Abstract
The objective of the present study was to investigate brain activity abnormalities in the early stage of Parkinson’s disease (PD). To achieve this goal, eyes-closed resting state electroencephalography (EEG) signals were recorded from 15 early-stage PD patients and 15 age-matched healthy controls. The AR Burg method and the wavelet packet entropy (WPE) method were used to characterize EEG signals in different frequency bands between the groups, respectively. In the case of the AR Burg method, an increase of relative powers in the δ- and θ-band, and a decrease of relative powers in the α- and β-band were observed for patients compared with controls. For the WPE method, EEG signals from patients showed significant higher entropy over the global frequency domain. Furthermore, WPE in the γ-band of patients was higher than that of controls, while WPE in the δ-, θ-, α- and β-band were all lower. All of these changes in EEG dynamics may represent early signs of cortical dysfunction, which have potential use as biomarkers of PD in the early stage. Our findings may be further used for early intervention and early diagnosis of PD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a common progressive neurodegenerative disorder of the central nervous system (Valls-Sole et al. 2002). Nowadays, PD affects about 1 % of the worldwide population over 55 years of age, and the number of PD patients in the elderly population has increased all the years recorded (Betarbet et al. 2002; Wooten et al. 2004). The motor clinical symptoms of PD, such as resting tremor, rigidity and postural instability (Savitt et al. 2006), result from dopaminergic deficiency in the basal ganglia. In addition, PD is also characterized by the presence of non-motor impairments including cognitive dysfunction, even in the early stage of PD (Cooper et al. 1991). However, the diagnosis of PD based on clinical symptoms is very difficult, especially in the early stage when there is no remarkable motor features and obvious cognitive dysfunction. Nonetheless, characterization of PD from the perspective of neuroscience provides us with an alternative way for exploring and quantifying corresponding brain functional neuronal mechanisms and improving diagnostic certainty (Valls-Sole et al. 2002).
Electroencephalography (EEG) is a non-invasive technique that records the electrical field produced by the neural electrical activity in the brain with good temporal resolution and high test-retest reliability, which is increasingly recognized as a fundamental hallmark of cortical integrative functions. It has been shown that quantifying EEG rhythms could provide an important biomarker for a lot of neuropsychiatric disorders, such as schizophrenia, major depressive disorder, Alzheimer’s disease, epilepsy and so on (Gandal et al. 2012; Hampel et al. 2010; Kheiri et al. 2012; Leuchter et al. 2009). This is critical for early intervention and early diagnosis, which is important for the efficient treatment of disease.
Several studies have shown EEG rhythm abnormalities in PD patients by using traditional fast Fourier transform method (FFT) or nonlinear time series methods (e.g. correlation dimension, LZ complexity) (de Weerd et al. 1990; Neufeld et al. 1988, 1994; Pezard et al. 2001; Soikkeli et al. 1991; Stoffers et al. 2007; Stam et al. 1995; Tanaka et al. 2000). These studies indicated that quantifying EEG may be useful in studying cognitive decline in PD, however, it is still unclear what kinds of measures might be useful. Moreover, studies usually included patients in advanced stages of PD. Hence, it is not known whether these quantitative EEG measures could reflect the changes in the early stage of PD patients relative to controls. Therefore, it is still necessary to use new analysis methods to analyze EEG signals of patients in the early stage of PD. New analysis methods together with EEG in the early stage of PD may contribute to reveal more important information underlying brain dysfunctions, which would be lost if analysis were restricted to traditional methods.
Nowadays, many novel methods are suggested for EEG signal processing. The AR Burg method is a recently developed technique to estimate power spectrum, which is widely used to estimate EEG of migraine, epileptic and alcoholic subjects (Akben et al. 2011; Faust et al. 2008). Compared with classical spectrum estimation methods (e.g. FFT), it can reduce the spectral losses and give better frequency resolution (Akin et al. 2000), and furthermore, it is very close to the true values. From a physiological point of view, spectral power in the EEG describes the activity of cortical pyramidal cells arranged in parallel and space-averaged over cortex (Nunez et al. 2001). Wavelet packet entropy (WPE) is a subtler multi-resolution method based on wavelet packet transform (WPT) and Shannon entropy (Coifman et al. 1990; Ocak et al. 2009). WPT is applied to extract different kinds of dynamic EEG rhythms accurately, and when combined with Shannon entropy, WPE can characterize the complexity of EEG rhythms.
