux profiles taken from coi1-1 mutants suggest that the modulation of auxin and H+ flux by JA requires the function of COI1. The auxin and H+ flux in pin2 and aux1 mutants with and without JA treatment suggest that JA can affect polar auxin transport by regulating PIN-mediated auxin efflux and the AUX-mediated influx pathways. Furthermore, the expression levels of PIN1, PIN2, PIN3, PIN7, AUX1, and TIR1 genes were reduced under MeJA treatment, and expression levels of CYP79B2 and CYP79B3 were elevated. Together, these results suggest that JA signaling may modulate the auxin signaling pathways by regulating the expression of key genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jasmonate (JA) functions as an important molecular signal that is induced by insect herbivory. JA plays a well-established role in mediating both plant defense responses and developmental processes (Creelman and Mullet 1997), such as root growth, tuberization, tendril coiling, senescence, and fertility (McConn and Browse 1996; Schommer and others 2008; Staswick and others 2009). Among these typical effects of JA, growth inhibition has been well characterized (Dathe and others 1981).
Plants perceive JA-Ile by coronatine insensitive 1 (COI1) (Xie and others 1998; Yan and others 2009; Sheard and others 2010). COI1 encodes an F-box protein which is part of a Skp1/Cullin/F-box complex (SCFCOI1) (Feys and others 1994; Xie and others 1998). SCF complexes act as E3 ubiquitin ligases that target jasmonate-ZIM domain proteins (JAZs) for degradation by the 26S proteasome (Chini and others 2007; Thines and others 2007) which leads to the releases of transcription factors that regulate downstream signal cascades and modulate plant defense responses and development (Chen and others 2011; Song and others 2013). The coi1 mutant is deficient in all JA responses, including both the root growth inhibition and defense gene expression (Feys and others 1994).
Among the numerous factors that are known to affect root growth, PM H+-ATPase-mediated H+ secretion is essential for optimal primary root growth and root hair development (Rober-Kleber and others 2003; Haruta and Sussman 2012). For example, under moderate water stress, polar auxin transport (PAT) is enhanced, inducing PM H+-ATPase-mediated proton secretion in the root tip, which regulates root growth during adaption to water stress (Xu and others 2013). In other work, MeJA treatment has also been shown to activate PM H+-ATPase activity in roots of Mung Bean (Wen and others 2006). However, it is not clear how the activity of PM H+-ATPase is integrated into root growth under MeJA treatment.
In addition to the role of PM H+-ATPase in root elongation, several plant hormones are also involved in regulating root growth, with auxin being the key regulator (Vanneste and Friml 2009). Auxin participates in almost every process of root growth and development, including the pattern formation of roots (Blilou and others 2005), cell division and cell expansion in the primary root (Stepanova and others 2008), and elongation and differentiation of cells leaving the root meristem (Rahman and others 2007). The polar transport of auxin regulates auxin distribution and guides root elongation and formation (Grieneisen and others 2007). Chen indicated that MeJA suppresses root growth, which was correlated to PAT in the root tip (Chen and others 2011). Further, Sun and others (2011) confirmed that exogenous MeJA treatment inhibited PIN2 endocytosis (at low JA concentration), or reduced PIN2 accumulation in the plasma membrane (at high JA concentration). In this study, however, MeJA suppressed endocytosis and abolished plasma membrane accumulation of PIN2 in coi1-1 plants, suggesting that PIN2-mediated auxin transport plays a key role in regulating the root elongation under MeJA treatment, and COI1 likely modulates PIN2 activity during this procedure. Although, these studies provide detailed molecular evidence for the alterations of auxin transporters in response to MeJA, to date the direct evidence of changes in PAT has not been reported. Another problem with previous studies is that the method used for detection of auxin levels was based on a destructive assay with poor temporal resolution (Ulmasov and others 1997; Petersson and others 2009; Pan and others 2010; Sun and others 2011, Qi and others 2012).
With the development of self-referencing (SR) auxin (IAA) microsensors, Mancuso and others (2005) and McLamore and others (2010) measured the auxin flux along maize root tips without any damage to the root tissue. This real time, non-invasive microsensor technique has been validated with independent measurements using HPLC (Mancuso and others 2005; Subraya and others 2013). In this study, a SR non-invasive micro-test technique (NMT), was used to directly measure transmembrane auxin flux rate and H+ flux rate along Arabidopsis thaliana roots before and after MeJA treatment. The transcription expression levels of transport and perception response genes under exogenous MeJA treatment were also evaluated. We show that MeJA directly reduced PIN-mediated transmembrane auxin flux, and increased H+ secretion along the roots. The alteration of auxin flux and H+ flux was correlated with the function of COI1. Our results provide new physiological evidence for the functional relationship between JA and auxin in regulating root growth.
