Abstract
A novel carbon paste electrode modified with graphene nanosheets and an ionic liquid (n-hexyl-3-methylimidazolium hexafluoro phosphate) was fabricated and used for the electrochemical study of mangiferin for the first time. This modified electrode offers a considerable improvement in voltammetric sensitivity toward mangiferin, compared to the bare electrode. Square wave voltammetry (SWV) exhibits a linear dynamic range from 5.0 × 10−8 to 2.0 × 10−4 M and a detection limit of 20.0 nM for mangiferin. Finally, the proposed method was successfully applied to the determination of mangiferin in real samples such as serum and urine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Graphene is an one-atom-thick planar sheet of sp2-bonded carbon atoms that is densely packed in a honey-comb crystal lattice. It is the latest nano scale form of carbon to be discovered and is the current hottest topic in material science. Due to its unique electronic properties, rich edge defects, large surface area, and strong mechanical strength [1]; it exhibits remarkable electrocatalytic and sensing properties [2–7].
Ionic liquids (ILs) have been generating increasing interest over the last decade [8–10]. ILs have a great potential for possible electrochemical applications because these compounds possess high thermal stability, no volatility, high polarity, large viscosity, high intrinsic conductivity, and wide electrochemical windows [11–13].
Mangiferin (Scheme 1) was originally isolated from Mangifera indica L. (Anacardiaceae) [14] and can be found in at least 16 plant families including Anacardiaceae, Iridaceae, and Gentianaceae [15]. As the active flavonoid compound of many eutherapeutic traditional Chinese herbs, mangiferin is receiving considerable attention in medical and nutritional research. Mangiferin is reported to have various pharmacological effects, including antioxidation [16], antitumor activity [17], anti-HIV activity [17], immunomodulation [18], anti-inflammation [19], antidiabetic activity [20], and hepatoprotective activity [21].
HPLC alone or HPLC combined with mass spectrometry [22] is widely employed for determination of mangiferin quality and quantity in pharmaceutical, pharmacokinetic, or pharmacological studies. Although HPLC is a reliable method and LC–MS has very powerful resolution for the detection of mangiferin and other flavonoid compounds, they remain a costly procedure. Compare to HPLC methods, electrochemical methods may be more applicable because they are less expensive, more convenient, more selective, and sensitive. There is no need for derivatization or time-consuming extraction steps in comparison with other techniques because of less sensitivity of electroanalytical methods to the matrix effects.
Electrochemical techniques in the field of pharmaceutical analysis have developed due to their simplicity, reasonable accuracy and precision, low cost, and rapidity [23–33].
Carbon-paste electrodes (CPEs) are widely utilized to perform the electrochemical determinations of a variety of biological and pharmaceutical species owing to their low residual current and noise, ease of fabrication, wide anodic and cathodic potential ranges, rapid surface renewal, and low cost. Moreover, chemically modified electrodes (CMEs) can be easily prepared by adding different substances to the bulk of CPEs in order to increase sensitivity, selectivity, and rapidity of determinations [34–44].
In the present work, we describe the preparation of a new carbon paste electrode modified with an ionic liquid and graphene nanosheets (IL-G-CPE) and investigate its performance for the determination of mangiferin in aqueous solutions for the first time.
Experimental
Apparatus and chemicals
The electrochemical measurements were performed with an Autolab potentiostat/galvanostat (PGSTAT 302N, Eco Chemie, the Netherlands). The experimental conditions were controlled with General Purpose Electrochemical System (GPES) software. A conventional three electrode cell was used at 25 ± 1 °C. An Ag/AgCl/KCl (3.0 M) electrode, a platinum wire, and IL-G-CPE were used as the reference, auxiliary, and working electrodes, respectively. A Metrohm 710 pH meter was used for pH measurements.
Mangiferin was purchased from Sigma Aldrich Company (USA), and all of the other reagents were of analytical grade and were obtained from Merck (Darmstadt, Germany). The buffer solutions were prepared from orthophosphoric acid and its salts in the pH range of 2.0–11.0.