The purpose of our study was to investigate whether patients in the early stage of non-demented PD shows electrophysiological indices of brain dysfunctions. To achieve this goal, we used both the AR burg method and the WPE method to quantify EEG signals, and further explored the differences between early-stage non-demented PD patients group and age-matched healthy controls group from the aspects of both power and complexity in the pattern of eye-closed resting state oscillatory brain activity. To our knowledge, this is the first time that these two methods are applied to study the brain activity in the early stage of PD.
Materials and methods
Subjects
Fifteen voluntary patients with a clinical diagnosis of idiopathic PD (7 males and 8 females, mean age 57.2 ± 7.7 years, range 47–69 years) were involved in our study. The mean duration of PD was 3.7 ± 1.6 years (range 1–6.2 years). The Hoehn and Yahr (H-Y) stage (Hoehn et al. 1967) was 1-2, 6 were in stage 1, 5 were in stage 1.5, 4 were in stage 2. The scores for the Mini-Mental-Status examination (MMSE) were 28.1 ± 1.2 (range 27–30), which was within normal limits. Exclusion criteria included atypical parkinsonism, use of neuroleptic drugs, antidepressants, dopamine blocking agents, or alcohol abuse, presence of other neurological or psychiatric conditions, and any other severe illness. All PD patients were receiving levodopa (L-dopa) drugs (Sinemet) in order to reduce the heterogeneity in the medication, and withdrew from L-dopa for at least 12 h before the study.
Fifteen age-matched healthy subjects (9 males and 6 females, mean age 58.4 ± 8.6, range 48–68 years) served as controls. They were healthy and intellectual, with no symptoms or history of neurological or psychiatric illness. All of them were normal by neurological examination. The scores of the MMSE for controls were 29.0 ± 0.8 (range 28–30). Both PD subjects and controls were right handed and without deficits in hearing.
Our study was performed with the approval of the local ethics committee. All subjects volunteered for this experiment. They were introduced to the nature of the experiment and their informed written consent was obtained according to the declaration of Helsinki.
EEG recordings
Subjects were seated upright in a recliner in a quiet and dimly shielded room with eyes closed to attain a state of relaxed wakefulness. They were told in advance that any movements should be tried to avoid during the experiment, such as body actions, eye movement/blink. EEG recording was continuous during the whole experiment. The recording provided us with sufficient data to intercept EEG signals free of artifacts.
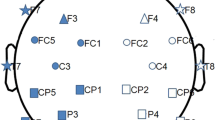
During recording, an EEG technician and neurophysiologist were present to monitor the quality of on-line EEG signals and to observe the performance status of subjects. When there was a sign of behavior, we usually made a mark in time without disturbing the subject. Furthermore, if there were sustained movements and/or EEG drowsiness, subject would be cued verbally and coached until recordings were free from artifacts. EEG signals were recorded on a Bio-logic Ceegraph-Vision system using 20 Ag–AgCl electrodes set on the scalp according to the international 10–20 system, as shown in Fig. 1. Linked ears were used as a reference. EEG impedances were kept below 5 \(k \Upomega\). EEG signals were recorded at a sampling rate 256 Hz.
The digitized EEG data were processed in a MATLAB environment (version 7.12.0.635, R2011a). Manual off-line inspection was first performed to locate and remove EEG signals related to behaviors according to the markers we had made. Furthermore, in order to eliminate the sustained effect of behaviors or sound cues on the EEG signals, the following 5-min length data after the markers were usually not taken into consideration. Clearly visible disturbances in the data were also deleted manually. Then, the data were digitally filtered with cut-off frequencies at 0.5–55 Hz in order to reduce artifacts components caused by residual myoelectricity and noise. Electrooculogram artifacts were further corrected according to the method proposed by Elbert et al. (1985). For each subject, we intercepted 80 s epoch from multichannel EEG signals free of artifacts for analysis.