Methods
Plant Material and Growth Conditions
In this study, WT A. thaliana lines (Col-0, Columbia ecotype) and mutant lines pin2 (eir1-4, Columbia background), aux1-7 (Columbia background), and coi1-1 (Columbia background) were used. The seeds were surface-sterilized for 10 min in 0.5 % NaClO water solution, washed four times in sterile water, and plated on half Murashige and Skoog (1/2MS) medium. Plants were stratified at 4 °C for 2 days in darkness, and then transferred to an incubator set at 22 ± 1 °C, with white light illumination (150 μmol m−2 s−1) in a long-day cycle (16/8 h). The relative humidity was 70 %. Three-day-old seedlings of Col-0 or coi1-1 were transferred to plates with varying concentrations of MeJA (0, 5, 10, 25, 50, and 100 μM), and then cultured for an additional 3 days. The homozygous coi1-1 seedlings were screened according to the methods of Feys and others (1994). The root length of Col-0 and homozygous coi1-1 seedlings was measured using Image-Pro Plus (Media Cybernetics Company).
Inhibitor Treatment
The seedlings were fixed on the bottom of a 35-mm dish and incubated in the test buffer solution (0.1 mM KCl, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM NaCl, 0.3 mM MES, 0.2 mM Na2SO4, and pH 6.0) for 20 min in light. Then 100 μM vanadate was added to the buffer according to Undurraga and others (2012). After 30 min incubation with vanadate, the samples were washed several times with the buffer. Then the samples were used for IAA flux or H+ flux measurement.
NMT Technique for Measuring Auxin Flux and H+ Flux
Net H+ flux was measured using a NMT, as described by Xu and others (2006). This technique, also known as the self-referencing microelectrode technique, is used to study the flux of specific molecules from single cells and/or tissues. The technique uses computer-controlled stepper motors to oscillate an ion-selective microelectrode near the surfaces of cells and tissues (within 1–2 µm of the surface), providing direct measurement of ion flux based on Fick’s first law of diffusion.
The H+ electrode was fabricated as described by Yan and others (2015). Pre-pulled and silanized glass micropipettes (2–4 μm aperture) were first filled with a backfilling solution (40 mM KH2PO4 and 15 mM NaCl, pH 7.0). Then, the micropipettes were front-filled with selective liquid ion exchange (LIX) cocktails to a column length of approximately 25 μm. LIX was obtained from Sigma Aldrich (Sun and others 2009). A Ag/AgCl electrode holder was inserted from the back of the electrode until the tip of the wire was immersed in the electrolyte solution. DRIREF-2 (World Precision Instruments) was used as a reference electrode. The electrodes were calibrated prior to the experiment in test buffer (0.1 mM KCl, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM NaCl, 0.3 mM MES, and 0.2 mM Na2SO4) with pH 6.0 and pH 7.0. Only electrodes with Nernstian slopes between 53 and 62 mV/[log-C (±z)] were used in the current study (z is the valence of the ion and C is the ion concentration).
In the test, 7-day-old seedling roots were fixed on the bottom of a 35-mm dish incubated in the test buffer (0.1 mM KCl, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM NaCl, 0.3 mM MES, and 0.2 mM Na2SO4), pH 6.0 for 20 min. Then the H+ flux of individual roots was measured using NMT. The sensor was placed near the roots (1–2 μm), and controlled by the stepper motors oscillated in the direction tangential to the root surface with an excursion distance of 20 μm. H+ fluxes were measured along the root tip, concentrating on the following zones (distance reported is the linearized distance from the root cap junction): 0, 100, 200, 300, 400, 600, 700, 800, 900, 1000, 1100, and 1200 μm from the root cap junction. Each plant was measured once. Then the roots were treated with 50 μM MeJA for 1 h, and the H+ flux was measured again. The final flux values at each zone were reported as the mean of six individual plants from each treatment. The H+ flux was calculated according to Porterfield and others (2009).
here, J is the free proton flux (pmol cm−2 s−1), D is the proton molecular diffusion coefficient (9.22 × 10−5 cm2 s−1), [H+] is the measured proton concentration gradient (pmol ml−1), K a is the acid dissociation constant for the buffer (MES), and ΔX is the excursion distance for the microelectrode oscillation (cm).
Net auxin fluxes were measured using the NMT (NMT-100, YoungerUSA LLC., Amherst, MA) with ASET 2.0 (Science wares, Falmouth, MA) and iFluxes 1.0 Software (Younger USA, LLC.). The IAA sensor construction and surface modification were based on the method of McLamore and others (2010). Sample preparation and measurement were the same as described above in the H+ measurement. The polarization voltage was +700 mV, and YG003-Y05 (YoungerUSA, LLC.) was used as the reference electrode to complete the circuit. The IAA electrode was calibrated with 0, 2, 4, 6, and 8 μM IAA in PBS buffer. Only electrodes with a linear calibration slope (R 2 > 0.99) were used. Fick’s first law of diffusion (J = −D × ΔC/ΔX) was used to calculate the auxin flux; where J is the free auxin flux (fmol cm−2 s−1), D is the molecular diffusion coefficient (7 × 10−6 cm2 s−1) (Sussman and Goldsmith 1981), ΔC is the auxin concentration gradient (fmol), and ΔX is the excursion distance for the microelectrode oscillation (20 μm).