Synthesis of graphene nanosheets
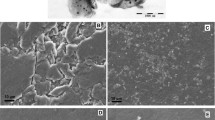
Graphene nanosheets were synthesized from natural graphite flakes based on the modified Hummers and Offeman’s method [45, 46]. In a typical synthesis process, 1.0 g of pristine graphite flakes was immersed in 50 mL of formic acid, and then sonicated for 2 h at room temperature. These resulting graphite plates were washed with acetone, and then dried in an oven at 95 °C for 12 h. Then, 100 mL H2SO4 (95 %) was added into a 500-mL flask, and cooled by immersion in an ice bath followed by stirring. About 1.0 g treated graphite powder and 0.5 g NaNO3 were added under vigorous stirring to avoid agglomeration. After the graphite powder was well dispersed, 3 g KMnO4 was added gradually under stirring and cooling so that the temperature of the mixture was maintained below 10 °C. The mixture was stirred for 2 h and diluted with deionised double-distilled water (in an ice bath). After that, 25 ml 15 % H2O2 was slowly added to the mixture until the color of the mixture changed to brilliant yellow, indicating fully oxidized graphite. The as-obtained graphite oxide slurry was re-dispersed in deionised double-distilled water and then exfoliated to generate graphene oxide nanosheets by sonication for 2 h. Then, the solution was filtered and washed with diluted HCl solution to remove metal ions. Finally, the product was washed with deionised double-distilled water until the solution became acid free, and dried under vacuum at 50 °C. A typical TEM for synthesized graphene nanosheets is shown in Fig. 1.
Preparation of the electrode
IL-G-CPEs were prepared by mixing 0.2 g of graphene with 0.8 g graphite powder and approximately, ∼0.8 mL of ionic liquids with a mortar and pestle. The paste was then packed into the end of a glass tube (ca. 3.4 mm i.d. and 15 cm long). A copper wire inserted into the carbon paste provided the electrical contact.
For comparison, ionic liquid/carbon paste electrode in the absence of graphene (IL-CPE) consistent of ionic liquid and graphite powder, graphene carbon paste electrode (G-CPE) consistent of graphene, graphite powder and paraffin oil, and bare carbon paste electrode (CPE) consistent of graphite powder and paraffin oil were also prepared in the same way.
Procedure of real samples preparation
The serum sample was centrifuged and then after filtering, diluted with PBS (pH 6.0) without any further treatment. The diluted serum sample was spiked with different amounts of mangiferin.
Urine samples were stored in a refrigerator immediately after collection. Ten milliliters of the sample was centrifuged for 15 min at 2,000 rpm. The supernatant was filtered out using a 0.45-μm filter. Then, different volumes of the solution were transferred into a 10-mL volumetric flask and were diluted to the mark with PBS (pH 6.0). The diluted urine sample was spiked with different amounts of mangiferin.
Result and discussion
Electrochemical behavior of mangiferin at the surface of various electrodes
Figure 2 displays cyclic voltammetric responses from the electrochemical oxidation of 100.0 μM mangiferin at the surface of IL-G-CPE (curve d), IL-CPE (curve c), G-CPE (curve b), and bare CPE (curve a). The results showed that the oxidation of mangiferin is very weak at the surface of the bare CPE, but in the presence of ILs in CPE could enhance the peak current and decrease the oxidation potential (decreasing the overpotential). A substantial negative shift of the currents starting from oxidation potential for mangiferin and dramatic increase of the current indicates the catalytic ability of IL-G-CPE (curve d) and IL-CPE (curve c) to mangiferin oxidation. The results showed that the combination of G and the ionic liquid (curve d) definitely improved the characteristics of mangiferin oxidation. However, IL-G-CPE shows much higher anodic peak current for the oxidation of mangiferin compared to IL-CPE, indicating that the combination of G and IL has significantly improved the performance of the electrode toward mangiferin oxidation.
