Abstract
For the purpose of enhancing the secondary metabolite content in micropropagated Stevia rebaudiana plants without inhibiting plant growth, node explants were cultured on woody plant medium (WPM) containing alginate (ALG), casein hydrolysate (CH), pectin (PEC), yeast extract (YE), methyl jasmonate (MeJA), salicylic acid (SA), or chitosan (CHI). The highest shoot number; shoot length, node number, and leaf number; leaf length; and stem diameter were observed on WPM containing 1.0 g/L YE; 100 µM CHI; 0.5 g/L CH; and 1.0 g/L ALG, respectively. The root regeneration frequency reached 100 % on WPM supplemented with 0.5, 1.0, or 2.0 g/L PEC, 1.0 g/L YE, or 50 µM CHI and the control. The highest root number was obtained on WPM containing 0.5 g/L PEC, while the longest root length was observed on WPM containing 1.0 g/L YE. The highest biomass accumulation was observed with treatment of 100 µM CHI. According to the high-performance liquid chromatography results, except for the treatments with 200 µM CHI, 100 or 200 µM MeJA, and 200 µM SA, the remaining elicitor treatments increased stevioside production compared to the control. The production of stevioside increased from 1.56 mg/g dry weight (DW) to 14.69 and 14.54 mg/g DW in the in vitro plantlets exposed to 0.5 g/L ALG and 2.0 g/L YE, respectively. Rebaudioside A was observed on only 0.5 g/L ALG-treated plants as 0.55 mg/g DW. The stevioside content of field-grown plants was identified as 15.06 mg/g DW. The present findings provide important information regarding the effect of elicitors on plant growth and secondary metabolite production of in vitro micropropagated S. rebaudiana.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stevia rebaudiana belongs to the family Asteraceae and is native to Paraguay. Its leaves contain about thirty-four steviol glycosides (SGs) (Ceunen and Geuns 2013). Of those, the four major SGs are stevioside, rebaudioside A, rebaudioside C, and dulcoside A. The sweetness of those compounds relative to sucrose is 210, 242, 30, and 30 times, respectively (Brandle and Rosa 1992). As stevia has no carbohydrates or fat, it is an excellent alternative calorie-free sweetener (Singh and Rao 2005). Stevioside has the ability to reduce blood glucose levels in patients with Type II diabetes and mildly reduce blood pressure in patients with hypertension (Humphrey et al. 2006). Moreover, Stevia-based natural sweeteners is also an excellent sugar substitute in foods such as confectioneries, fruit juices, jams, biscuits, chocolates, vegetables and other food stuffs (Singh and Rao 2005).
The amount of SGs is influenced by the genotype, propagation methods, environmental conditions, and agronomic practices (Brandle and Rosa 1992). Plant tissue culture techniques can provide the production of plantlets which are genetically uniform and have homogeneous secondary metabolite content within a short time under controlled physical conditions. Hence, a more predictable and stable secondary metabolite content can be obtained using these techniques. Despite these advantages of plant tissue culture, the commercially production of plant secondary metabolites using in vitro technology remained limited to shikonin, paclitaxel, resveratrol, artemisinin, ginsenosides and ajmalicinin (Yue et al. 2014). Thus, various tissue culture techniques are developed to enhance the yield of secondary metabolites. One of the most common applications for this purpose is the use of elicitors (Sivanandhan et al. 2013).
Elicitors are compounds triggering the formation of secondary metabolites, which have been widely used for the overproduction of secondary metabolites in in vitro plant cultures (Dörnenburg and Knorr 1995; Ramakrishna and Ravishankar 2011; Murthy et al. 2014). The most common elicitors used for the enhancement of secondary metabolite production in plant tissue culture studies are alginate (ALG), casein hydrolysate (CH), pectin (PEC), yeast extract (YE), methyl jasmonate (MeJA), salicylic acid (SA), and chitosan (CHI) (Xu et al. 2015). Elicitors can be classified according to their origin as biotic and abiotic compounds. Plant hormones (SA, jasmonates, etc.) may be also considered as elicitors. CHI, ALG, PEC, and YE are biotic elicitors, and they have a biological origin. CHI and ALG originate from the cell wall components of microorganisms, while PEC derives from plant cell walls. They are signaling molecules within the elicitation pathways and can induce a similar plant defense response to pathogens or herbivore infections (exogenous elicitors). In some cases, they are released from the attacked plant through the enzymes of the pathogen (endogenous elicitors) (Angelova et al. 2006; Baenas et al. 2014; Gadzovska Simic et al. 2014). YE is a complex biological preparation (containing several components), and although the molecular structures of the active ingredients are unknown, it can elicit plant defense responses (Angelova et al. 2006; Baenas et al. 2014). Signal molecules, such as jasmonates [jasmonic acid (JA) and MeJA] and SA, induce a plant’s defense responses to pathogens and insects. Although jasmonates both accumulate and are produced in plants, the exogenous application of JA and SA has been shown to elicit secondary metabolite accumulation caused by the induction of a defense response (Rhee et al. 2010; Sharma et al. 2015). To aid in the growth and development of excised tissues and promote cell growth, and the production of secondary metabolites, CH could be added to the medium. The most likely cause of the enhanced cell growth by CH is the effects of one or more of the amino acids in the protein hydrolysate (Li and Zhang 2006).