Quantitative RT-PCR
Seven-day-old seedlings were treated with 50 μM MeJA for 3 h, root tissue was harvested and frozen in liquid nitrogen. Total RNA was extracted using the Total RNA extraction kit (Takara). After DNase treatment, 500 ng of total RNA was used for reverse transcription. qRT-PCR was performed using the Power SYBR Green PCR Master Mix kit (Applied Biosystems) on a 7300 Real-Time PCR system (Applied Biosystems) according to the manufacturer’s instructions. Normalization was performed using control genes ACTIN-2 and ACTIN-7. An arbitrary value of 1 was assigned to non-treated samples.
The primers used in the qRT-PCR were as follows:
PIN1-F | GGTCGGAACTCTAACTTTGGTC | PIN1-R | CAGCTCCAGCAGCAGTTCCAGC |
PIN2-F | CCTCGCCGCACTCTTTCTTTGG | PIN2-R | CCGTACATCGCCCTAAGCAATGG |
PIN3-F | AGCACCTGACAACGATCAAGGCG | PIN3-R | GTTCTCCTCCGAAATCTCCACT |
PIN7-F | TGTTTGGGGATCCAACGGATC | PIN7-R | TACCCTCTCCGACTCTTCTTC |
AUX1-F | GTTCGTATACGTGAAGGGAGTA | AUX1-R | CACACACTACATATTATCGACT |
CYP79B2-F | CACGATGATGCTCGCGAGACT | CYP79B2-R | TCACTTCACCGTCGGGTAGAGA |
CYP79B3-F | AGTCACTTCCGAACACTCA | CYP79B3-R | TCGCAGGTTACCATATTCC |
TIR1-F | CTTAACCGAGCTGTTCCAC | TIR1-R | AGTAAACATCTGGTCGCACT |
ACTIN2-F | TTGACTACGAGCAGGAGATGG | ACTIN2-R | ACAAACGAGGGCTGGAACAAG |
ACTIN7 -F | CCATTCAGGCCGTTCTTTC | ACTIN7 -R | CGTTCTGCGGTAGTGGTGA |
Histochemical GUS Assay
Seven-day-old seedlings were fumigated with 5 μM MeJA or alcohol (CK) for 24 h. Then, the treated seedlings were incubated overnight at 37 °C in X-Gluc buffer (100 mM sodium phosphate, pH 7.0, 0.1 mM EDTA, 0.5 mM ferricyanide, 0.5 mM ferrocyanide, 0.1 % Triton X-100, and 1 mM X-Gluc). Individual representative seedlings were photographed.
Data Analysis
The significance of arithmetic mean values for all data sets was assessed by two-way ANOVA and multiple comparisons. The differences at P < 0.05 were considered statistically significant.
Results
Root Tip Response to Exogenous MeJA Treatment
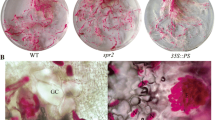
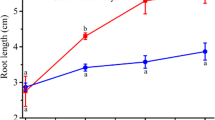
To investigate how JA inhibits root growth, we first measured the primary root length of wild-type (Col-0) and coi1-1 Arabidopsis seedlings under MeJA treatment (0, 5, 10, 25, 50, and 100 μM). As expected, the root elongation of Col-0 was inhibited in a dose-sensitive manner. However, the root growth of JA-insensitive coi1-1 mutants was largely unaffected by MeJA, indicating that the suppressed effect of MeJA on root elongation requires the function of COI1 (Fig. 1a, b).