Analysis method
AR Burg method
The AR Burg method is a powerful tool to estimate the power spectrum of signals. The spectrum estimation procedure has two steps. First, estimate the parameters of the model-based method from a given data sequence x(n), 0 ≤ n ≤ N − 1. Then, compute the power spectral density (PSD) estimate from these estimates.
The AR method is a model-based (parametric) method, which is based on modeling the data sequence x(n) as the output of a linear system characterized by a rational structure. Since AR parameters can be estimated easily by solving linear equations, the AR method is one of the most frequently used parametric methods. In the AR method, data can be modeled as the output of a causal, all-pole, discrete filter whose input is white noise. The AR method of order p (AR(p)) is expressed as the following equation
where a(k) are the AR coefficients and w(n) is the white noise of variance equal to σ2. AR(p) model can be characterized by AR parameters \(\left\{ {a[1],a[2],{\ldots},a[p],\sigma ^2 } \right\}. \) PSD is
where \(A(f) = 1 + a_1 e^{ - j2\pi f} + {\cdots} + a_p e^{ - j2\pi fp}.\)
In our study, AR coefficients are estimated by the Burg method, which is a recursive algorithm (Kay et al. 1988). It ensures a stable AR model and is computationally efficient (Akben et al. 2011). The Burg method is based on minimizing the forward and backward prediction errors and estimates the reflection coefficient.
From the estimates of AR parameters by the Burg algorithm, PSD estimation is formed as (Akben et al. 2011; Kay et al. 1981, 1988)
where \(\hat e_p \) is the total least squares error. The model order p of the AR method is determined by using Akaike information criterion (AIC). In our study, the model order is taken as p = 10.
Wavelet packet entropy method
The wavelet analysis is a widely used method in processing non-stationary signals. Wavelet coefficients can efficiently provide both full information and a direct estimation of local energies at different scales.
WPT can be viewed as a generalization of classical wavelet transform (WT), both of which are orthogonal wavelet decomposition procedure where a signal is passed through several filters. However, there are more processing filters used in WPT than that is used in WT. Figure 2a shows the decomposition process of a signal using WT into approximation and detail coefficients. Because WT decomposes only approximations of the signal and successive details are never reanalyzed, important information located in higher frequency components will be lost. To obtain good and adjustable frequency resolutions at high frequencies, WPT method is proposed (Coifman et al. 1990). Unlike WT, WPT not only decomposes approximations of the signal but also details. It holds the important information located in higher frequency components than WT in certain applications. The top level of WPT is the time representation of the signal, whereas the bottom level has better frequency resolution. Therefore, WPT can provide a better multi-resolution and time-frequency analysis for non-stationary data. Many literatures have demonstrated that WPT is one of the most promising methods for the feature extraction from EEG signals (Adeli et al. 2003; Ocak et al. 2009; Yang et al. 2006).
For n-level decomposition, WPT generates a full decomposition tree, as depicted in Fig. 2b. Corresponding to the wavelet, there are two finite impulse filters, i.e. a low-pass filter h(k) and a high-pass filter g(k). Using these two filters, the wavelet packet function can be defined as
where ψ (t) is the mother wavelet function. The recursive relations between the jth level and the (j + 1)th level for signal S(t) are
Thus, wavelet coefficients C j k at position k of level j can be expressed as
A detailed mathematical description of WPT is shown in literature (Coifman et al. 2008; Mallat 1989). In our study, wavelet packet algorithm is implemented by using DB5 function as mother wavelet with a scale of 32 (Wiklund et al. 1997; Torrence et al. 1998).
Then, the wavelet energy corresponding to the position indices at the sth level can be calculated as
and the total energy can be obtained from
where position index \(i = 0,{\ldots},2^s - 1.\) The relative wavelet energy in our work is defined as
The total relative energy can be easily found to be ∑ i p i = 1. Then, following the definition of Shannon entropy (Wiklund et al. 1997), WPE and WPE for each resolution level \((i = 1,2,{\ldots},N)\) can be defined as (Wang et al. 2011; Yang et al. 2006)
If the entropy value is greater than one, the component has a potential to reveal more information about the signal and it needs to be decomposed further in order to obtain simple frequency component of the signal (Shinde et al. 2004). WPE gives a measure of signal disorder or complexity, and provides distinctive features about the signal.