High number of papers has been published about the production of SGs in in vitro stevia cultures, but the results obtained by these studies remain highly contradictory. While some researchers reported little or no SGs in the callus culture, suspension culture (Bondarev et al. 2001; Mathur and Shekhawat 2013), etiolated in vitro regenerants, callus culture grown in the light, etiolated heterotrophic callus (Ladygin et al. 2008), hairy root culture (Yamazaki and Flores 1991; Pandey et al. 2016), in vitro root culture (Reis et al. 2011) of S. rebaudiana, some researchers identified SGs in leaf-derived callus cultures (Sivaram and Mukundan 2003), salts (NaCl and Na2CO3), proline or polyethylene glycol treated callus and suspension culture (Gupta et al. 2014, 2015). On the other hand, it has been reported that in vitro shoots of S. rebaudiana have the ability to produce SGs (Yamazaki and Flores 1991; Bondarev et al. 2001; Aman et al. 2013; Dey et al. 2013; Singh et al. 2014). The effect of CH and YE on the in vitro shoot multiplication of S. rebaudiana (Sridhar and Aswath 2014) and the effect of CH on the accumulation of stevioside and rebaudioside A in the callus culture of S. rebaudiana have been described (Hsing et al. 1983). However, to the best of our knowledge, there is no information concerning the composition of stevioside and rebaudioside A in the in vitro shoots grown on elicitor-supplemented media.
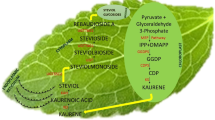
It is well known that SGs synthesis occurs exclusively in the mesophyll cells of S. rebaudiana leaves; with all the steps up to kaurene occurring in plastids, one of the 2 oxidation steps takes place on the surface of the endoplasmic reticulum, and the glycosylation takes place in the cytoplasm (Brandle et al. 2002; Brandle and Telmer 2007). Chloroplasts are vital in precursor synthesis; hence, tissue such as roots and lower stems, which contain no chlorophyll, contain little or merely trace amounts of glycosides (Singh and Rao 2005). SGs are also found in the stems and flowers, but to a lesser degree (Ceunen and Geuns 2013). Because of this reason, in the present study, in vitro-grown whole plantlets were used for increasing the production of SGs. Studies regarding the increasing of SGs contents in whole in vitro plantlets of S. rebaudiana without the adverse effect on the plantlets’ growth are of great importance. The aim of the present study was to increase stevioside and rebaudioside A production in in vitro-grown plantlets by supplying different elicitors in the medium, to get information about the effect of these elicitors on the growth of in vitro plantlets, and to determine the optimal treatment both secondary metabolite production and plant growth.
Materials and methods
Plant materials
In vitro propagated plantlets, which were obtained from single seed descent seedlings, of S. rebaudiana Bertoni in the Bioengineering Department of Ege University were used as the plant material.
Preparation of elicitors
YE, CH, PEC, and ALG (Sigma Aldrich) were dissolved with double distilled water and the pH of the elicitor solutions was adjusted to 5.8. The MeJA and SA (Sigma Aldrich) were dissolved in 96 % ethanol and the solutions were then diluted with double distilled water in concentrations as required. Finally, the pH of both solutions was adjusted to 5.8. Stock solution of CHI (from crab shells) was prepared in 2.0 mL of 1 % (v/v) acetic acid (Sigma-Aldrich) by stirring with a glass rod and then diluted with double distilled water. Finally, the pH of the solution was adjusted to 5.8.
The stock solutions of ALG, MeJA, SA, and CHI were filter-sterilized through a 0.22 µm syringe Millipore filter (Minisart®, Sartorius, Germany), and then added to the autoclaved WPM aseptically at the desired concentrations. The stock solutions of PEC, YE and CH were added to the media at the desired concentrations before autoclaving at 121 °C for 15 min. In the present study, ALG, CH, PEC or YE at the concentrations of 0.5–2.0 g/L and CHI, MeJA or SA at the concentrations of 50–100 µM were added individually in semi-solidified WPM as elicitors.
Elicitor treatment and culture conditions
The node explants with an axillary buds (1.0–1.5 cm in length) were cultured in glass tubes (23/24 × 140 mm, Lab Associates b.v., Oudenbosch, The Netherlands) containing 10 mL of woody plant medium (WPM; Lloyd and McCown 1980) supplemented with ALG, CH, PEC or YE at the concentrations of 0.5–2.0 g/L and CHI, MeJA or SA at the concentrations of 50–100 µM, 3 % (w/v) sucrose and solidified with 0.65 % (w/v) plant agar (Duchefa Biochemie B.V., The Netherlands) (pH 5.8).
The experiments were conducted in triplicate with ten explants in each replication. Thirty explants were tested in total per treatment. All of the cultures were maintained in a growth room at 25 ± 1 °C under a cool white fluorescent light (50 μmol/m2 s) for a light/dark photoperiod of 16:8.
After 4 weeks of treatments, all of the plantlets were harvested, and divided into leaves, roots, and stems to identify the fresh biomass weights of each replication. After freeze drying, dry weights (DWs) for each application were determined. After that, the stevioside and rebaudioside A contents were investigated using a high-performance liquid chromatography (HPLC).
Stevioside and rebaudioside A analysis
The leaves of 4 week-old S. rebaudiana plantlets grown on WPM supplemented with elicitors or control plantlets were collected and the content of stevioside and rebaudioside A was analyzed using HPLC.
Chemicals
HPLC-grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). The ultrapure water was provided from an in-house ultrapure water system (Sartorius Arium 611, Sartorius Stedim Biotech, Göttingen, Germany).