Effect of exogenous MeJA on Arabidopsis thaliana root length. a Pictures were taken from 6-day-old seedlings of wild-type (Col-0), and coi1-1 grown on vertical plates with and without MeJA (50 μM). b MeJA induced inhibition of root growth in WT (Col-0) and coi1-1 seedlings. WT and coi1-1 seeds were germinated on 1/2 MS medium containing different concentrations of MeJA (0, 5, 10, 25, 50, and 100 μM), and seedling root length was measured at 6 days after germination (n = 40; mean ± SD; statistical analysis was performed by ANOVA, a P < 0.05).The experiments are representative of at least three independent experiments
MeJA Inhibited the Root Growth by Modulating Auxin Transport
To determine whether the JA-suppressed root growth was related to auxin transport, the auxin flux rate was measured in different functional regions of the root apex using NMT on the seedlings of Col-0 and the JA perception mutant coi1-1. As shown in Fig. 2a, b, the auxin flux rate in the roots of Col-0 plants is positive in the meristem (0–200 μm) and transition zone (200–600 μm), suggesting strong auxin activity in those regions. The peak value of auxin efflux was observed at 300 μm behind the root tip. In the elongation zone (600–1000 μm) and mature zone (>1000 μm), the auxin flux rate was negative, indicating a net auxin influx. The auxin flux profile of the coi1-1 mutant was similar to that from Col-0 seedlings. When the seedling roots of Col-0 and coi1-1 were exposed to exogenous 50 μM MeJA, the auxin efflux rate of WT was significantly repressed in the transition zone, whereas in the elongation and mature zones, the auxin flux rate was not significantly different. Conversely, in the roots of coi1-1 seedlings, the auxin flux rate was not affected by MeJA treatment. To investigate whether the auxin flux was related to the PM H+-ATPase, the auxin flux along vanadate pretreated Col-0 roots was further monitored. We found that the auxin efflux increased along these roots, with the most pronounced regions being the meristem and transition zones.
Effect of exogenous MeJA on the auxin flux rate and H+ flux rate. a Auxin flux profile along the roots apex of Col-0 (black), coi1-1 (red) and vanadate pre-treated Col-0 (navy) Arabidopsis lines with (open) and without (solid) MeJA treatment (50 μM). n = 6; bars indicate standard errors. c H+ flux profile along the root apex of Col-0 (black), coi1-1 (red) and vanadate-treated Col (navy) Arabidopsis lines with (open) and without (solid) MeJA treatment (50 μM). n = 6; bars indicate standard errors. b, d Mean auxin flux rate (b) and H+ flux rate (d) in transition zones. Statistical analysis was performed by ANOVA (n = 6; mean ± SD, a P < 0.05) (Color figure online)
Uptake of IAA from the extracellular space to the cytoplasm is known to be correlated with apoplastic pH (Yang and others 2006; Taiz and Zeiger 2006). Thus, we measured transmembrane H+ flux along the seedling roots. As shown in Fig. 2c, d, the H+ flux along the roots was converse to IAA. In the meristem and transition zones, the H+ flux showed a strong negative value (indicating influx). However, in elongation and mature zones, the flux was positive (indicative of efflux). Vanadate treatment enhanced the H+ influx in the meristem and transition zones in Col-0 roots. The H+ flux in coi1-1 roots was similar to that of Col-0. Exogenous MeJA significantly increased H+ secretion with the most striking area being the transition zone. As expected, exogenous application of MeJA did not significantly affect the net H+ flux along coi1-1 roots.
MeJA-Regulated Auxin and Proton Flux in A. thaliana Roots in Auxin Transport Mutants
PAT involves the PIN family of auxin efflux carriers and also the AUX1/LAX family of auxin influx carriers. Furthermore, we investigated whether these auxin transporters mediated MeJA-suppressed auxin transport. As shown in Fig. 3a, b, before MeJA treatment, the auxin flux rate in the roots of pin2 and aux1-7 seedlings was significantly lower than that that in Col-0 plants in the transition zone. However in the meristem zone, elongation zone, and mature zone, the auxin flux rate in pin2 and aux1-7 showed no significant difference compared to WT. MeJA treatment reduced the auxin flux rate in the meristem and transition zones in roots of both pin2 and aux1-7 seedlings. To our surprise, the auxin flux profile of pin2 mutants under MeJA treatment was similar to Col-0 under MeJA treatment. However, MeJA largely reduced the auxin flux along the whole root of aux1-7, especially in the transition zone.
Effect of MeJA on auxin and proton flux rates in root apexes of auxin transport mutants. a Auxin flux profile along the root apex of Col-0 (black), pin2 (blue), and aux1–7 (olive) Arabidopsis lines with (open) and without (solid) MeJA treatment (50 μM); n = 6, bars indicate standard errors. c Proton flux profile along the root apex of Col-0 (black) pin2 (blue) and aux1–7 (olive) Arabidopsis lines with (open) and without (solid) MeJA treatment (50 μM). n = 6, bars indicate standard errors. b, d Mean b auxin flux rate and d proton flux rate in transition zone of the root. Statistical analysis was performed by ANOVA (n = 6; mean ± SD, a P < 0.05) (Color figure online)
Further, we observed that the H+ flux rate along pin2 and aux1-7 roots was significantly lower than that in Col-0 roots, indicating that PIN or AUX1 mutation may affect the activity of PM H+-ATPase. JA treatment increased the H+ secretion in both the pin2 and aux1-7 roots, with the transition zone being most affected (Fig. 3c, d).