Effect of variables
The electrochemical behavior of mangiferin (pK a1 = 6.52, pK a2 = 7.97, pK a3 = 9.44, pK a4 = 12.10 [47]), is dependent on the pH value of the aqueous solution, therefore, pH optimization of the solution seems to be necessary. The effect of solution pH on electrochemical responses of mangiferin at the surface of IL-G-CPE was investigated over a pH range of 2.0 to 10.0 using cyclic voltammetry. The results showed that the peak current reached to a maximum value at pH 6.0. The results also showed a linear negative shift for the variation of the anodic peak potential of mangiferin with increasing the solution pH (from 2.0 to 10.0) with a slope of −49 mV/pH unit, which indicates that the total numbers of electrons and protons taking part in the charge transfer were the same for mangiferin.
The effect of potential scan rates on the oxidation current of mangiferin has been studied (Fig. 3). The results showed that increasing in the potential scan rate induced an increase in the peak current. In addition, the oxidation process is diffusion controlled as deduced from the linear dependence of the anodic peak current (I p) on the square root of the potential scan rate (ν 1/2) over a wide range from 10 to 300 mV s−1.
Figure 4 shows the cyclic voltammogram of an IL-G-CPE obtained in 0.1 M PBS (pH 6.0) containing 0.1 mM mangiferin, with a sweep rate of 10 mV s−1. The points show the rising part of the voltammogram (known as the Tafel region), which is affected by the electron transfer kinetics between mangiferin and IL-G-CPE. If deprotonation of mangiferin is a sufficiently fast step, the number of electrons involved in the rate determining step can be estimated from the slope of the Tafel plot. The inset of Fig. 4 shows a Tafel plot that was drawn from points of the Tafel region of the cyclic voltammogram. The Tafel slope of 0.091 V obtained in this case agrees well with the involvement of one electron in the rate determining step of the electrode process, assuming a charge transfer coefficient of α = 0.35.
Chronoamperometric measurements
Chronoamperometric measurements of mangiferin at IL-G-CPE were carried out by setting the working electrode potential at 0.45 V (at the first potential step) and at 0.0 V (at second potential step) vs. Ag/AgCl/KCl (3.0 M) for the various concentrations of mangiferin in PBS (pH 6.0; Fig. 5). For an electroactive material (mangiferin in this case) with a diffusion coefficient of D, the current observed for the electrochemical reaction at the mass transport limited condition is described by the Cottrell equation [48]. Experimental plots of I vs. t −1/2 were employed, with the best fits for different concentrations of mangiferin (Fig. 5a). The slopes of the resulting straight lines were then plotted vs. mangiferin concentration (Fig. 5b). From the resulting slope and Cottrell equation the mean value of the D was found to be 4.38 × 10−6 cm2/s.
Chronoamperograms obtained at IL-G-CPE in 0.1 M PBS (pH 6.0) for different concentration of mangiferin. The numbers 1–7 correspond to 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, and 1.2 mM of mangiferin. Insets A plots of I vs. t −1/2 obtained from chronoamperograms 1–7. B Plot of the slope of the straight lines against mangiferin concentration
Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy was also employed to study the oxidation of mangiferin at the surface of various electrodes. Figure 6 shows Nyquist diagrams of IL-G-CPE (curve d), IL-CPE (curve c), G-CPE (curve b), and bare CPE (curve a) in the presence of mangiferin at pH 6.0. Nyquist diagram consists of a semicircle and straight line with a slope of nearly 45° that is due to the occurrence of mass transport process via diffusion. The semicircle diameters of Nyquist plot reflect the electron transfer resistance (R ct), which is from the electron transfer of the mangiferin solution. The results showed that for IL-G-CPE (curve d), the diameter of the semicircle is smaller than the other electrodes, which is due to the presence of high conductive IL and G in the carbon paste. All these results indicated that mangiferin can successfully oxidize on the surface of IL-G-CPE.