HPLC sample preparation
The leaf samples, approximately 50 mg, were sonicated 3 times with 5 mL of methanol for 20 min. The clear extracts were combined and diluted with methanol to 20 mL. Prior to HPLC analysis, the samples were filtered through a 0.45 µm PTFE filter (Sartorius AG, Göttingen, Germany) to remove any undissolved particles.
Liquid chromatography with photodiode array detection analysis
Liquid chromatography with photodiode array detection (LC-DAD) analyses were performed with a Thermo Surveyor Plus HPLC instrument (Thermo Scientific, Bremen, Germany), equipped with a quaternary pump, an autosampler, a column oven, and a diode array detector. For all of the separations, a Teknokroma RP C18 column (250 × 4.6 mm, particle size 5 µm, Teknochroma, Barcelona, Spain) was used. Liquid chromatography separations were carried out using the following solvents: ultrapure water (A) and acetonitrile (B) and the gradient elution was performed as: 0–1 min 65A/35B, for 4 min to 63A/27B, for 2.5 min to 60A/40B, 0.5 min to 5A/95B and kept at that composition for 3 min and changed to initial ratios (65A/35B) of method in 1 min. Prior to the next injection, the column was equilibrated for 3 min at the beginning conditions (65A/35B). The flow rate was 1 mL/min and the column temperature was 40 °C. Detection was performed at 210 nm and the UV spectra of all of the samples were scanned between 200 and 360 nm.
Quantifications of stevioside and rebaudioside A were carried out using calibration curves generated by a SG mixture which contained 50 % rebaudioside A and 36 % stevioside by mass. These percentages were determined according to the relative peak areas of the SGs based on the LC-DAD signals. The retention time of rebaudioside A was 5.75 min and stevioside was 6.15 min. The calibration curve for rebaudioside A was constituted with standard solutions between 1000.000–15.625 µg/mL and 720.00–11.250 µg/mL for stevioside. The regression coefficient of the calibration curve for rebaudioside A was 0.998 and the formula was peak area of rebaudioside A = 8000.76 × rebaudioside A concentration (µg/mL). Similar to rebaudioside A, the regression coefficient of stevioside was 0.998 and the formula was peak area of stevioside = 7850.76 × stevioside concentration (µg/mL).
Statistical analysis
Observations were recorded 4 weeks after the culture initiation. The experiments were arranged in a completely randomized design, and all of the treatments were replicated in triplicate, each with ten explants. The statistical analysis of the data on various parameters was subjected to Statistical Package for the Social Sciences Version 16.0 (SPSS Inc., Chicago, USA). The significance of the differences among the means was determined using the Student–Newman–Keuls test at P = 0.05.
Results and discussion
Effect of elicitors on in vitro shoot growth
The stevia plant has bilateral nodes. After approximately 3 days of node culture, generally, 2 shoots emerge from these nodes when plant growth regulators (PGRs) are not used. In the present study, since the PGRs were not used, the node explants exhibited nearly 2 shoots with all of the applications except with 200 µM CHI; 50, 100 and 200 µM MeJA, and 100 and 200 µM SA. The highest shoot number (3.9 shoots per explant) was observed on WPM containing 1.0 g/L YE (Table 1). The shoot number results were statistically significant close to each other except with 200 µM CHI; 50, 100 and 200 µM MeJA, and 100 and 200 µM SA treatments (Table 1).
The shoot length per explant showed a considerable variation, which ranged between 0.78 cm (200 µM SA) and 14.72 cm (100 µM CHI) (Table 1). In general, the shoot growth with treatments of ALG, CH, PEC, YE, CHI, and the control was better than those treated with MeJA and SA (Table 1).
The node number per explant is a significant factor for the proliferation rate, when the target is micropropagation. Therefore, the mean number of nodes per explant was recorded as well. With 100 µM CHI, the highest node number (10.70 nodes per explant), and in parallel with this the highest leaf number (23.40 leaves per explant), were recorded (Table 1). In addition, the longest leaf length (1.02 cm) was obtained with 0.5 g/L of CH treatment followed by 0.5 and 2.0 g/L of PEC treatments. Furthermore, WPM supplemented with 1.0 g/L ALG gave the best response regarding the stem diameter per explant (0.90 cm per explant).
In the present study, with progressively increasing MeJA concentrations in the WPM, the MeJA treatments did not support shoot growth after 2 weeks of in vitro culture. More specifically, with the concentrations of 100 and 200 µM, the shoots turned brownish and did not continue development into a healthy shoot. Due to leaf necrosis, the stevioside and rebaudioside A analysis could not be performed at these concentrations. The culture of the explants on SA resulted in the development of very thin and short shoots with short internodes and small leaves. Therefore, MeJA and SA treatments were found to be unsuitable for the aim of the present study.
Table 2 shows the effect of the elicitors on root formation. Except for the treatments with MeJA, a high percentage of root formation (60–100 %) occurred with the remaining treatments. The regeneration percentage reached 100 % on the WPM supplemented with 0.5, 1.0, and 2.0 g/L PEC; 1.0 g/L YE; 50 µM CHI; and the elicitor-free WPM (Table 2). Adding MeJA to the media decreased the not only shoot formation but also root formation. The highest root number (6.13 roots per explant) was observed with 0.5 g/L PEC treatment followed by 1.0 g/L YE, 1.0 g/L ALG and 100 µM CHI treatments. No statistical differences was observed between 1.0 and 0.5 g/L YE treatments with regard to root length and. The longest root length was obtained with these treatments as 7.04 and 6.60 cm, respectively.