MeJA Activated the Auxin Biosynthesis Gene and Repressed the Auxin Transport Relevant Genes
To determine how MeJA alters the auxin flux rate, the transcription expression levels of two Arabidopsis cytochrome P450s genes CYP79B2 and CYP79B3 (Zhao and others 2002), which convert tryptophan (Trp) to indole-3-acetaldoxime (IAOx) in vitro, were measured. The expression levels of auxin transporter-related genes PIN1, PIN2, PIN3, PIN7, AUX1, and the auxin perception gene TIR1 were also quantified by qRT-PCR. The Dr5:GUS was also monitored before and after MeJA treatment. As shown in Fig. 4a, b, 50 μM MeJA treatment up-regulated the expression levels of CYP79B2 and CYP79B3, consistently, MeJA fumigation promoted IAA accumulation in the root cap. However, MeJA treatment down-regulated the transcription expression level of auxin transport-related genes PIN1, PIN2, PIN3, PIN7, and AUX1, and the IAA perception gene TIR1.
JA regulated the expression of auxin biosynthesis and transport-related genes at the transcription level. a Quantitative real-time PCR analysis of transcript levels of the auxin biosynthesis-related genes in Col-0 plants after MeJA treatment. Seven-day-old seedlings were treated with 50 μM MeJA for 3 h and root tissues were collected for RNA extraction and qRT-PCR assay. The transcript levels of CYP79B2 and CYP79B3 were normalized to the ACTIN7 expression. Error bars represent the SD of triplicate reactions. The experiment was repeated three times with similar results. b Histochemical staining of GUS activity in the seven-day-old seedlings upon MeJA treatment. c Quantitative real-time PCR analysis of transcript levels of the auxin transport-related genes in Col-0 plants after MeJA treatment
Discussion
Plants have a limited arsenal of resources for growth and defense. When defense reactions increase in intensity, growth and development suffer. Under insect herbivore predation, plants with inherently slow growth rates survive better than those with fast growth rates; slow rates in turn favor large investments in anti-herbivore defenses (Coley and others 1985). JA functions as an important molecular signal induced by insect herbivory (Turner and others 2002; Li and others 2002; Wasternack and Kombrink 2010), regulating plant defense response, and also root growth and development (Creelman and Mullet 1997). COI1 is a critical component of the JA receptor, and COI1 mutants are insensitive to JA, including resistance to JA inhibited root growth and defects in the expression of JA-regulated genes (Feys and others 1994). Similar to the above, in the present study, MeJA also inhibited primary root elongation in Col-0 seedlings, but did not affect coi1-1 seedlings.
Plant root development is subject to hormonal control which is coordinated by several phytohormones (Wolters and Jürgens 2009). Hentrich and others (2013) have recently shown that the MeJA-triggered inhibition of root elongation growth depends on the crosstalk between JA-signaling and auxin biosynthesis. MeJA or coronatine treatment promotes the production of auxin, the Arabidopsis cytochrome P450s genes CYP79B2, CYP79B3, the YUC genes, and ASA1, TAA1 genes are involved in auxin biosynthesis (Stepanova and others 2008; Sun and others 2009; Qi and others 2012; Hentrich and others 2013; Yang and others 2014). Our results also showed that MeJA up-regulated the CYP79B3, suggesting that MeJA treatment elevated auxin biosynthesis. However, whether the auxin flux along the roots was involved in this JA signaling has yet to be fully uncovered. Therefore, we monitored the auxin levels along the roots using the NMT. We found that in the roots of Col-0 plants, MeJA significantly affected auxin transport in the meristem and transition zones, but not in the elongation and mature zones, which was similar to the results of McLamore (McLamore and others 2010), suggesting that these two zones play important roles in MeJA-regulated root development. Data from Baluska and others (2010) also support this argument, which demonstrated that the transition zone has a unique role in the determination of cell fate and root growth (Baluska and others 2010). However, MeJA did not significantly affect auxin transport along the roots of coi1-1 seedlings, suggesting that JA regulates the auxin transport required for the function of COI1, which was consistent with the growth phenotype under MeJA treatment.
The subcellular distribution of the auxin transporter-related PIN family plays a critical role in auxin gradient-mediated developmental processes (Wisniewska and others 2006). Sun and others (2011) indicated that MeJA affected PIN2 endocytosis or reduced PIN2 accumulation in the plasma membrane. Under Alternaria brassicicola stress, a JA-mediated signal pathway, A. thaliana up-regulated the auxin biosynthesis and down-regulated the auxin polar transport in roots. These results imply that the modulation of auxin transport in roots may be related to root growth. In these studies, however, the direct observation of auxin transport was not observed. In the current study, we found that before MeJA treatment, the auxin flux rates in the meristem and transition zones of pin2 and aux1-7 were significantly lower than Col-0 roots, suggesting that PAT in pin2 and aux1-7 roots was reduced. However, the auxin flux has been shown to be less pronounced and not significantly different in elongation and mature zones, which may be due to the normal tissue-specific distribution/localization of PIN2 within the root. After MeJA treatment, the auxin flux rate in pin2 roots had no significant difference to Col-0 plants. However, the auxin transport in aux1-7 seedling roots was significantly lower than that in Col-0 and pin2 plants, suggesting that PIN family transporters play a central role in modulating MeJA-suppressed auxin transport.