Calibration plot and limit of detection
The peak current of mangiferin oxidation at the surface of the modified electrode can be used for determination of mangiferin in solution. Therefore, square wave voltammetry (SWV) experiments were done for different concentrations of mangiferin (Fig. 7). The oxidation peak currents of mangiferin at the surface of a modified electrode were proportional to the concentration of the mangiferin within the ranges 5.0 × 10−8 to 2.0 × 10−4 M with detection limit (3σ) of 20.0 nM.
SWVs of IL-G-CPE in 0.1 M PBS (pH 6.0) containing different concentrations of mangiferin. Numbers 1–14 correspond to 0.05, 0.1, 0.5, 1.0, 5.0, 10.0, 25.0, 50.0, 75.0, 100.0, 125.0, 150.0, 175.0, and 200.0 μM of mangiferin. Inset B show the plots of the peak current as a function of mangiferin concentration in the range of 0.05–200.0 μM
The repeatability and stability of IL-G-CPE
The long-term stability of the IL-G-CPE was tested over a 3-week period. When CVs were recorded after the modified electrode was stored in atmosphere at room temperature, the peak potential for mangiferin oxidation was unchanged and the current signals showed less than 2.6 % decrease relative to the initial response. The antifouling properties of the modified electrode toward mangiferin oxidation and its oxidation products were investigated by recording the cyclic voltammograms of the modified electrode before and after use in the presence of mangiferin. Cyclic voltammograms were recorded in the presence of mangiferin after having cycled the potential 20 times at a scan rate of 30 mV s−1. The peak potentials were unchanged and the currents decreased by less than 2.4 %. Therefore, at the surface of IL-G-CPE, not only the sensitivity increase, but the fouling effect of the analyte and its oxidation product also decreases.
Real sample analysis
In order to evaluate the analytical applicability of the proposed method, also it was applied to the determination of mangiferin in serum and urine samples. The results are given in Table 1.
Conclusion
Electrochemical behavior of mangiferin was studied for the first time at the surface of a graphene nanosheet/ionic liquid carbon paste electrode. The proposed modified electrode presented a low detection limit and good linear range and reproducibility which make it a suitable mangiferin sensor for practical applications.
References
Westervelt RM (2008) Science 320:324
Mallesha M, Manjunatha R, Nethravathi C, Suresh GS, Rajamathi M, Melo JS, Venkatesha TV (2011) Bioelectrochemistry 81:104
Gan T, Sun J, He M, Wang L (2013) Ionics. doi:10.1007/s11581-013-0951-9
Wan Q, Liu Y, Wang Z, Wei W, Li B, Zou J, Yang N (2013) Electrochem Commun 29:29
Sun W, Liu J, Ju X, Zhang L, Qi X, Hui N (2013) Ionics 19:657
Xi F, Zhao D, Wang X, Chen P (2013) Electrochem Commun 26:81
Sun W, Liu J, Wang X, Li T, Li G, Wu J, Zhang L (2012) Mater Sci Eng 32:2129
Afkhami A, Madrakian T, Shirzadmehr A, Bagheri H, Tabatabaee M (2012) Ionics 18:881
Banks CE, Compton RG (2006) Analyst 131:15
Ensafi AA, Rezaei B, Krimi-Maleh H (2011) Ionics 17:659
Sun W, Guo CX, Zhu Z, Li CM (2009) Electrochem Commun 11:2105