Statistical analysis
Each subject was characterized by relative power or WPE computed from EEG signals. All statistical analysis with regard to group differences was conducted by independent-samples t test. P values less than 0.01 was considered statistically significant difference between PD patients and controls. Because t test was applied with criterion variables of power in four different frequency bands and WPE in five different frequency bands, Bonferroni correction of P values was required for multiple comparisons correction. Therefore, in different frequency band, we tested the statistical significance of power at the alpha level 0.01/4 = 0.0025, and WPE at the alpha level 0.01/5 = 0.002 after correction.
Results
AR Burg method for spectral analysis
EEG contains different specific frequency bands. Features in sub-bands are particularly important to characterize different brain states. The standard frequency bands of interest were δ-band (0–4 Hz), θ-band (4–8 Hz), α-band (8–13 Hz) and β-band (13–30 Hz), respectively. The relative power in each sub-band can be obtained by dividing the power in each sub-band by the total power estimated by the AR Burg method.
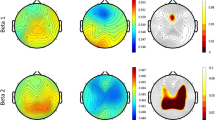
Figure 3 shows the δ-, θ-, α- and β-band relative powers of 20 EEG channels for two groups. It can be found that there was an increase in δ-band relative power of 20 EEG channels for PD patients, while a decrease in α- and β-band relative powers. However, there was no consistent trend of θ-band relative powers for different electrodes. Most EEG electrodes showed an increase in θ-band relative power for PD patients, while several other electrodes decreased, such as Fp1, Fp2, F7, F3, Fz and Oz.
Further spectral analysis results in the δ-, θ-, α- and β-band are summed up in Fig. 4 and Table 1. It can be seen that there were an increase in δ- and θ-band relative powers, and a decrease in α- and β-band relative powers for PD patients compared with controls. Moreover, differences were statistically significant in δ-, α- and β-band relative powers between PD patients and controls observed by the AR Burg method (P < 0.0025).
Wavelet packet entropy for complexity analysis
EEG signals were decomposed by five levels in our study. The lowest frequency resolution can be estimated as
where f s is sampling rate 256 Hz. There are 25 = 32 sub-bands of wavelet packet at the 5th level whose corresponding frequency ranges are \(f_1:[0,4],\, f_2:[4,8],\, f_3:[8,12],\, f_4:[12,16], \ldots,\, f_{31}:[120,124],\, f_{32}:[124,128].\) The new frequency bands have similar bands with the traditional frequency bands derived from the clinical EEG signal analysis. Specifically, δ-band is f 1:1–4 Hz, θ-band is f 2 :4–8 Hz, α-band is f 3: 8–12 Hz, β-band is f 4 − f 8:12–32 Hz, and γ-band is f 9 − f 32: >32 Hz. Thus, specific rhythm of EEG signals can be extracted by WPT.
Figure 5a is the brain topography of WPE for two groups. Compared with controls, it can be observed that there was an increase in WPE of different EEG channels in PD patients except channel Fz, Cz and Oz. In addition, notched boxplot was used for visualizing the distributions of WPE values averaged over 20 EEG electrodes for PD patients and controls, as shown in Fig. 5b. It can be found that the increase of WPE in the patients was significant different compared with controls (P < 0.001).
We further investigated how WPE distributes over different sub-bands. Figure 6 shows the WPE values of different sub-bands. For two groups, WPE in all sub frequency bands were not equal to each other, which was relatively bigger in lower sub-bands (f 1–f 17) than that in higher sub-bands (f 18–f 32). For different sub-bands, the comparative results of WPE values between PD patients and controls were different. For example, in the relatively lower sub-bands (f 1–f 8), WPE of PD patients was smaller than that of controls, while it was higher in sub-bands (f 9–f 12). For sub-bands (f 13–f 14), WPE of patients was lower relative to controls again. There were peak values of WPE for two groups, with a higher WPE in f 15 and lower WPE in f 16 for patients compared to controls. For sub-bands (f 17–f 32), there were almost no difference between two groups, except f 27 and f 31. In these two sub-bands, WPE of patients was bigger than that of controls.