Further analysis of relevant auxin transporter genes showed that MeJA treatment down-regulated the expression of PIN1, PIN2, PIN3, PIN7, and AUX1, suggesting that MeJA treatment not only reduced the auxin transport in the central microtubule organization from leaves to roots, but also inhibited the redistribution of the PIN2-dependent auxin transport stream, and PIN2-, PIN3-, PIN7-mediated auxin lateral redistribution. MeJA down-regulated PAT, which may have reduced the auxin concentration in the elongation zone of roots, and this could repress root elongation. MeJA also down-regulated the transcription level of the auxin perception gene TIR1, suggesting that MeJA reduced the sensitivity of roots to IAA. Taken together, the results of auxin transport and auxin biosynthesis suggest that elevation of auxin content in the root tip may be a compensatory response of reduced PAT.
JA treatment caused H+ secretion in the roots of Col-0, pin2 and aux1-7 seedlings but not in coi1-1 roots, suggesting that JA regulates H+ transport required for the function of COI1. Vanadate treatment promoted H+ influx along the roots, indicating that H+ secretion was mediated by PM H+-ATPase, which was consistent with previous results (Wen and others 2006). It is worth noting that in pin2 and aux1-7 seedling roots, the H+ flux was lower than that in Col-0 roots, this may be due to the reduced activity of PM H+-ATPase in the pin2 mutant (Xu and others 2012), which means that deficiencies in auxin transport affect the PM H+-ATPase activity in the roots.
MeJA induced large H+ efflux along the roots, which has important physiological consequences. The auxin uptake is mainly ensured by two mechanisms, diffusion of the protonated form of IAA across the plasma membrane and AUX1/LAX family protein-mediated deprotonated auxin influx (Rubery and Sheldrake 1974; Goldsmith 1982; Delbarre and others 1996; Yang and others 2006). AUX1 activity was demonstrated to be pH dependent (Yang and others 2006; Cho and others 2012; Taiz and Zeiger 2006), our results suggest that when large quantities of H+ efflux out of the cell, a proton motive force (ΔE + ΔpH) was established. This promoted auxin transmembrane influx through passive diffusion [protonated form of auxin (IAAH) or the secondary active transport (2H+-IAA−)]. This is one possible reason that MeJA reduced the auxin flux in meristem and transition zones. In addition, because JA-triggered inhibition of plant growth was related to mitosis (Zhang and Turner), whereas, the cell division could be affected by the cytosolic pH (Morisawa and Steinhardt 1982), our results suggested that the large MeJA induced H+ efflux may interfere with normal cell mitosis, which in turn affects root growth.
In conclusion, we observed the direct changes of auxin flux along the roots in response to MeJA treatment. The results provide insight into the physiological mechanisms underlying JA-induced inhibition of primary root growth of Arabidopsis plants. As shown in Fig. 5, in the presence of COI1, MeJA repressed the PIN family-mediated auxin polar transport, otherwise, MeJA-activated PM H+-ATPase activity, resulting in significant H+ efflux, which affected auxin influx. The reduced auxin transport affected auxin content in the elongation zone, which finally inhibited the root elongation.