Effect of elicitors on biomass accumulation
The fresh and dry weights of the leaves, stems, roots and shoots/plantlets (leaves + stems + roots) were evaluated separately, and the results are shown in Table 3. Different responses were observed among the treatments in terms of fresh and dry weights. In parallel with the highest number of leaves, the highest leaf fresh (112.0 mg per explant) and dry (11.3 mg per explant) weights were also observed with 100 µM CHI treatment followed by 1.0 g/L ALG and, 50 and 200 µM CHI treatments. All of the concentrations of CHI and 1.0 g/L ALG were statistically significant close to each other with regard to leaf dry weight.
Although the highest shoot length was obtained with 100 µM CHI, the highest stem fresh (232.8 mg per explant) and dry (26.6 mg per explant) weights were observed with 1.0 g/L ALG due to the formation of the highest stem diameter (0.90 cm/explant) per explant. Even though the highest root induction, root number per explant, and root length per explant were not observed with 100 µM CHI, this treatment resulted in the highest fresh (146.8 mg per explant) and dry (6.8 mg per explant) weights because of the regeneration of thicker roots compared to the other treatments. Supplementation with CHI generally resulted in an increase in the shoot/plantlet fresh and dry weights. The highest shoot/plantlet fresh (433.4 mg per explant) and dry (40.3 mg per explant) weights were observed with 100 µM CHI. In previous studies, a positive effect of CHI on in vitro plant growth was demonstrated in various plants (Ait Barka et al. 2004; Nge et al. 2006; Pornpienpakdee et al. 2010). Polysaccharide CHI is known to induce an increase in the lignin biosynthesis and cell wall lignification of plants. This results in stronger cell walls which pathogens are less able to penetrate (Vasconsuelo et al. 2003). This may explain why the CHI treatments were found to support good shoot growth. Treatment with CHI might stimulate lignification, resulting in better shoot growth and an increase in weight.
Effect of elicitors on stevioside and rebaudioside A production
There are some studies regarding elicitor treatment to shoot or whole plant cultures. In those studies, the shoots or in vitro plantlets were exposed to elicitors for different periods in liquid culture, and increments in the secondary metabolite accumulation were reported. However, the cultures were performed in 2 stages. In the first stage, the shoots or plantlets were grown on semi-solid media and in the second stage, they were transferred into liquid media containing elicitors (Kim et al. 2004; Shabani et al. 2009; Putalun et al. 2010; Thaweesak et al. 2011; Pérez-Alonso et al. 2012; Skrzypczak-Pietraszek et al. 2014). In the present study, we focused on finding suitable elicitors supporting the growth of shoots/plantlets and enhancing the secondary metabolite biosynthesis in 1 stage. The results indicated that the application of elicitors effects not only the growth of shoots/plantlets but also the production of stevioside. As seen in Table 4, except for the treatments with 200 µM CHI, 100 and 200 µM MeJA, and 200 µM SA, the remaining treatments positively affected the stevioside content compared to the control (Table 4).
The major component of the cell walls in brown algae is ALG, which is a natural polysaccharide that acts on the plant cells as an endogenous elicitor (Akimoto et al. 1999; Xu et al. 2015). The anionic function in the carboxylic groups of ALG affects the plant cell membrane or may even change the steric structure of the saccharide elicitor, causing an increase in the sensitivity of their recognition by the plant cells (Akimoto et al. 1999). With regards to the effects of ALG, stevioside production was stimulated by ALG treatments without any loss in shoot growth. The shoot growth was nearly the same compared to the control. At the 3 different concentrations (0.5, 1.0, and 2.0 g/L), the 0.5 g/L dose of ALG was more effective than the 1.0 and 2.0 g/L concentrations with regards to the stevioside yield. The highest stevioside content (14.69 mg/g DW) was observed and the stevioside content increased 9.43-fold compared to the control plants with a concentration of 0.5 g/L. The rebaudioside A content was observed only in this treatment as 0.55 mg/g DW (Table 4).
With the CH treatments, the secondary metabolite production showed a negative correlation with the shoot growth. Although the CH treatments generally reduced the shoot growth compared to the control, the stevioside content increased 8.38-fold (13.05 mg/g DW) with treatment of 0.5 g/L CH (Table 4). Although a subsequent decrease in the stevioside production was observed with concentrations of 1.0 and 2.0 g/L, both provided higher metabolite content than the control plants (Table 4). The stimulation of stevioside and rebaudioside A accumulation with CH has also been observed in S. rebaudiana callus culture. The addition of CH to the callus culture medium increased the accumulation of both compounds in S. rebaudiana but retarded the growth of callus. The stevioside and rebaudioside A contents of the callus tissue cultured on the medium supplemented with 2 g/L CH were twice as much as that of control, which means approximately 4 times to that of the leaves obtained from field-grown plants (Hsing et al. 1983).
PEC is a natural polysaccharide elicitor derived from plant cell walls (Sharma et al. 2011). Like other biotic elicitors (ALG, CHI, and YE), it is a signaling molecule within elicitation pathways and can induce similar plant defense response (Angelova et al. 2006; Baenas et al. 2014; Gadzovska Simic et al. 2014). In the present study, PEC at all of the concentrations enhanced stevioside production at nearly the same amounts when compared to the control (Table 4). The stevioside yield ranged between 9.23 and 10.74 mg/g DW, which was 5.93- and 6.90-folds higher than that of the control culture, respectively (Table 4).
The YE is often used as a biotic elicitor in secondary metabolite production (Cai et al. 2012). In the present study, YE treatments also enhanced the metabolite content. The stevioside content increased to 14.54 mg/g DW, which is 9.34-fold higher than the control plants at 2.0 g/L (Table 4).