Zhuang RR, Jian FF, Wang KF (2010) Ionics 16:661
Shiddiky MJA, Torriero AAJ (2011) Biosens Bioelectron 26:775
Haynes LJ (1963) Adv Carbohydr Chem 18:227
Sanugul K, Akao T, Li Y, Kakiuchi N, Nakamura N, Hattori M (2005) Biol Pharm Bull 28:1672
Sanchez GM, Re L, Giuliani A, Nunez-Selles AJ, Davison GP, Leon Fernandez OS (2000) Pharm Res 42:565
Guha S, Ghosal S, Chattopadhyay U (1996) Chemotherapy 42:443
Garcia D, Escalante M, Delgado R, Ubeira FM, Leiro J (2003) Phytother Res 17:1203
Lee HZ, Lin WC, Yeh FT, Lin CN, Wu CH (1998) Eur J Pharm 353:303
Miura T, Iwamoto N, Kato M, Ichiki H, Kubo M, Komatsu Y (2001) Biol Pharm Bull 24:091
Yoshikawa M, Ninomiya K, Shimoda H, Nishida N, Matsuda H (2002) Biol Pharm Bull 25:72
Liu Y, Xu F, Zeng X, Yang L, Deng Y, Wu Z, Feng Y, Li X (2010) J Chromatogr B 878:3345
Huang W, Gan T, Luo S, Zhang S (2013) Ionics 19:1303–1307
Mokhtari A, Karimi-Maleh H, Ensafi AA, Beitollahi H (2012) Sens Actuators B 169:96
Biniak S, Pakuła M, Darlewski W, S’wia˛tkowski A, Kula P (2009) J Appl Electrochem 39:593
Gopu G, Muralidharan B, Vedhi C, Manisankar P (2012) Ionics 18:231
Tajik S, Taher MA, Beitollahi H (2013) Sens Actuators B 188:923
Yari A, Sepahvand R (2011) Microchim Acta 174:321
Xu G, Tang L, Liu H (2013) Ionics 19:309
Taleat Z, Mazloum Ardakani M, Naeimi H, Beitollahi H, Nejati M, Zare HR (2008) Anal Sci 24:1039
Beitollahi H, Mohadesi A, Khalilizadeh Mahani S, Karimi-Maleh H, Akbari A (2012) Ionics 18:703
Mohammadi S, Beitollahi H, Mohadesi A (2013) Sens Lett 11:388
Zhou J, Wang F, Zhang K, Song G, Liu J, Ye B (2012) Microchim Acta 178:179
Beitollahi H, Raoof JB, Hosseinzadeh R (2011) Electroanalysis 23:1934
Noroozifar M, Khorasani-Motlagh M, Bemanadi Parizi M, Akbari R (2013) Ionics 19:1317
Beitollahi H, Raoof JB, Hosseinzadeh R (2011) Anal Sci 27:991
Aydoğdu G, Zeybek DK, Zeybek B, Pekyardımcı Ş (2013) J Appl Electrochem 43:523
Mazloum-Ardakani M, Beitollahi H, Amini MK, Mirkhalaf F, Abdollahi-Alibeik M (2010) Sens Actuators B 151:243
Raoof JB, Ojani R, Beitollahi H (2007) Electroanalysis 19:1822
Beitollahi H, Ghorbani F (2013) Ionics 19:1673
Raoof JB, Ojani R, Beitollahi H, Hossienzadeh R (2006) Electroanalysis 18:1193
Chicharro M, Zapardiel A, Bermejo E, Moreno M, Madrid E (2002) Anal Bioanal Chem 373:277
Raoof JB, Ojani R, Beitollahi H, Hosseinzadeh R (2006) Anal Sci 22:1213
Wang Z, Liu G, Zhang, Wang H (2013) Ionics 19:1687
Hummers WS, Offeman RE (1958) J Am Chem Soc 80:1339
Kovtyukhova NI, Ollivier P, Martin RB, Mallouk TE, Chizhik SA, Buzaneva EV, Gorchinskiy AD (1999) Chem Mater 11:771
Gómez-Zaleta B, Ramírez-Silva MT, Gutiérrez A, González-Vergara E, Güizado-Rodríguez M, Rojas-Hernández A (2006) Spectrochim Acta 64:1002
Bard AJ, Faulkner LR (2001) Electrochemical methods fundamentals and applications, 2nd edn. Wiley, New York
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tajik, S., Taher, M.A. & Beitollahi, H. The first electrochemical sensor for determination of mangiferin based on an ionic liquid–graphene nanosheets paste electrode. Ionics 20, 1155–1161 (2014). https://doi.org/10.1007/s11581-013-1063-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-1063-2