Proposed model of how JA affected the root growth. Based on previous studies of the interaction of JA and auxin (Sun and others 2011; Qi and others 2012; Zhang and Turner 2008), the hypothetical signal sequences of MeJA-inhibited root growth was as follows: in the presence of COI1, MeJA reduced PINs- and AUX1-mediated auxin polar transport. MeJA also activated PM H+-ATPase, leading to large H+ efflux which promoted auxin influx. The reduced auxin polar transport finally inhibited root elongation
References
Baluska F, Mancuso S, Volkmann D, Barlow PW (2010) Root apex transition zone: a signalling-response nexus in the root. Trends Plant Sci 15:402–408
Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433:39–44
Chen Q, Sun J, Zhai Q, Zhou W, Qi L, Xu L, Wang B, Chen R, Jiang H, Qi J, Li X, Palme K, Li C (2011) The basic Helix-Loop-Helix transcription factor MYC2 directly represses PLETHORA expression during Jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell 23:3335–3352
Chini A, Fonseca S, Fernández G, Adie B, Chico JM, Lorenzo O, Garcia-Casado G, López-Vidriero I, Lozano FM, Ponce MR, Micol JL, Solano R (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448:666–671
Cho D, Villiers F, Kroniewicz L, Lee S, Seo YJ, Hirschi KD, Leonhardt N, Kwak JM (2012) Vacuolar CAX1 and CAX3 influence auxin transport in guard cells via regulation of apoplastic pH. Plant Physiol 160:1293–1302
Coley PD, Bryant JP, Chapin FS (1985) Resource availability and plant anti herbivore defense. Science 23:895–900
Creelman RA, Mullet JE (1997) Oligosaccharins, brassinolides, and jasmonates: Nontraditional regulators of plant growth, development, and gene expression. Plant Cell 9:1211–1223
Dathe W, Rönsch H, Preiss A, Schade W, Sembdner G, Schreiber K (1981) Endogenous plant hormones of the broad bean, Vicia faba L. (-)-jasmonic acid, a plant growth inhibitor in pericarp. Planta 153:530–535
Delbarre A, Müller P, Imhoff V, Guern J (1996) Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 198:833–844
Feys B, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6:751–759
Goldsmith MHM (1982) A saturable site responsible for polar transport of indole-3-acetic acid in sections of maize coleoptiles. Planta 155:68–75
Grieneisen VA, Xu J, Marée AF, Hogeweg P, Scheres B (2007) Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449:1008–1013
Haruta M, Sussman MR (2012) The effect of a genetically reduced plasma membrane proton motive force on vegetative growth of Arabidopsis. Plant Physiol 158:1158–1171
Hentrich M, Böttcher C, Düchting P, Cheng Y, Zhao Y, Berkowitz O, Masle J, Medina J, Pollmann S (2013) The jasmonic acid signaling pathway is linked to auxin homeostasis through the modulation of YUCCA8 and YUCCA9 gene expression. Plant J 74:626–637
Li L, Li C, Lee GI, Howe GA (2002) Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc Natl Acad Sci 99:6416–6421
Mancuso S, Marras AM, Magnus V, Baluska F (2005) Noninvasive and continuous recordings of auxin fluxes in intact root apex with a carbon nanotube-modified and self-referencing microelectrode. Anal Biochem 341:344–351
McConn M, Browse J (1996) The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell 8:403–416
McLamore ES, Diggs A, Calvo Marzal P, Shi J, Blakeslee JJ, Peer WA, Murphy AS, Porterfield DM (2010) Non-invasive quantification of endogenous root auxin transport using an integrated flux microsensor technique. Plant J 63:1004–1016
Morisawa MS, Steinhardt RA (1982) Changes in intracellular pH of Physarum plasmodium during the cell cycle and in response to starvation. Exp Cell Res 140:341–352
Pan X, Welti R, Wang X (2010) Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography–mass spectrometry. Nat Protoc 5:986–992
Petersson SV, Johansson AI, Kowalczyk M, Makoveychuk A, Wang JY, Moritz T, Grebe M, Benfey PN, Sandberg G, Ljung K (2009) An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell 21:1659–1668
Porterfield DM, McLamore ES, Banks MK (2009) Microsensor technology for measuring H+ flux in buffered media. Sens Actuator B 136:383–387
Qi L, Yan J, Li Y, Jiang H, Sun J, Chen Q, Li H, Chu J, Yan C, Sun X, Yu Y, Li C, Li C (2012) Arabidopsis thaliana plants differentially modulate auxin biosynthesis and transport during defense responses to the necrotrophic pathogen Alternaria brassicicola. New Phytol 195:872–882
Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI (2007) Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J 50:514–528
Rober-Kleber N, Albrechtova JT, Fleig S, Huck N, Michalke W, Wagner E, Speth V, Neuhaus G, Fischer-Iglesias C (2003) Plasma membrane H+-ATPase is involved in auxin-mediated cell elongation during wheat embryo development. Plant Physiol 131:1302–1312
Rubery PH, Sheldrake AR (1974) Carrier-mediated auxin transport. Planta 118:101–121
Schommer C, Palatnik JF, Aggarwal P, Chételat A, Cubas P, Farmer EE, Nath U, Weigel D (2008) Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol 6:1991–2001
Sheard LB, Tan X, Mao H, Withers J, Ben-Nissan G, Hinds TR, Kobayashi Y, Hsu FF, Sharon M, Browse J, He SY, Rizo J, Howe GA, Zheng N (2010) Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468:400–405