In plant pathogen fungi, the main structural component of the cell wall is CHI, which mimics its effects and activates the biosynthesis of defense-related secondary metabolites in plants (Benhamou and Thériault 1992; Zhang et al. 2007; Tocci et al. 2010; Wiktorowska et al. 2010). Hence, it displays elicitor activity and is a substitute for fungal elicitors in the elicitation effect (Zhao et al. 2005). SGs are similar to other secondary plant metabolites, in that they behave in a defensive manner, by feeding deterrents or antimicrobial agents against specific herbivores, pests, or pathogens (Nanayakkara et al. 1987; Brandle et al. 1998; Richman et al. 1999). Hence, with the exception of treatments with 200 µM CHI, the stevioside content was seen to increase in the shoots treated with 50 and 100 µM CHI (Table 4). The stevioside content reached 7.02 mg/g DW (4.51-fold higher than the control plants) at 100 µM dose (Table 4).
MeJA is widely synthesized in higher plants and has an important signaling role in the elicitation of plant defense systems (Sharma et al. 2015). Therefore, MeJA can be applied exogenously to increase the production of secondary metabolites. In the present study, exogenously applied MeJA adversely affected shoot/plantlet growth. Tiny shoots with necrotic leaves formed with all of the concentrations of MeJA. The metabolite yield of the 100 and 200 µM MeJA-treated plantlets was not evaluated because all of the leaves showed necrosis. Treatments with 50 µM MeJA resulted in an 8.05-fold stevioside (12.53 mg/g DW) increase when compared to the control (Table 4). The negative effect of MeJA on shoot growth could be due to high MeJA concentrations and long exposure time.
Salicylic acid has an important role in the defense against attacks by microbes and herbivores, and against abiotic stresses, such as wounding and ozone exposure; hence, it is very often used in plant in vitro cultures as an elicitor (Muffler et al. 2011). When compared to the control, the shoot growth was very thin, the stevioside content reached 13.84 mg/g DW (8.89-fold higher compared to the control plants) with the 100 µM treatment (Table 4).
In the previous studies, the stevioside contents in in vitro-grown plants have been reported as 3.4 mg/g DW (Bondarev et al. 2001), 3.30 mg/g DW (Ladygin et al. 2008), 82.48 µg/g DW (Aman et al. 2013), 2.9 mg/g DW (Dey et al. 2013), 10.20 mg/g DW (Khalil et al. 2016). Bondarev et al. (2001) found stevioside contents as 0.015 mg/g DW in suspension culture, 0.06 mg/g DW in morphogenic callus and 0.387 mg/g DW in shoots, regenerated from callus. They also established a callus culture in continuous darkness; however, they determined no stevioside in this culture. Ladygin et al. (2008) reported stevioside contents as 0.28 mg/g DW in etiolated in vitro regenerants, 0.06 mg/g DW in green morphogenic callus and trace in etiolated callus. Reis et al. (2011) established successfully an adventitious root culture of S. rebaudiana in a roller bottle system, but no stevioside was synthesized in this culture. Pandey et al. (2016) reported the stevioside production as 1.72 mg/g DW in hairy root culture. Mathur and Shekhawat (2013) observed the content of stevioside as 381.03 µg/g DW in cell suspension culture. Gupta et al. (2014) increased the concentration of stevioside from 0.20 to 1.34 % (6.7-fold higher than the control culture) in callus culture exposed to abiotic stress induced 0.025 % Na2CO3 and from 1.02 to 1.33 % (1.30-fold higher than the control culture) in suspension culture exposed to 0.025 % Na2CO3 (on 15th day). In another study, they cultured in vitro raised nodal explants of Stevia on MS medium supplemented with 2.0 mg/L kinetin and different concentrations of NaCl (0.05–0.20 %), Na2CO3 (0.025–0.10 %), Proline (2.5–10 mM) and Polyethylene glycol (2.5–10 %) for 4 weeks (Gupta et al. 2016). The content of stevioside was increased from 0.49 to 1.37 % (2.39-fold higher than the control culture) in in vitro shoots exposed to 0.05 % Na2CO3. In the present study, much higher contents of stevioside could be achieved and the stevioside content reached its maximum value with the 0.5 g/L ALG- and 2.0 g/L YE-treated in vitro plantlets as 14.69 and 14.54 mg/g DW, respectively (Table 4). These amounts are nearly 9.5-fold higher than the control plants (1.56 mg/g DW) and nearly same with the field-grown plants (15.06 mg/g DW). Gupta et al. (2015) increased the concentration of stevioside from 0.20 to 1.47 % (7.35-fold higher than the control culture) in callus culture exposed to 7.5 % PEG and from 0.23 to 1.83 % (7.96-fold higher than the control culture) in suspension culture exposed to 5 % PEG (on 10th day). Although our results regarding the stevioside content seem lower, the increasing content rate of stevioside in our study (approximately 9.5-fold higher than the control plants) was higher than the results (7.35- and 7.96-fold higher than the controls of callus and suspension cultures) obtained by Gupta et al. (2016). However they obtained important results regarding rebaudioside A content in callus culture, suspension culture and in vitro-grown shoots exposed to above mentioned treatments (Gupta et al. 2014, 2015, 2016). In the present study, the rebaudioside A content was observed only in 0.5 g/L ALG treatments as 0.55 mg/g DW (Table 4).