Based on the results of WPE in 32 sub-bands, WPE values for 5 basic EEG bands were computed, as shown in Fig. 7. It can be clearly found that WPE in the δ-band, θ-band, α-band and β-band for PD patients were all lower than that for controls, while in the γ-band, it was higher compared to controls. Statistical results (see Table 2) revealed that differences of WPE values derived from EEG signals between patients and controls in 5 basic EEG bands were all statistically significant (P < 0.002), which demonstrates that the brain activity of PD patients was obviously more complex than that of controls in lower EEG bands.
Discussion
It is widely known that PD is a predominantly motor disorder caused by dopaminergic deficiency in the basal ganglia (Pezard et al. 2001). However, non-motor impairment involved cognitive dysfunction has frequently been observed in the early stages of PD (Cooper et al. 1991). Cognitive status was correlated with electrophysiological signals (e.g. EEG) over specific frequency band. Consequently, an understanding of the neural basis in PD is essential, both from a prognostic perspective as well as for the development of targeted therapeutic strategies. It has been shown that EEG analysis, such as linear or non-linear methods, could depict more global indices of brain functions, which can reflect the disturbed subcortico-cortical mechanisms in patients with advanced PD and/or dementia (de Weerd et al. 1990; Neufeld et al. 1988, 1994; Soikkeli et al. 1991; Stam et al. 1994, 1995; Tanaka et al. 2000). Thus, the goal of our study is to search for the EEG abnormalities in less advanced cases of PD without dementia. Furthermore, most of the patients in our study are in the early stage. It has been demonstrated that the changes of brain dynamics in the early stage of PD can be characterized by the AR Burg method and WPE measure relative to controls.
As for the AR Burg method, we found an increase of the relative powers in the δ- and θ-band, and a decrease in the α- and β-band for PD patients compared to controls, relating to slowing of resting state oscillatory brain activity. The increase of θ-band relative power for PD patients was not significantly different relative to controls, while the differences in the δ-, α- and β-band were statistically significant.
Over the years, several studies have focus on spectral analysis of the brain activity in PD patients, which is still a controversial issue. Pezard et al. (2001) used traditional FFT method to characterize spectral power in different EEG frequency bands, and found a significant decrease of β-band power in non-demented PD patients of the early stages relative to controls. In addition, Olde Dubbelink et al. (2013) used another brain activity recording technique, i.e. Magnetoencephalography (MEG), to study early changes in early-stage non-demented PD patients, which revealed an increase in θ power over time, along with a decrease in α power in contrast to healthy controls. The results of our recorded EEG signals in the early stage of PD observed by the AR Burg method are in line with these previous studies. Moreover, Stoffer et al. (2007) noted extensive changes in oscillatory brain activity from MEG signals when comparing untreated patients in the first clinical stages of PD to controls, which showed widespread increase in θ power as well as overall decrease in β power that are similar to our results, while an enhancement of α power that is just the opposite of our findings.
So far, there are only a few studies related to PD in the early stage, and the majority of previous findings in PD have focus on the non-demented or demented patients without exact stages. Recent studies using EEG or MEG have noted that there was stronger increase of power in demented as compared to non-demented PD patients (Caviness et al. 2007; Neufeld et al. 1994; Soikkeli et al. 1991), and the latter showed the increase of power in low frequency domain (Bosboom et al. 2006; Kotini et al. 2005; Serizawa et al. 2008; Moazami-Goudarzi et al. 2008), or even in all frequency bands (Tanaka et al. 2000) in comparison with controls. For example, Soikkeli et al. (1991) reported an increase of θ activity and a decrease of β power in demented PD patients using FFT analysis of EEG data obtained with a single occipital electrode, and Neufeld et al. (1994) also suggested consistent trend of increase in the δ- and θ-band and a significant decrease in the α-band for demented PD patients, but both of them did not obtain similar results in non-demented patients relative to controls. For non-dement PD, Serizawa and Moazami-Goudarzi et al. presented higher spectral power over low frequency domain and diffuse slowing of EEG compared with healthy controls (Serizawa et al. 2008; Moazami-Goudarzi et al. 2008). MEG studies carried out by Bosboom and Kotini et al. showed similar results that relative θ power was diffusely increased and β power concomitantly decreased in the non-demented PD patients relative to controls (Bosboom et al. 2006; Kotini et al. 2005). These results indicated that PD must be associated with the slowing of oscillatory brain activity, which are quite comparable with our findings. On the contrary, Tanaka et al. (2000) reported that there was an increase of α power relative to controls, which is quite different from previous studies including ours.