Song SS, Qi TC, Fan M, Zhang X, Gao H, Huang H, Wu DW, Guo HW, Xie DX (2013) The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet 9:e1003653
Staswick PE, Su W, Howell SH (2009) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci 89:6837–6840
Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jürgens G, Alonso JM (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133:177–191
Subraya KK, Diggs A, Porterfield DM (2013) Amperometric biosensor approaches for quantification of indole 3-acetic acid in plant stress responses. Commun Soil Sci Plan 44:1749–1763
Sun J, Xu Y, Ye S, Jiang H, Chen Q, Liu F, Zhou W, Chen R, Li X, Tietz O, Wu X, Cohen JD, Palme K, Li C (2009) Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell 21:1495–1511
Sun J, Chen Q, Qi L, Jiang H, Li S, Xu Y, Liu F, ZhouW Pan J, Li X, Palme K, Li C (2011) Jasmonate modulates endocytosis and plasma membrane accumulation of the Arabidopsis PIN2 protein. New Phytol 191:360–375
Sussman MR, Goldsmith MH (1981) The action of specific inhibitors of auxin transport on uptake of auxin and binding of N-1-naphthylphthalamic acid to a membrane site in maize coleoptiles. Planta 152:13–18
Taiz L, Zeiger E (2006) Plant physiology, 4th edn. Sinauer Associates, US, Sunderland
Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448:661–665
Turner JG, Ellis C, Devoto A (2002) The jasmonate signal pathway. Plant Cell 14:S153–S164
Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9:1963–1971
Undurraga SF, Santos MP, Paez-Valencia J, Yang H, Hepler PK, Facanha AR, Hirschi KD, Gaxiola RA (2012) Arabidopsis sodium dependent and independent phenotypes triggered by H+-PPase up-regulation are SOS1 dependent. Plant Sci 183:96–105
Vanneste S, Friml J (2009) Auxin: a trigger for change in plant development. Cell 136:1005–1016
Wasternack C, Kombrink E (2010) Jasmonates: structural requirements for lipid-derived signals active in plant stress responses and development. ACS Chem Biol 5:55–77
Wen B, Bin J, Wang X (2006) Effects of methyl jasmonate and abscisic acid treatments on the hydrolysis activity of plasma membrane H+-ATPase in Mung Bean (Vigna radiata L). Plant Physiol J 42:855–859
Wisniewska J, Xu J, Seifertova D, Brewer PB, Ruzicka K, Blilou I, Rouquie D, Benkova E, Scheres B, Friml J (2006) Polar PIN localization directs auxin flow in plants. Science 312:883
Wolters H, Jürgens G (2009) Survival of the flexible: Hormonal growth control and adaptation in plant development. Nat Rev Genet 10:305–317
Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998) COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280:1091–1094
Xu Y, Sun T, Yin L (2006) Application of non-invasive microsensing system to simultaneously measure both H+ and O2 fluxes around the pollen tube. J Integr Plant Biol 48:823–832
Xu W, Jia L, Baluška F, Ding G, Shi W, Ye N, Zhang J (2012) PIN2 is required for the adaptation of Arabidopsis roots to alkaline stressby modulating proton secretion. J Exp Bot 63:6105–6114
Xu W, Jia L, Shi W, Liang J, Zhou F, Li Q, Zhang J (2013) Abscisic acid accumulation modulates auxin transport in the root tip to enhance proton secretion for maintaining root growth under moderate water stress. New Phytol 197:139–150
Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, Wang Z, Xie D (2009) The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21:2220–2236
Yan SL, Luo ST, Dong SS, Zhang T, Sun JR, Wang NN, Yao HJ, Shen YB (2015) Heterotrimeric G-proteins involved in the MeJA regulated ion flux and stomatal closure in Arabidopsis thaliana. Funct Plant Biol 42:126–135
Yang Y, Hammes UZ, Taylor CG, Schachtman DP, Nielsen E (2006) High-affinity auxin transport by the AUX1 influx carrier protein. Curr Biol 16:1123–1127
Yang ZB, Geng X, He C, Zhang F, Wang R, Horst WJ, Ding Z (2014) TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell 26:2889–2904
Zhang Y, Turner JG (2008) Wound-induced endogenous jasmonates stunt plant growth by inhibiting mitosis. PLoS One 3:e3699
Zhao Y, Hull AK, Gupta NR, Goss KA, Alonso J, Ecker JR, Normanly J, Chory J, Celenza JL (2002) Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev 16:3100–3112
Acknowledgments
We would like to thank Professor Daoxin Xie for providing seeds of COI1 mutations coi1-1. We would like to thank Professor Hongwei Guo for providing seeds of aux1–7. We also would like to thank Xuyue (Beijing) Science and Technology Company for their technical support. This work was financially supported by the National ‘863’ Plant Project (No. 2011AA10020102) and the National Natural Science Foundation of China (31270655) and we would also like to thank Dr. Melanie Correll for proofreading the manuscript prior to submission.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors have no conflict of interest.
Additional information
Ting Zhang and Suli Yan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yan, S., Zhang, T., Dong, S. et al. MeJA Affects Root Growth by Modulation of Transmembrane Auxin Flux in the Transition Zone. J Plant Growth Regul 35, 256–265 (2016). https://doi.org/10.1007/s00344-015-9530-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-015-9530-9