Because of the different genotypes, geographical origins, propagation methods, environmental conditions, and agronomic practices, the stevioside contents reported in the intact plants or field-grown plants showed quite varieties such as 8.75 g/100 g (Woelwer-Rieck et al. 2010), 5.8 % (Gardana et al. 2010), 1.79 mg/g (Pandey et al. 2016), 24.9, 21.6 and 30.2 mg/g (Bondarev et al. 2001), 25.40 mg/g (Ladygin et al. 2008). The quantity of stevioside obtained from the present study generally remained lower compared to field-grown plants. However, in case of selection of the mother plant materials with high SGs contents, it may be also possible to achieve high SGs production in in vitro-grown plantlets.
Conclusion
In the present study, it was demonstrated that elicitor (ALG, CH, PEC, YE, MeJA, SA, and CHI) application to micropropagated S. rebaudiana plants enhanced stevioside synthesis when compared to the untreated in vitro plantlets. The maximum stevioside production was found in the in vitro plantlets treated with 0.5 g/L ALG or 2.0 g/L YE (14.69 and 14.54 mg/g DW, respectively). This result is approximately 9.4-fold higher than that in the control plants and these amounts of stevioside were nearly same with the field-grown plants. The results obtained from the present study can act as a roadmap for the further studies regarding the increase in stevioside production. Elicitor treatment can be applied to large-scale in vitro propagated plantlets of S. rebaudiana in bioreactors and the production of stevioside in desired concentrations can be optimized under controlled conditions. In future, studies may be conducted to direct the metabolic pathway to rebaudioside A production or the established system can be exploited to investigate stevioside synthetic pathways. Moreover, the elicitors can be applied under field conditions.
References
Ait Barka E, Eullaffroy P, Clément C, Vernet G (2004) Chitosan improves development, and protects Vitis vinifera L. against Botrytis cinerea. J Plant Cell Rep 22(8):608–614
Akimoto C, Aoyagi H, Tanaka H (1999) Endogenous elicitor-like effects of alginate on physiological activities of plant cells. Appl Microbiol Biotechnol 52:429–436
Aman N, Hadi F, Khalil SA, Zamir R, Ahmad N (2013) Efficient regeneration for enhanced steviol glycosides production in Stevia rebaudiana (Bertoni). C R Biol 336:486–492
Angelova Z, Georgiev S, Roos W (2006) Elicitation of plants. Biotechnol Biotechnol Equip 20(2):72–83. doi:10.1080/13102818.2006.10817345
Baenas N, García-Viguera C, Moreno DA (2014) Elicitation: a tool for enriching the bioactive composition of foods. Molecules 19:13541–13563. doi:10.3390/molecules190913541
Benhamou N, Thériault G (1992) Treatment with chitosan enhances resistance of tomato plants to the crown and root rot pathogen Fusarium oxysporum f. sp. radicis-lycopersici. Physiol Mol Plant Pathol 41(1):33–52. doi:10.1016/0885-5765(92)90047-Y
Bondarev N, Reshetnyak O, Nosov A (2001) Peculiarities of diterpenoid steviol glycoside production in in vitro cultures of Stevia rebaudiana Bertoni. Plant Sci 161:155–163. doi:10.1016/S0168-9452(01)00400-9
Brandle JE, Rosa N (1992) Heritability for yield, leaf: stem ratio and stevioside content estimated from a landrace cultivar of Stevia rebaudiana. Can J Plant Sci 72:1263–1266
Brandle JE, Telmer PG (2007) Steviol glycoside biosynthesis. Phytochemistry 68:1855–1863. doi:10.1016/j.phytochem.2007.02.010
Brandle JE, Starratt AN, Gijzen M (1998) Stevia rebaudiana: its agricultural, biological and chemical properties. Can J Plant Sci 78:527–536. doi:10.4141/P97-114
Brandle JE, Richman A, Swanson AK, Chapman BP (2002) Leaf ESTs from Stevia rebaudiana: a resource for gene discovery in diterpene synthesis. Plant Mol Biol 50:613–622. doi:10.1023/A:1019993221986
Cai Z, Kastell A, Mewis I, Knorr D, Smetansk I (2012) Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Organ Cult 108:401–409. doi:10.1007/s11240-011-0051-3
Ceunen S, Geuns JMC (2013) Influence of photoperiodism on the spatio-temporal accumulation of steviol glycosides in Stevia rebaudiana (Bertoni). Plant Sci 198:72–82. doi:10.1016/j.plantsci.2012.10.003
Dey A, Kundu S, Bandyopadhyay A, Bhattacharjee A (2013) Efficient micropropagation and chlorocholine chloride induced stevioside production of Stevia rebaudiana Bertoni. C R Biol 336:17–28. doi:10.1016/j.crvi.2012.11.007
Dörnenburg H, Knorr D (1995) Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzyme Microb Technol 17:674–684
Gadzovska Simic S, Tusevski O, Maury S, Delaunay A, Joseph C, Hagège D (2014) Effects of polysaccharide elicitors on secondary metabolite production and antioxidant response in Hypericum perforatum L. shoot cultures. Sci World J 2014:609649. doi:10.1155/2014/609649