With regard to the WPE method, we observed that EEG signals of PD patients in the early stage were characterized by a higher WPE over the global frequency domain. WPE is an index that can quantify the complexity of the signal, which is model independent. The higher WPE indicated EEG signals for PD patients were more complex compared to controls, which means that the quality of prediction of EEG dynamics decreased more rapidly in PD patients than that in controls. Pezard et al. (2001) also found that EEG of early-stage PD patients was characterized by a higher entropy using local entropy, which is comparable with our results observed by relative WPE. However, some previous studies got different results by using nonlinear times series analysis methods. For example, Muller et al. (2001) found that in the resting state, correlation dimension of EEG signals revealed only slight differences between the early-stage PD patients and healthy controls, while Stam et al. (1995) found that there was a decrease in Kolmogorov entropy and correlation dimension for PD patients, which indicated a complexity decrease in PD patients relative to controls. In addition, MEG studies by Gomez et al. (2011) also showed that the oscillatory brain activity of PD patients in the early stage was less complex than that of controls by using LZ complexity measure.
Furthermore, we also found that WPE in the δ-, θ-, α- and β-band for PD patients were all lower relative to controls, while it was higher in the γ-band. The increase of WPE in the γ-band for PD patients indicated the γ-band activity was more complex, while the other four basic bands all become relatively ordered. Recent studies both on animals and humans have suggested that γ-band activity played an important role in attention as well as working and long-term memory (Herrmann et al. 2004). Hence, it can be concluded that γ-band activity getting more complex may be considered as a reflection of dysfunction in PD patients. Besides, it has been reported that β-band oscillatory activity in the basal ganglia associated with the pathology that gave rise to tremor in PD (Levy et al. 2000). β-band activity getting more ordered detected in our studies may be related to the symptoms of resting tremor in PD patients. Moreover, it has been shown the resting tremor in PD was associated with an altered balance between β and γ oscillations in the motor circuits of the subthalamic nucleus (STN) (Weinberger et al. 2009). By computing WPE, we have shown a decrease of WPE in the β-band and an increase in the γ-band for PD patients compared with controls. This can be regarded as a marker of the altered balance between β and γ oscillations.
In addition, several confounding factors, such as experiment state (e.g. rest of specific task), ages, disease severity, medication and size, should be considered that may have influence on the studies. It has been shown that brain oscillatory was abnormal in PD patients during performance of specific tasks (Muller et al. 2001; Wang et al. 2012), but different task performance design of these studies made it difficult for us to compare and interpret. This is one of the main reasons why most of the recent studies choose eye-closed resting state to investigate. In addition, it has been reported that a loss of physiological complexity often accompanied aging (Kyriazis 2003), so we selected aged-matched subject as controls. In our study, to minimize the effects of medication on experiment results, although EEG was recorded at least 12 h after the last dose of medication, we could not fully rule out medication effects on the power or complexity differences between patients and controls. Besides, the variance with earlier reports may also possibly be due to the size of the patient group, the disease severity of the selected patients, or even the property of the algorithm itself in different studies.
Conclusions
In conclusion, this study presents the AR Burg method and WPE as novel methods to study the oscillatory brain activity from EEG signals in the early-stage PD patients without dementia. Our findings demonstrated the usefulness of the AR Burg method and the WPE method in detecting abnormalities dynamics associated with PD. Relative power and wavelet entropy depicted several significant differences of EEG between PD patients of the early stage and controls. The decrease in β-band relative power and the increase of WPE may represent the early signs of subcortico-cortical dysfunction in PD patients. PD is a complicated neurodegenerative disease, and there must be a lot of information underlying brain activity. Therefore, as a step forward, it is necessary to apply new analysis methods to extract more typical features from EEG signals of PD patients in the early stage, and further make classification analysis based on those characteristic indices, which may have potential use as biomarkers of PD in the early stage and provide an objective technique for the early diagnosis of PD.