Gardana C, Scaglianti M, Simonetti P (2010) Evaluation of steviol and its glycosides in Stevia rebaudiana leaves and commercial sweetener by ultra-high-performance liquid chromatography-mass spectrometry. J Chromatogr A 1217:1463–1470. doi:10.1016/j.chroma.2009.12.036
Gupta P, Sharma S, Saxena S (2014) Effect of salts (NaCl and Na2CO3) on callus and suspension culture of Stevia rebaudiana for steviol glycoside production. Appl Biochem Biotechnol 172(6):2894–2906. doi:10.1007/s12010-014-0736-2
Gupta P, Sharma S, Saxena S (2015) Biomass yield and steviol glycoside production in callus and suspension culture of Stevia rebaudiana treated with proline and polyethylene glycol. Appl Biochem Biotechnol 176(3):863–874. doi:10.1007/s12010-015-1616-0
Gupta P, Sharma S, Saxena S (2016) Effect of abiotic stress on growth parameters and steviol glycoside content in Stevia rebaudiana (Bertoni) raised in vitro. J Appl Res Med Aromat Plants. doi:10.1016/j.jarmap.2016.03.004
Hsing YI, Su WF, Chang WC (1983) Accumulation of stevioside and rebaudioside A in callus culture of Stevia rebaudiana Bertoni. Bot Bull Acad Sin 24:115–119
Humphrey TV, Richman AS, Menassa R, Brandle JE (2006) Spatial organisation of four enzymes from Stevia rebaudiana that are involved in steviol glycoside synthesis. Plant Mol Biol 61:47–62. doi:10.1007/s11103-005-5966-9
Khalil SA, Kamal N, Sajid M, Ahmad N, Zamir R, Ahmad N, Ali S (2016) Synergism of polyamines and plant growth regulators enhanced morphogenesis, stevioside content, and production of commercially important natural antioxidants in Stevia rebaudiana Bert. In Vitro Cell Dev Biol Plant 52:174–184. doi:10.1007/s11627-016-9749-6
Kim OT, Kim MY, Hong MH, Ahn JC, Hwang B (2004) Stimulation of asiaticoside accumulation in the whole plant cultures of Centella asiatica (L.) Urban by elicitors. Plant Cell Rep 23:339–344. doi:10.1007/s00299-004-0826-7
Ladygin VG, Bondarev NI, Semenova GA, Smolov AA, Reshetnyak OV, Nosov AM (2008) Chloroplast ultrastructure, photosynthetic apparatus activities and production of steviol glycosides in Stevia rebaudiana in vivo and in vitro. Biol Plant 52:9–16. doi:10.1007/s10535-008-0002-y
Li L, Zhang CR (2006) Production of puerarin and isoflavones in cell suspension cultures of Pueraria lobata (Willd.): effects of medium supplementation with casein hydrolysate and coconut milk. J Environ Biol 27(1):21–26
Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Int Plant Prop Soc 30:421–427
Mathur S, Shekhawat GS (2013) Establishment and characterization of Stevia rebaudiana (Bertoni) cell suspension culture: an in vitro approach for production of stevioside. Acta Physiol Plant 35:931–939. doi:10.1007/s11738-012-1136-2
Muffler K, Leipold D, Scheller MC, Haas C, Steingroewer J, Bley T, Neuhaus HE, Mirata MA, Schrader J, Ulber R (2011) Biotransformation of triterpenes. Process Biochem 46:1–15
Murthy HN, Lee EJ, Paek KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult 118:1–16. doi:10.1007/s11240-014-0467-7
Nanayakkara NP, Klocke JA, Compadre CM, Hussain RA, Pezzuto JM, Kinghorn AD (1987) Characterization and feeding deterrent effects on the aphid, Schizaphis graminum, of some derivatives of the sweet compounds, stevioside and rebaudioside A. J Nat Prod 50(3):434–441. doi:10.1021/np50051a015
Nge KL, New N, Chandrkrachang S, Stevens WF (2006) Chitosan as a growth stimulator in orchid tissue culture. Plant Sci 170:1185–1190
Pandey H, Pandey P, Pandey SS, Singh S, Banerjee S (2016) Meeting the challenge of stevioside production in the hairy roots of Stevia rebaudiana by probing the underlying process. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-016-1020-7
Pérez-Alonso N, Capote A, Gerth A, Jiménez E (2012) Increased cardenolides production by elicitation of Digitalis lanata shoots cultured in temporary immersion systems. Plant Cell Tissue Organ Cult 110:153–162. doi:10.1007/s11240-012-0139-4
Pornpienpakdee P, Singhasurasak R, Chaiyasap P, Pichyangkura R, Bunjongrat R, Chadchawan S, Limpanavech P (2010) Improving the micropropagation efficiency of hybrid Dendrobium orchids with chitosan. Sci Hortic 124:490–499. doi:10.1016/j.scienta.2010.02.008
Putalun W, Udomsin O, Yusakul G, Juengwatanatrakul T, Sakamoto S, Tanaka H (2010) Enhanced plumbagin production from in vitro cultures of Drosera burmanii using elicitation. Biotechnol Lett 32:721–724. doi:10.1007/s10529-010-0202-3
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6(11):1720–1731. doi:10.4161/psb.6.11.17613
Reis RV, Borges APPL, Chierrito TPC, de Souto ER, de Souza LM, Iacomini M, de Oliveira AJB, Gonçalves RAC (2011) Establishment of adventitious root culture of Stevia rebaudiana Bertoni in a roller bottle system. Plant Cell Tissue Organ Cult 106:329–335. doi:10.1007/s11240-011-9925-7
Rhee HS, Cho HY, Son SY, Yoon SYH, Park JM (2010) Enhanced accumulation of decursin and decursinol angelate in root cultures and intact roots of Angelica gigas Nakai following elicitation. Plant Cell Tissue Organ Cult 101:295–302. doi:10.1007/s11240-010-9688-6
Richman AS, Gijzen M, Starratt AN, Yang Z, Brandle JE (1999) Diterpene synthesis in Stevia rebaudiana: recruitment and up-regulation of key enzymes from the gibberellin biosynthetic pathway. Plant J 19(4):411–421. doi:10.1046/j.1365-313X.1999.00531.x
Shabani L, Ehsanpour AA, Asghari G, Emami J (2009) Glycyrrhizin production by in vitro cultured Glycyrrhiza glabra elicited by methyl jasmonate and salicylic acid. Russ J Plant Physiol 56(5):621–626. doi:10.1134/S1021443709050069
Sharma M, Sharma A, Kumar A, Kumar Basu S (2011) Enhancement of secondary metabolites in cultured plant cells through stress stimulus. Am J Plant Physiol 6(2):50–71. doi:10.3923/ajpp.2011.50.71
Sharma M, Ahuja A, Gupta R, Mallubhotla S (2015) Enhanced bacoside production in shoot cultures of Bacopa monnieri under the influence of abiotic elicitors. Nat Prod Res 29(8):745–749. doi:10.1080/14786419.2014.986657
Singh SD, Rao GP (2005) Stevia: the herbal sugar of 21st century. Sugar Tech 7(l):17–24
Singh P, Dwivedi P, Atri N (2014) In vitro shoot multiplication of Stevia and assessment of stevioside content and genetic fidelity of the regenerants. Sugar Tech 16(4):430–439. doi:10.1007/s12355-013-0292-z
Sivanandhan G, Kapil Dev G, Jeyaraj M, Rajesh M, Arjunan A, Muthuselvam M, Manickavasagam M, Selvaraj N, Ganapathi A (2013) Increased production of withanolide A, withanone, and withaferin A in hairy root cultures of Withania somnifera (L.) Dunal elicited with methyl jasmonate and salicylic acid. Plant Cell Tissue Organ Cult 114:121–129. doi:10.1007/s11240-013-0297-z
Sivaram L, Mukundan U (2003) In vitro culture studies on Stevia rebaudiana. In Vitro Cell Dev Biol Plant 39:520–523. doi:10.1079/IVP2003438
Skrzypczak-Pietraszek E, Słota J, Pietraszek J (2014) The influence of l-phenylalanine, methyl jasmonate and sucrose concentration on the accumulation of phenolic acids in Exacum affine Balf. f. ex Regel shoot culture. Acta Biochim Pol 61(1):47–53
Sridhar TM, Aswath CR (2014) Influence of additives on enhanced in vitro shoot multiplication of Stevia rebaudiana (Bert.)—An important anti diabetic medicinal plant. Am J Plant Sci 5:192–199
Thaweesak J, Seiichi S, Hiroyuki T, Waraporn P (2011) Elicitation effect on production of plumbagin in in vitro culture of Drosera indica L. J Med Plants Res 5(19):4949–4953
Tocci N, Ferrari F, Santamaria AR, Valletta A, Rovardi I, Pasqua G (2010) Chitosan enhances xanthone production in Hypericum perforatum subsp. angustifolium cell cultures. Nat Prod Res 24(3):286–293. doi:10.1080/14786410903006353
Vasconsuelo A, Giuletti AM, Picotto G, Rodriguez-Talou J, Boland R (2003) Involvement of the PLC/PKC pathway in Chitosan-induced anthraquinone production by Rubia tinctorum L. cell cultures. Plant Sci 165:429–436
Wiktorowska E, Długosz M, Janiszowska W (2010) Significant enhancement of oleanolic acid accumulation by biotic elicitors in cell suspension cultures of Calendula officinalis L. Enzyme Microb Technol 46:14–20. doi:10.1016/j.enzmictec.2009.09.002
Woelwer-Rieck U, Lankes C, Wawrzun A, Matthias W (2010) Improved HPLC method for the evaluation of the major steviol glycosides in leaves of Stevia rebaudiana. Eur Food Res Technol 231:581–588. doi:10.1007/s00217-010-1309-4
Xu S, Zhou W, Pottinger S, Baldwin IT (2015) Herbivore associated elicitor-induced defences are highly specific among closely related Nicotiana species. BMC Plant Biol 15(2):1–13. doi:10.1186/s12870-014-0406-0
Yamazaki T, Flores HE (1991) Examination of steviol glucosides production by hairy root and shoot cultures of Stevia rebaudiana. J Nat Prod 54:986–992. doi:10.1021/np50076a010
Yue W, Ming QL, Lin B, Rahman K, Zheng CJ, Han T, Qin LP (2014) Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit Rev Biotechnol 36(2):215–232. doi:10.3109/07388551.2014.923986
Zhang CH, Fevereiro PS, He G, Chen Z (2007) Enhanced paclitaxel productivity and release capacity of Taxus chinensis cell suspension cultures adapted to chitosan. Plant Sci 172(1):158–163. doi:10.1371/journal.pone.0104005
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333. doi:10.1016/j.biotechadv.2005.01.003
Acknowledgments
This research was financed by Ahi Evran University Scientific Research Projects Commission (PYO-MÜH.4001.14.006). All of the experiments were carried out at the Bioengineering Department of Ege University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayraktar, M., Naziri, E., Akgun, I.H. et al. Elicitor induced stevioside production, in vitro shoot growth, and biomass accumulation in micropropagated Stevia rebaudiana . Plant Cell Tiss Organ Cult 127, 289–300 (2016). https://doi.org/10.1007/s11240-016-1049-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1049-7