References
Adeli H, Zhou Z et al (2003) Analysis of EEG records in an epileptic patient using wavelet transform. J Neurosci Methods 123:69–87
Akben S B, Subasi A et al (2011) Analysis of EEG signals under flash stimulation for migraine and epileptic patients. J Med Syst 35:437–443
Akin M, Kiymik MK (2000) Application of periodogram and AR spectral analysis to EEG signals. J Med Syst 24:247–256
Betarbet R, Sherer TB et al (2002) Animal models of Parkinson’s disease. Bioessays 24:308–318
Bosboom JL, Stoffers D et al (2006) Resting state oscillatory brain dynamics in Parkinson’s disease: an MEG study. Clin Neurophysiol 117(11):2521–2531
Caviness JN, Hentz JG et al (2007) Both early and late cognitive dysfunction affects the electroencephalogram in Parkinson’s disease. Parkinsonism Relat Disord 13(6):348–354
Coifman R, Meyer Y et al (1990) Signal processing and compression with wavelet packets. Yale University, New Haven
Coifman RR, Wickerhauser MV (2008) Entropy-based algorithms for best basis selection. IEEE Trans Inf Theory 38:713–718
Cooper JA, Sagar HJ et al (1991) Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain 114(Pt 5):2095–2122
de Weerd AW, Perquin WVM et al (1990) Role of the EEG in the prediction of dementia in Parkinson’s disease. Dementia 1:115–118
Elbert T, Lutzenberger W et al (1985) Removal of ocular artifacts from the EEG—a biophysical approach to the EOG. Electroencephalogr Clin Neurophysiol 60(5):455–463
Faust O, Acharya RU et al (2008) Analysis of EEG signals during epileptic and alcoholic states using AR modeling techniques. ITBM-RBM 29:44–52
Gandal MJ, Edgar JC et al (2012) Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 62:1504–1518
Gomez C, Olde Dubbelink KT et al (2011) Complexity analysis of resting-state MEG activity in early-stage Parkinson’s disease patients. Ann Biomed Eng 39(12):2935–2944
Hampel H, Frank R et al (2010) Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov 9:560–574
Herrmann CS, Munk MH et al (2004) Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci 8:347–355
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Kay SM (1988) Modern spectral estimation: theory and application. Prentice-Hall, New Jersey
Kay SM, Marple SL (1981) Spectrum analysis-a modern perspective. Proc IEEE 69:1380–1419
Kheiri F, Bragin A et al (2012) Non-linear classification of heart rate parameters as a biomarker for epileptogenesis. Epilepsy Res 100:59–66
Kotini A, Anninos P et al (2005) Low-frequency MEG activity and MRI evaluation in Parkinson’s disease. Brain Topogr 18(1):59–63
Kyriazis M (2003) Practical applications of chaos theory to the modulation of human ageing: nature prefers chaos to regularity. Biogerontology 4(2):75–90
Leuchter AF, Cook IA et al (2009) Effectiveness of a quantitative electroencephalographic biomarker for predicting differential response or remission with escitalopram and bupropion in major depressive disorder. Psychiatry Res 169:132–138
Levy R, Hutchison WD et al (2000) High-frequency synchronization of neuronal activity in the subthalamic nucleus of parkinsonian patients with limb tremor. J Neurosci 20:7766–7775
Mallat S (1989) A theory for multiresolution signal decomposition: the wavelet representation. IEEE Pattern Anal Mach Intell 11:674–693
Moazami-Goudarzi M, Sarnthein J et al (2008) Enhanced frontal low and high frequency power and synchronization in the resting EEG of parkinsonian patients. Neuroimage 41(3):985–997
Muller V, Lutzenberger W et al (2001) Investigation of brain dynamics in Parkinson’s disease by methods derived from nonlinear dynamics. Exp Brain Res 137(1):103–110
Neufeld MY, Inzelberg R et al (1988) EEG in demented and non-demented parkinsonian patients. Acta Neurol Scand 78:1–5
Neufeld MY, Blumen S et al (1994) EEG frequency analysis in demented and nondemented parkinsonian patients. Dementia 5:23–28
Nunez PL, Wingeier BM et al (2001) Spatial-temporal structures of human alpha rhythms: theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp 13(3):125–164
Ocak H (2009) Automatic detection of epileptic seizures in EEG using discrete wavelet transform and approximate entropy. Expert Syst Appl 36:2027–2036
Olde Dubbelink KTE, Stoffers D et al (2013) Cognitive decline in Parkinson’s disease is associated with slowing of resting-state brain activity: a longitudinal study. Neurobiol Aging 34(2):408–418
Pezard L, Jech R et al (2001) Investigation of non-linear properties of multichannel EEG in the early stages of Parkinson’s disease. Clin Neurophysiol 112:38–45
Savitt JM, Dawson VL et al (2006) Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest 116:1744–1754
Serizawa K, Kamei S et al (2008) Comparison of quantitative EEGs between parkinson disease and age-adjusted normal controls. J Clin Neurophysiol 25(6):361–366
Shinde A (2004) A wavelet packet based sifting process and its application for structural health monitoring. Master Thesis, Faculty of Worcester Polytechnic Institute
Soikkeli R, Partanen J et al (1991) Slowing of EEG in Parkinson’s disease. Electroencephalogr Clin Neurophysiol 79:159–165
Stam KJ, Tavy DL et al (1994) Non-linear dynamical analysis of multichannel EEG: clinical applications in dementia and Parkinson’s disease. Brain Topogr 7:141–150
Stam CJ, Jelles B et al (1995) Investigation of EEG non-linearity in dementia and Parkinson’s disease. Electroencephalogr Clin Neurophysiol 95:309–317
Stoffers D, Bosboom JL et al (2007) Slowing of oscillatory brain activity is a stable characteristic of Parkinson’s disease without dementia. Brain 130:1847–1860
Tanaka H, Koenig T et al (2000) Event-related potential and EEG measures in Parkinson’s disease without and with dementia. Dement Geriatr Cogn Disord 11:39–45
Torrence C, Compo GP (1998) A practical guide to wavelet analysis. Bull Am Meteorol Soc 79:61–78
Valls-Sole J, Valldeoriola F (2002) Neurophysiological correlate of clinical signs in Parkinson’s disease. Clin Neurophysiol 113:792–805
Wang D, Miao D et al (2011) Best basis-based wavelet packet entropy feature extraction and hierarchical EEG classification for epileptic detection. Expert Syst Appl 38:14314–14320
Wang M, Wang B et al (2012) A new quantitative evaluation method of spiral drawing for patients with Parkinson’s disease based on a polar coordinate system with varying origin. Physica A Stat Mech Appl 391(18):4377–4388
Weinberger M, Hutchison WD et al (2009) Increased gamma oscillatory activity in the subthalamic nucleus during tremor in Parkinson’s disease patients. J Neurophysiol 101:789–802
Wiklund U, Akay M et al (1997) Short-term analysis of heart-rate variability by adapted wavelet transforms. IEEE Eng Med Biol Mag 16:113–118, 138
Wooten GF, Currie LJ et al (2004) Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry 75:637–639
Yang BH, Yan GZ et al (2006) Feature extraction for EEG-based brain-computer interfaces by wavelet packet best basis decomposition. J Neural Eng 3:251–256
Acknowledgments
Project supported by the Key Program of the National Natural Science Foundation of China (Grant No. 50537030), the National Natural Science Foundation of China (Grant No. 61072012), and the Young Scientists Fund of the National Natural Science Foundation of China (Grant Nos. 61104032 and 50907044).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, CX., Wang, J., Yi, GS. et al. Investigation of EEG abnormalities in the early stage of Parkinson’s disease. Cogn Neurodyn 7, 351–359 (2013). https://doi.org/10.1007/s11571-013-9247-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11571-013-9247-z